Abstract
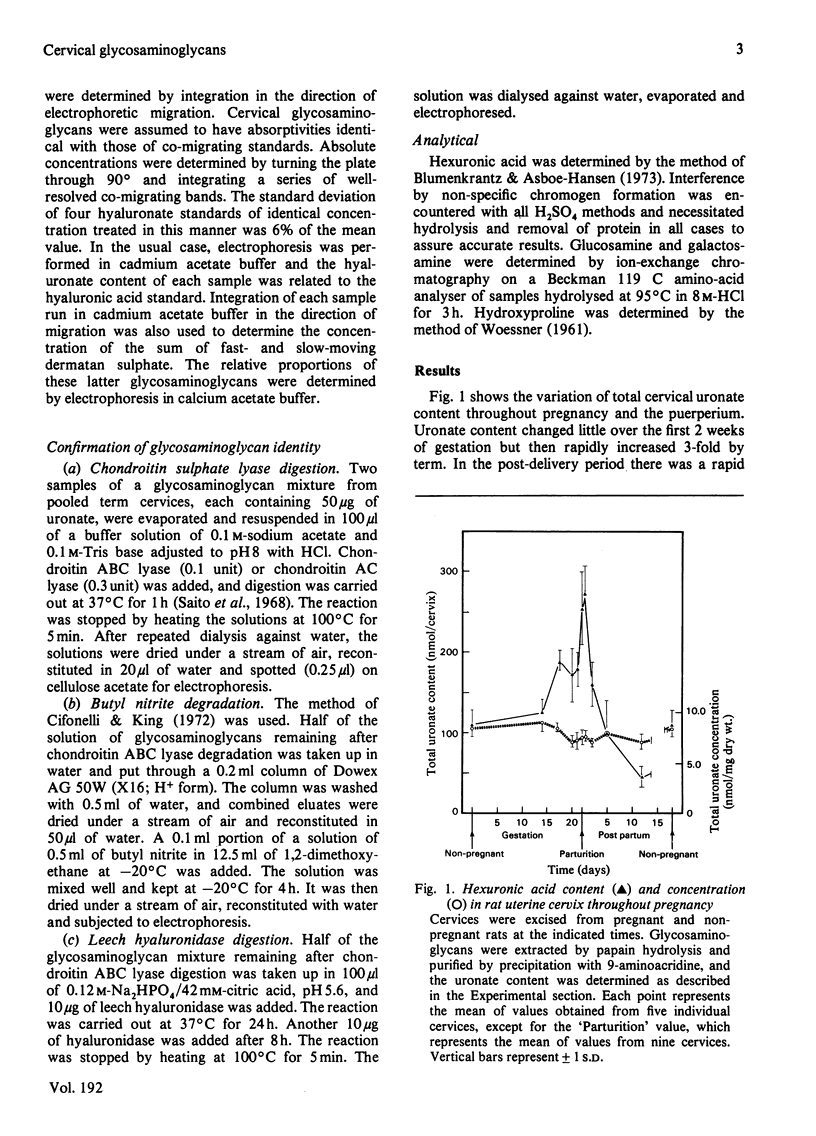
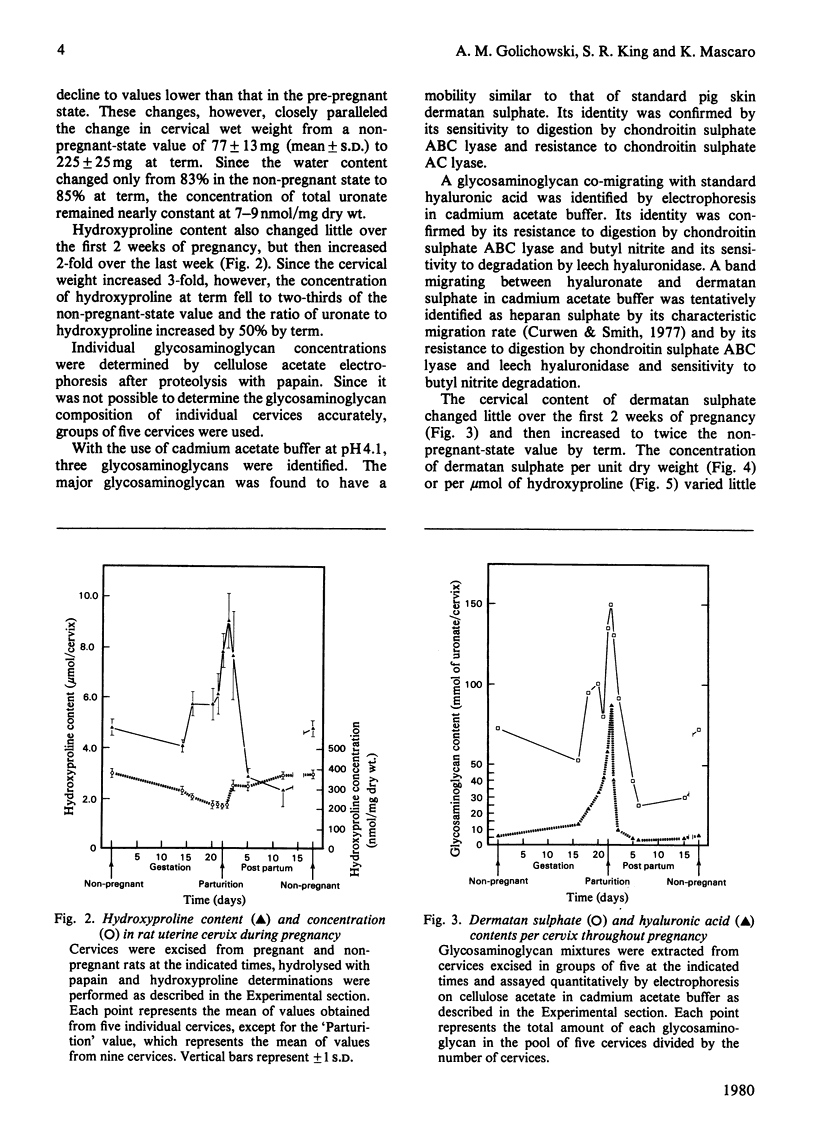
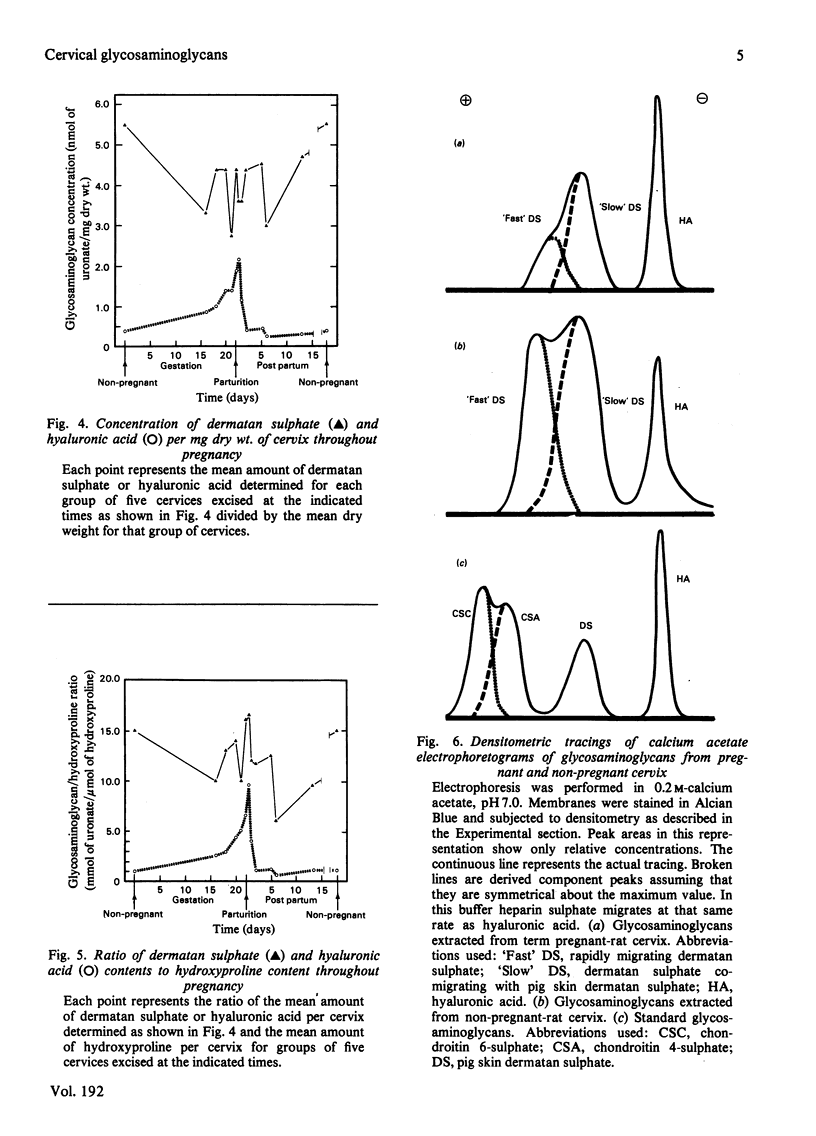
Non-pregnant and pregnant rats of known gestational age were killed at intervals and their uterine cervices were excised and digested with papain. Glycosaminoglycans thus extracted were separated by cellulose acetate electrophoresis and stained with Alcian Blue. Glycosaminoglycans were identified by comparison with standards and by serial degradation with chondroitin ABC lyase, butyl nitrite and leech hyaluronidase. Dermatan sulphate, hyaluronic acid and heparan sulphate were identified and quantitative determined by densitometry. The overall concentration of glycosaminoglycans changed little during pregnancy. A 3-fold total increase in uronic acid paralleled the increase in cervical weight. Hyaluronate content, however, increased 17-fold, and rose from 6% of total glycosaminoglycans in the non-pregnant state to 33% at term. Furthermore, the ratio of hyaluronate to hydroxyproline increased 10-fold. These changes are consistent with an accumulation of hyaluronate in the interstices between collagen fibres, resulting in the softening of this tissue that is seen in late pregnancy.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BUCKINGHAM J. C., SELDEN R., DANFORTH D. N. Connective tissue changes in the cervix during pregnancy and labor. Ann N Y Acad Sci. 1962 Sep 29;97:733–742. doi: 10.1111/j.1749-6632.1962.tb34682.x. [DOI] [PubMed] [Google Scholar]
- Blumenkrantz N., Asboe-Hansen G. New method for quantitative determination of uronic acids. Anal Biochem. 1973 Aug;54(2):484–489. doi: 10.1016/0003-2697(73)90377-1. [DOI] [PubMed] [Google Scholar]
- Bryant W. M., Greenwell J. E., Weeks P. M. Alterations in collagen organization during dilatation of the cervix uteri. Surg Gynecol Obstet. 1968 Jan;126(1):27–39. [PubMed] [Google Scholar]
