Abstract
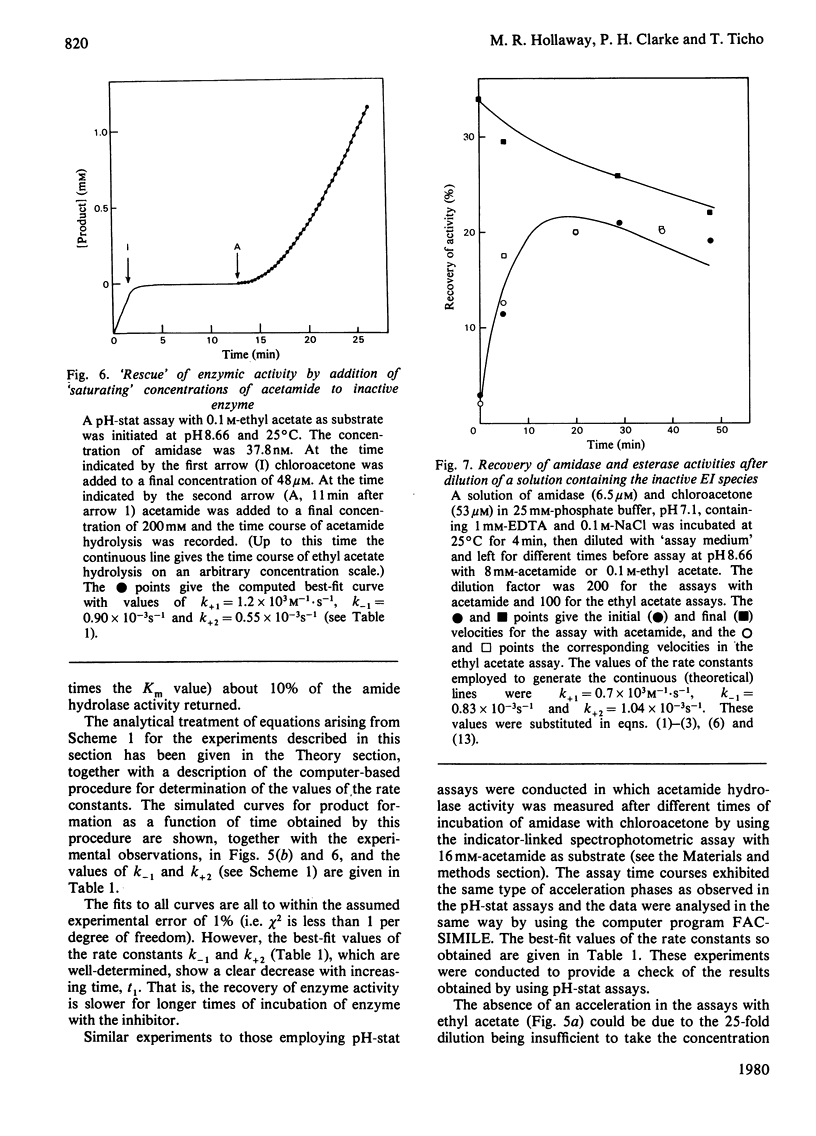
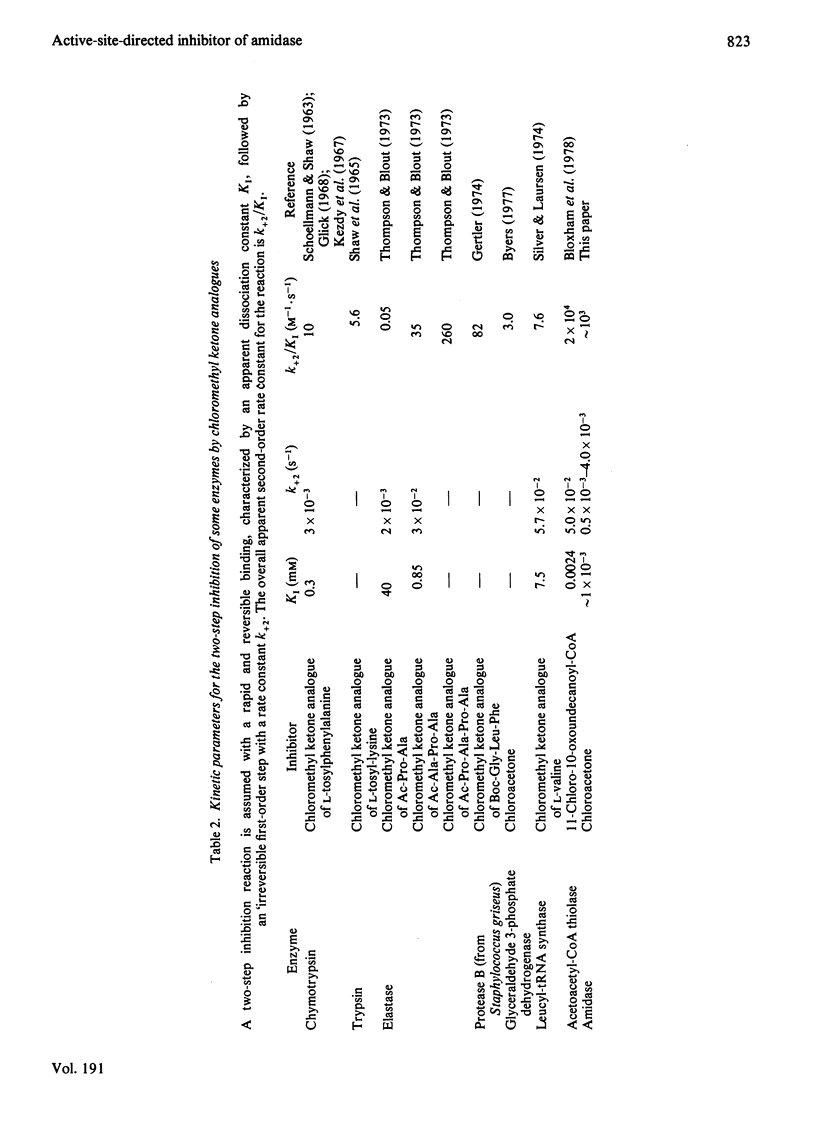
1. Chloroacetone (I) was shown to be an active-site-directed inhibitor of the aliphatic amidase (EC 3.5.1.4) from Pseudomonas aeruginosa strain PAC142.2. This inhibitor reacted with the enzyme in two stages: the first involving the reversible formation of an enzymically inactive species, EI, and the second the formation of a species, EX, from which enzymic activity could not be recovered. 3. Different types of kinetic experiment were conducted to test conformity of the reaction to the scheme: E + I k+1 Equilibrium k-1 EI Leads to K+2 EX A computer-based analysis of the results was carried out and values of the individual rate constants were determined. 4. No direct evidence for a binding step before the formation of EI could be obtained, as with [E]0 Less Than [I]0 the observed first-order rate constant for the formation of EI was directly proportional to the concentration of chloroacetone up to 1.2 mM (above this concentration the reaction became too rapid to follow even by the stopped-flow method developed to investigate fast inhibition). 5. The value of k+1 exhibited a bell-shaped pH-dependency with a maximum value of about 3 X 10(3) M-1. S-1 at pH6 and apparent pKa values of 7.8 and about 4.8.6. The values of k-1 and K+2 were similar and changed with the time of reaction from values of about 3 X 10(-3) S-1 (pH8.6) at short times to about one-sixth this value for longer periods of incubation. In this respect the simple reaction scheme is insufficient to describe the inhibition process. 7. The overall inhibition reaction is rapid, whether it is considered in relation to the expected chemical reactivity of chloroacetone, the rate of reaction of other enzymes with substrate analogues containing the chloromethyl group, or the rate of the amidase-catalysed hydrolysis of N-methylacetamide, a substrate that is nearly isosteric with chloroacetone. 8. Acetamide protected the amidase from inhibition by chloroacetone, and the concentration-dependence of the protection gave a value of an apparent dissociation constant similar to the Km value for this substrate. 9. Addition of acetamide to solutions of the species EI led to a slow recovery of activity. Recovery of active enzyme was also observed after dilution of a solution of EI in the absence of substrate. 10. The species EI is considered not to be a simple adsorption complex, and the possibilities are discussed that it may be a tetrahedral carbonyl adduct, a Schiff base (azomethine) or a complex in which the enzyme has undergone a structural change. The species EX is probably a derivative in which there is a covalent bond between a group in the enzyme and the C-1 atom of the inhibitor.