- Cifonelli J. A., King J. The distribution of 2-acetamido-2-deoxy-D-glucose residues in mammalian heparins. Carbohydr Res. 1972 Feb;21(2):173–186. doi: 10.1016/s0008-6215(00)82144-8. [DOI] [PubMed] [Google Scholar]
- Cretius K., Hannig K., Beier G. Untersuchungen zur Löslichkeit und zum Verhalten des Kollagens im nichtschwangeren und im schwangeren menschlichen Uterus. Arch Gynakol. 1966;203(4):329–353. doi: 10.1007/BF00673134. [DOI] [PubMed] [Google Scholar]
- Curwen K. D., Smith S. C. Quantitative microanalysis of aortic glycosaminoglycans. Anal Biochem. 1977 May 1;79(1-2):291–301. doi: 10.1016/0003-2697(77)90404-3. [DOI] [PubMed] [Google Scholar]
- DANFORTH D. N., BUCKINGHAM J. C., RODDICK J. W., Jr Connective tissue changes incident to cervical effacement. Am J Obstet Gynecol. 1960 Nov;80:939–945. doi: 10.1016/0002-9378(60)90472-5. [DOI] [PubMed] [Google Scholar]
- Damle S. P., Kieras F. J., Tzeng W. K., Gregory J. D. Isolation and characterization of proteochondroitin sulfate from pig skin. J Biol Chem. 1979 Mar 10;254(5):1614–1620. [PubMed] [Google Scholar]
- Danforth D. N., Veis A., Breen M., Weinstein H. G., Buckingham J. C., Manalo P. The effect of pregnancy and labor on the human cervix: changes in collagen, glycoproteins, and glycosaminoglycans. Am J Obstet Gynecol. 1974 Nov 1;120(5):641–651. doi: 10.1016/0002-9378(74)90608-5. [DOI] [PubMed] [Google Scholar]
- Flint M. Interrelationships of mucopolysaccharide and collagen in connective tissue remodelling. J Embryol Exp Morphol. 1972 Apr;27(2):481–495. [PubMed] [Google Scholar]