Full text
PDF




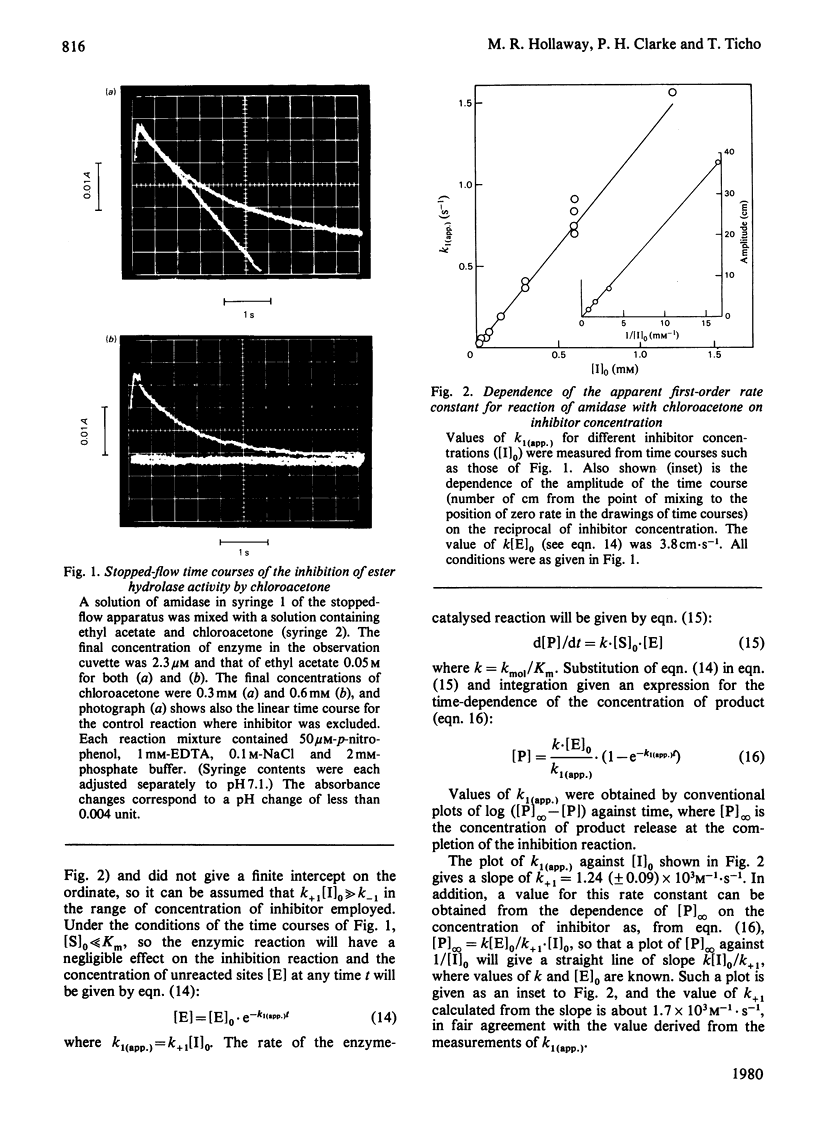
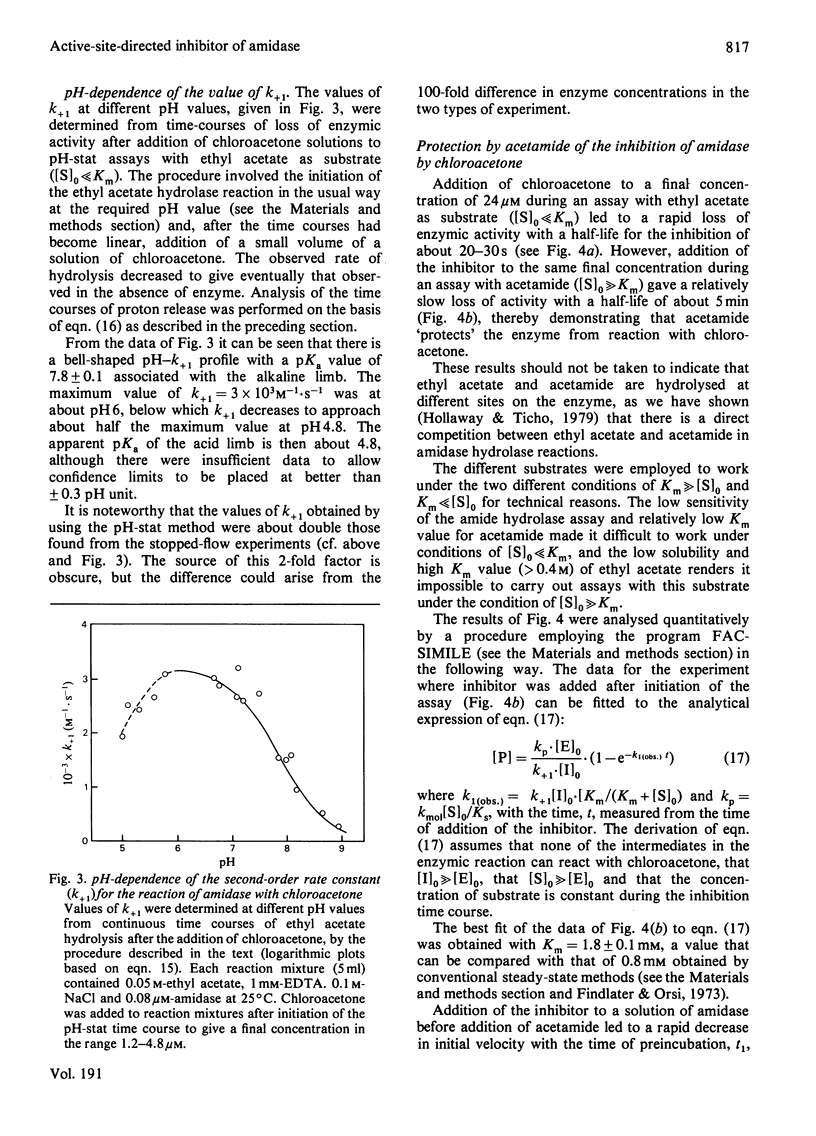
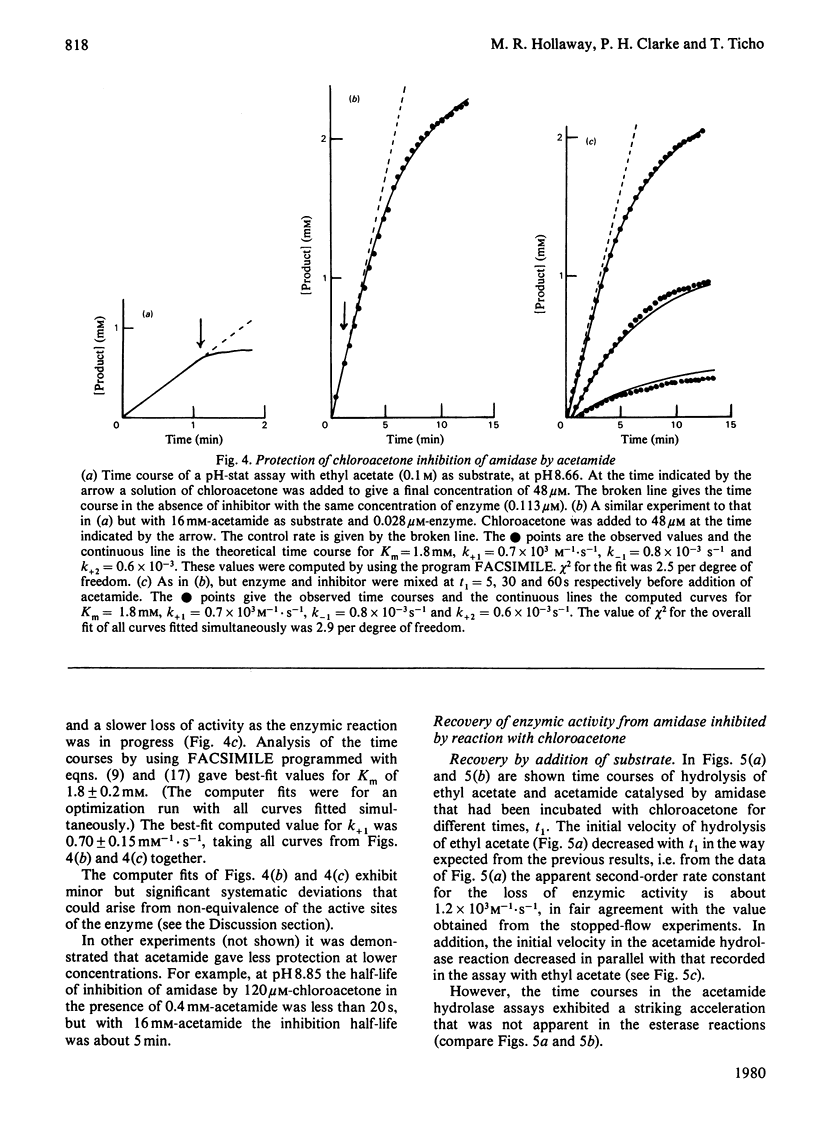
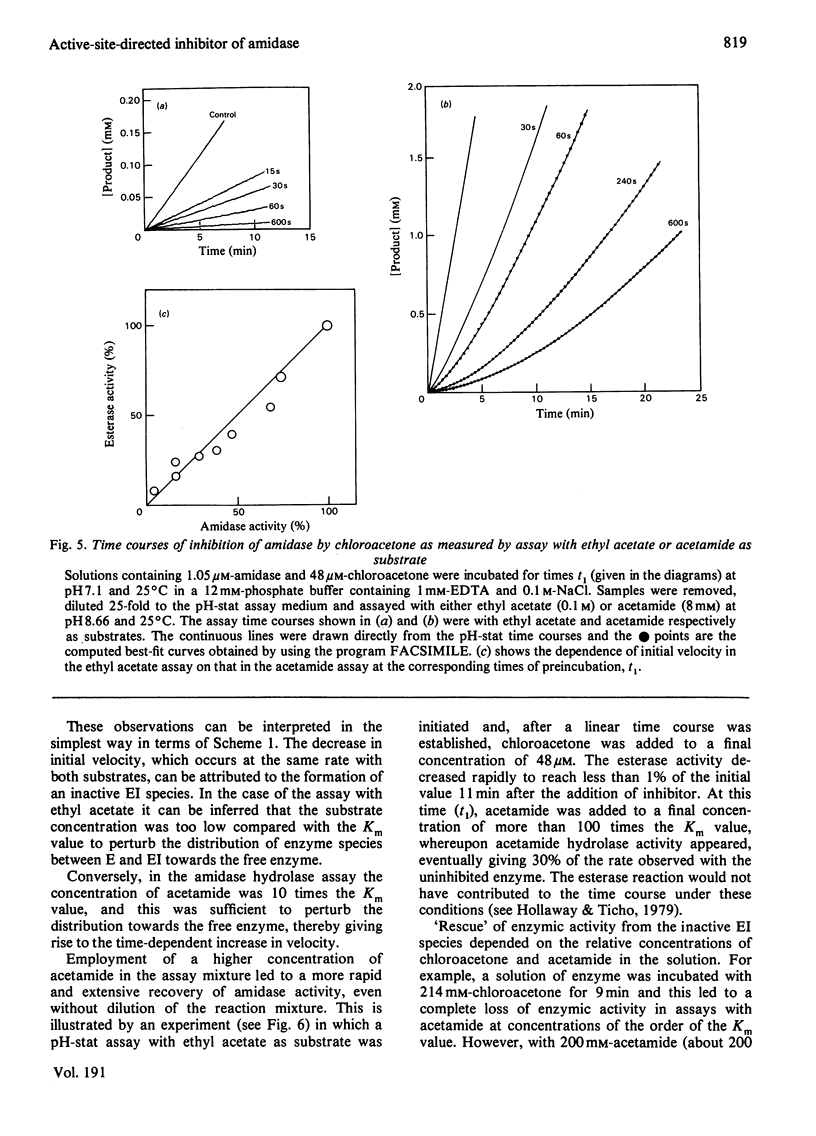



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BRAMMAR W. J., CLARKE P. H. INDUCTION AND REPRESSION OF PSEUDOMONAS AERUGINOSA AMIDASE. J Gen Microbiol. 1964 Dec;37:307–319. doi: 10.1099/00221287-37-3-307. [DOI] [PubMed] [Google Scholar]
- Bloxham D. P., Chalkley R. A., Coghlin S. J., Salam W. Synthesis of chloromethyl ketone derivatives of fatty acids. Their use as specific inhibitors of acetoacetyl-coenzyme A thiolase, cholesterol biosynthesis and fatty acid synthesis. Biochem J. 1978 Dec 1;175(3):999–1011. doi: 10.1042/bj1750999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J. E., Brown P. R., Clarke P. H. Butyramide-utilizing mutants of Pseudomonas aeruginosa 8602 which produce an amidase with altered substrate specificity. J Gen Microbiol. 1969 Aug;57(2):273–285. doi: 10.1099/00221287-57-2-273. [DOI] [PubMed] [Google Scholar]
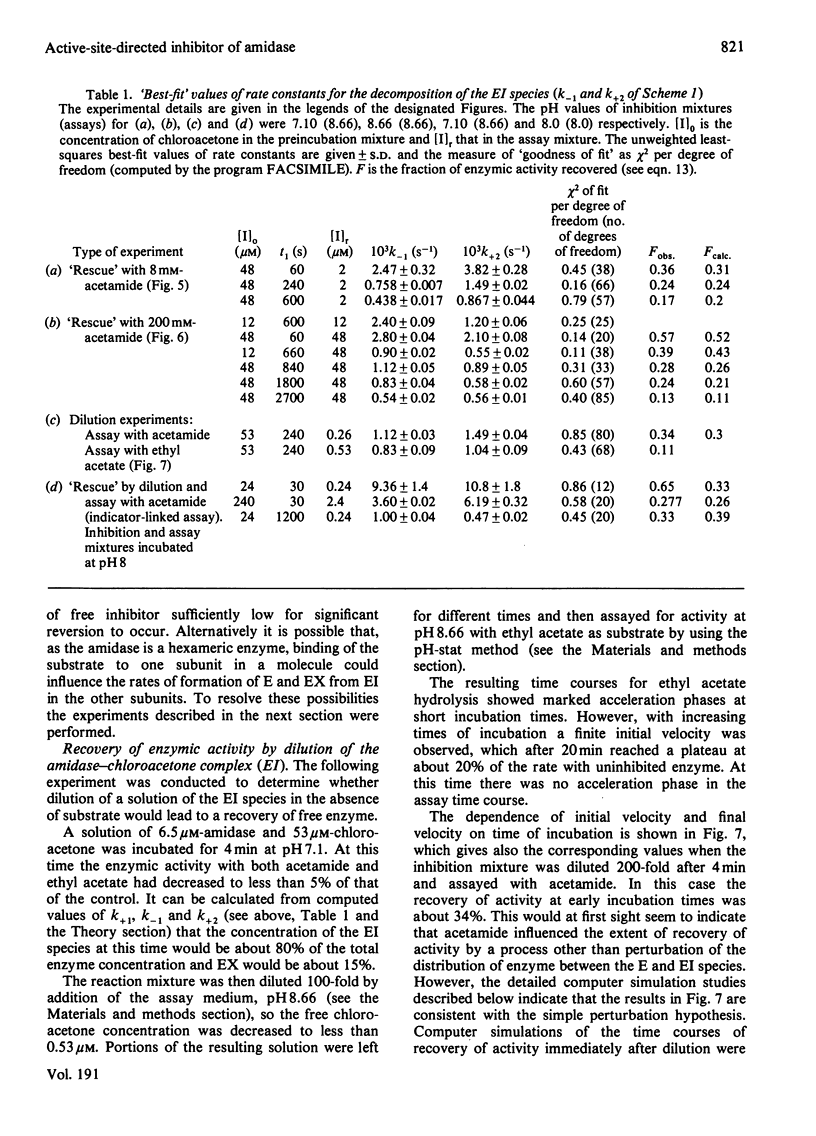
- Brown P. R., Clarke P. H. Amino acid substitution in an amidase produced by an acetanilide-utilizing mutant of Pseudomonas aeruginosa. J Gen Microbiol. 1972 Apr;70(2):287–288. doi: 10.1099/00221287-70-2-287. [DOI] [PubMed] [Google Scholar]
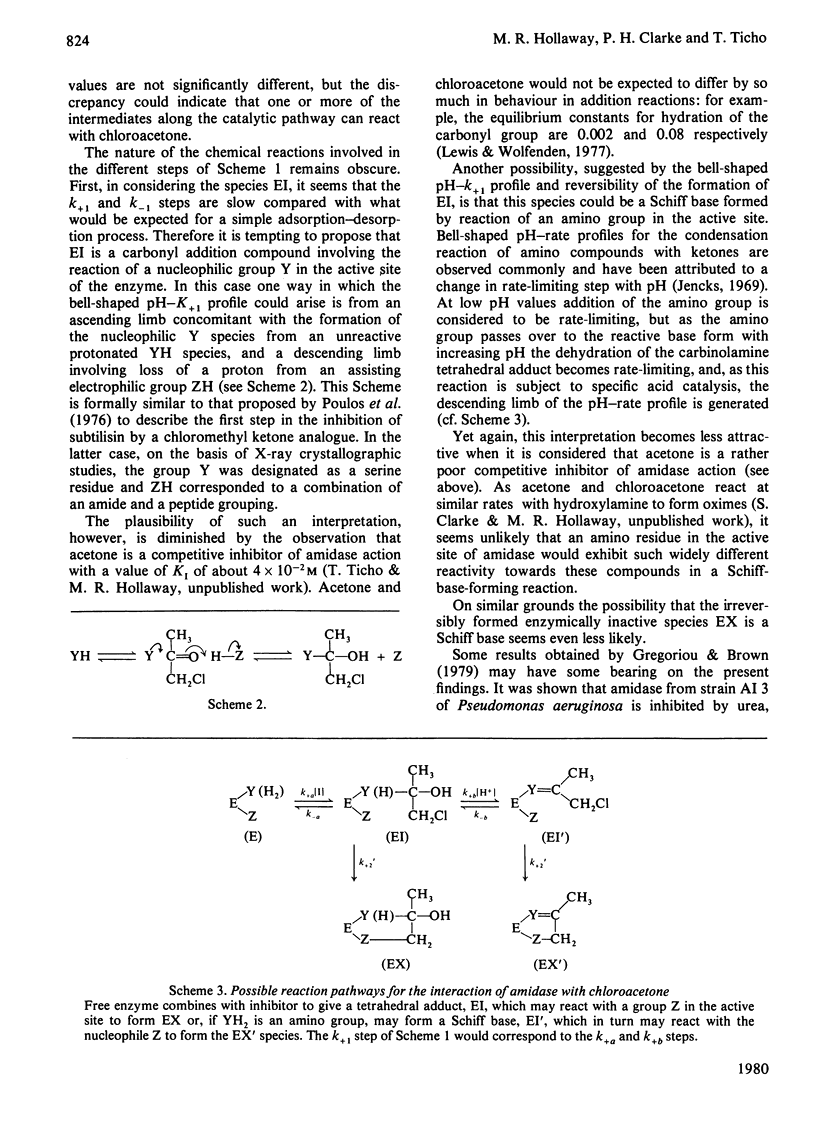
- Brown P. R., Smyth M. J., Clarke P. H., Rosemeyer M. A. The subunit structure of the aliphatic amidase from Pseudomonas aeruginosa. Eur J Biochem. 1973 Apr 2;34(1):177–187. doi: 10.1111/j.1432-1033.1973.tb02744.x. [DOI] [PubMed] [Google Scholar]
- Byers L. D. Criteria for evaluationg enzymic rate enhancements. The case of glyceraldehyde-3-phosphate dehydrogenase. J Am Chem Soc. 1977 Jun 8;99(12):4146–4149. doi: 10.1021/ja00454a039. [DOI] [PubMed] [Google Scholar]