- Gillard G. C., Merrilees M. J., Bell-Booth P. G., Reilly H. C., Flint M. H. The proteoglycan content and the axial periodicity of collagen in tendon. Biochem J. 1977 Apr 1;163(1):145–151. doi: 10.1042/bj1630145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HARKNESS M. L., HARKNESS R. D. Changes in the physical properties of the uterine cervix of the rat during pregnancy. J Physiol. 1959 Oct;148:524–547. doi: 10.1113/jphysiol.1959.sp006304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HARKNESS M. L., HARKNESS R. D. The collagen content of the reproductive tract of the rat during pregnancy and lactation. J Physiol. 1954 Mar 29;123(3):492–500. doi: 10.1113/jphysiol.1954.sp005066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HARKNESS M. L., HARKNESS R. D. The mechanical properties of the uterine cervix of the rat during involution after parturition. J Physiol. 1961 Apr;156:112–120. doi: 10.1113/jphysiol.1961.sp006661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HARKNESS R. D. Metabolism of collagen. Lect Sci Basis Med. 1955 1956;5:183–216. [PubMed] [Google Scholar]
- HARKNESS R. D., NIGHTINGALE M. A. The extensibility of the cervix uteri of the rat at different times of pregnancy. J Physiol. 1962 Feb;160:214–220. doi: 10.1113/jphysiol.1962.sp006842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopwood J. J., Dorfman A. Glycosaminoglycan synthesis by Wilms' tumor. Pediatr Res. 1978 Jan;12(1):52–56. doi: 10.1203/00006450-197801000-00013. [DOI] [PubMed] [Google Scholar]
- Hopwood J. J., Fitch F. W., Dorfman A., Kennedy J. P., Jr Hyaluronic acid synthesis in a cell-free system from rat fibrosarcoma. Biochem Biophys Res Commun. 1974 Nov 27;61(2):583–590. doi: 10.1016/0006-291x(74)90997-8. [DOI] [PubMed] [Google Scholar]
- KIMMEL J. R., SMITH E. L. Crystalline papain. I. Preparation, specificity, and activation. J Biol Chem. 1954 Apr;207(2):515–531. [PubMed] [Google Scholar]
- Kleissl H. P., van der Rest M., Naftolin F., Glorieux F. H., de Leon A. Collagen changes in the human uterine cervix at parturition. Am J Obstet Gynecol. 1978 Apr 1;130(7):748–753. doi: 10.1016/0002-9378(78)90003-0. [DOI] [PubMed] [Google Scholar]