- ELLMAN G. L. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959 May;82(1):70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- Findlater J. D., Orsi B. A. Transition-state analogs of an aliphatic amidase. FEBS Lett. 1973 Sep 1;35(1):109–111. doi: 10.1016/0014-5793(73)80588-5. [DOI] [PubMed] [Google Scholar]
- Gertler A. Inhibition of Streptomyces griseus protease B by peptide chloromethyl ketones: partial mapping of the binding site and identification of the reactive residue. FEBS Lett. 1974 Jul 1;43(1):81–85. doi: 10.1016/0014-5793(74)81110-5. [DOI] [PubMed] [Google Scholar]
- Glick D. M. Ligand-induced pK changes in chymotrypsin. Biochemistry. 1968 Oct;7(10):3390–3396. doi: 10.1021/bi00850a012. [DOI] [PubMed] [Google Scholar]
- Gregoriou M., Brown P. R. Inhibition of the aliphatic amidase from Pseudomonas aeruginosa by urea and related compounds. Eur J Biochem. 1979 May 2;96(1):101–108. doi: 10.1111/j.1432-1033.1979.tb13018.x. [DOI] [PubMed] [Google Scholar]
- Hollaway M. R., Ticho T. A competition time-course method for following enzymic reactions applied to the hydrolysis of acetamide catalysed by an aliphatic amidase. FEBS Lett. 1979 Oct 1;106(1):185–188. doi: 10.1016/0014-5793(79)80724-3. [DOI] [PubMed] [Google Scholar]
- KELLY M., CLARKE P. H. An inducible amidase produced by a strain of Pseudomonas aeruginosa. J Gen Microbiol. 1962 Feb;27:305–316. doi: 10.1099/00221287-27-2-305. [DOI] [PubMed] [Google Scholar]
- Kézdy F. J., Thomson A., Bender M. L. Studies on the reaction of chymotrypsin and L-1-chloro-3-tosylamido-4-phenyl-2-butanone. J Am Chem Soc. 1967 Feb 15;89(4):1004–1009. doi: 10.1021/ja00980a044. [DOI] [PubMed] [Google Scholar]
- Lewis C. A., Jr, Wolfenden R. Antiproteolytic aldehydes and ketones: substituent and secondary deuterium isotope effects on equilibrium addition of water and other nucleophiles. Biochemistry. 1977 Nov 1;16(22):4886–4890. doi: 10.1021/bi00641a022. [DOI] [PubMed] [Google Scholar]
- Lienhard G. E., Jencks W. P. Thiol addition to the carbonyl group. Equilibria and kinetics. J Am Chem Soc. 1966 Sep 5;88(17):3982–3994. doi: 10.1021/ja00969a017. [DOI] [PubMed] [Google Scholar]
- Poulos T. L., Alden R. A., Freer S. T., Birktoft J. J., Kraut J. Polypeptide halomethyl ketones bind to serine proteases as analogs of the tetrahedral intermediate. X-ray crystallographic comparison of lysine- and phenylalanine-polypeptide chloromethyl ketone-inhibited subtilisin. J Biol Chem. 1976 Feb 25;251(4):1097–1103. [PubMed] [Google Scholar]
- SCHOELLMANN G., SHAW E. Direct evidence for the presence of histidine in the active center of chymotrypsin. Biochemistry. 1963 Mar-Apr;2:252–255. doi: 10.1021/bi00902a008. [DOI] [PubMed] [Google Scholar]
- Silver J., Laursen R. A. Inactivation of aminoacyl-tRNA synthetases by amino acid chloromethylketones. Biochim Biophys Acta. 1974 Feb 27;340(1):77–89. doi: 10.1016/0005-2787(74)90175-0. [DOI] [PubMed] [Google Scholar]
- Sluyterman L. A. The rate-limiting reaction in papain action as derived from the reaction of the enzyme with chloroacetic acid. Biochim Biophys Acta. 1968 Jan 8;151(1):178–187. doi: 10.1016/0005-2744(68)90172-1. [DOI] [PubMed] [Google Scholar]
- Smyth P. F., Clarke P. H. Catabolite repression of Pseudomonas aeruginosa amidase: the effect of carbon source on amidase synthesis. J Gen Microbiol. 1975 Sep;90(1):81–90. doi: 10.1099/00221287-90-1-81. [DOI] [PubMed] [Google Scholar]
- Thompson R. C., Blout E. R. Peptide chloromethyl ketones as irreversible inhibitors of elastase. Biochemistry. 1973 Jan 2;12(1):44–47. doi: 10.1021/bi00725a008. [DOI] [PubMed] [Google Scholar]