- Kondo T. Histochemical studies on the human cervix uteri. 1. Acid mucopolysaccharides of the human cervix uteri in nonpregnancy. 2. Acid mucopolysaccharides of the human cervix uteri during pregnancy. Nagoya Med J. 1972 Feb;17(3):103–121. [PubMed] [Google Scholar]
- Maillot K. V., Zimmermann B. K. The solubility of collagen of the uterine cervix during pregnancy and labour. Arch Gynakol. 1976 Apr 29;220(4):275–280. doi: 10.1007/BF00673411. [DOI] [PubMed] [Google Scholar]
- Obrink B. A study of the interactions between monomeric tropocollagen and glycosaminoglycans. Eur J Biochem. 1973 Mar 1;33(2):387–400. doi: 10.1111/j.1432-1033.1973.tb02695.x. [DOI] [PubMed] [Google Scholar]
- Obrink B., Laurent T. C., Carlsson B. The binding of chondroitin sulphate to collagen. FEBS Lett. 1975 Aug 1;56(1):166–169. doi: 10.1016/0014-5793(75)80133-5. [DOI] [PubMed] [Google Scholar]
- Obrink B., Sundelöf L. O. Light scattering in the study of associating macromolecules. The binding of glycosaminoglycans to collagen. Eur J Biochem. 1973 Aug 17;37(2):226–232. doi: 10.1111/j.1432-1033.1973.tb02979.x. [DOI] [PubMed] [Google Scholar]
- Orkin R. W., Toole B. P. Hyaluronidase activity and hyaluronate content of the developing chick embryo heart. Dev Biol. 1978 Oct;66(2):308–320. doi: 10.1016/0012-1606(78)90240-3. [DOI] [PubMed] [Google Scholar]
- Rimmer D. M. The effect of pregnancy on the collagen of the uterine cervix of the mouse. J Endocrinol. 1973 Jun;57(3):413–418. doi: 10.1677/joe.0.0570413. [DOI] [PubMed] [Google Scholar]
- Saito H., Yamagata T., Suzuki S. Enzymatic methods for the determination of small quantities of isomeric chondroitin sulfates. J Biol Chem. 1968 Apr 10;243(7):1536–1542. [PubMed] [Google Scholar]
- Stanbury J. B., Embery G. An improved electrophoretic procedure for the detection of acidic glycosaminoglycans (mucopolysaccharides). Med Lab Sci. 1977 Jul;34(3):267–269. [PubMed] [Google Scholar]
- Stys S. J., Clewell W. H., Meschia G. Changes in cervical compliance at parturition independent of uterine activity. Am J Obstet Gynecol. 1978 Feb 15;130(4):414–418. doi: 10.1016/0002-9378(78)90282-x. [DOI] [PubMed] [Google Scholar]
- Toole B. P. Binding and precipitation of soluble collagens by chick embryo cartilage proteoglycan. J Biol Chem. 1976 Feb 10;251(3):895–897. [PubMed] [Google Scholar]
- Toole B. P., Lowther D. A. Dermatan sulfate-protein: isolation from and interaction with collagen. Arch Biochem Biophys. 1968 Dec;128(3):567–578. doi: 10.1016/0003-9861(68)90064-7. [DOI] [PubMed] [Google Scholar]
- Tsiganos C. P., Muir H. Studies on protein-polysaccharides from pig laryngeal cartilage. Heterogeneity, fractionation and characterization. Biochem J. 1969 Aug;113(5):885–894. doi: 10.1042/bj1130885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WOESSNER J. F., Jr The determination of hydroxyproline in tissue and protein samples containing small proportions of this imino acid. Arch Biochem Biophys. 1961 May;93:440–447. doi: 10.1016/0003-9861(61)90291-0. [DOI] [PubMed] [Google Scholar]
- ZARROW M. X., YOCHIM J. Dilation of the uterine cervix of the rat and accompanying changes during the estrous cycle, pregnancy and following treatment with estradiol, progesterone and relaxin. Endocrinology. 1961 Aug;69:292–304. doi: 10.1210/endo-69-2-292. [DOI] [PubMed] [Google Scholar]
- von Maillot K., Greiling H. Der Glykosoaminoglykan-Gehalt der Cervix uteri während Schwangerschaft und Geburt. Arch Gynakol. 1977 Jul 29;224(1-4):220–221. doi: 10.1007/BF00679534. [DOI] [PubMed] [Google Scholar]


