Abstract
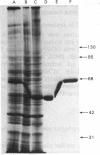
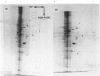
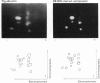
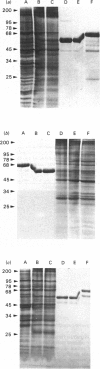
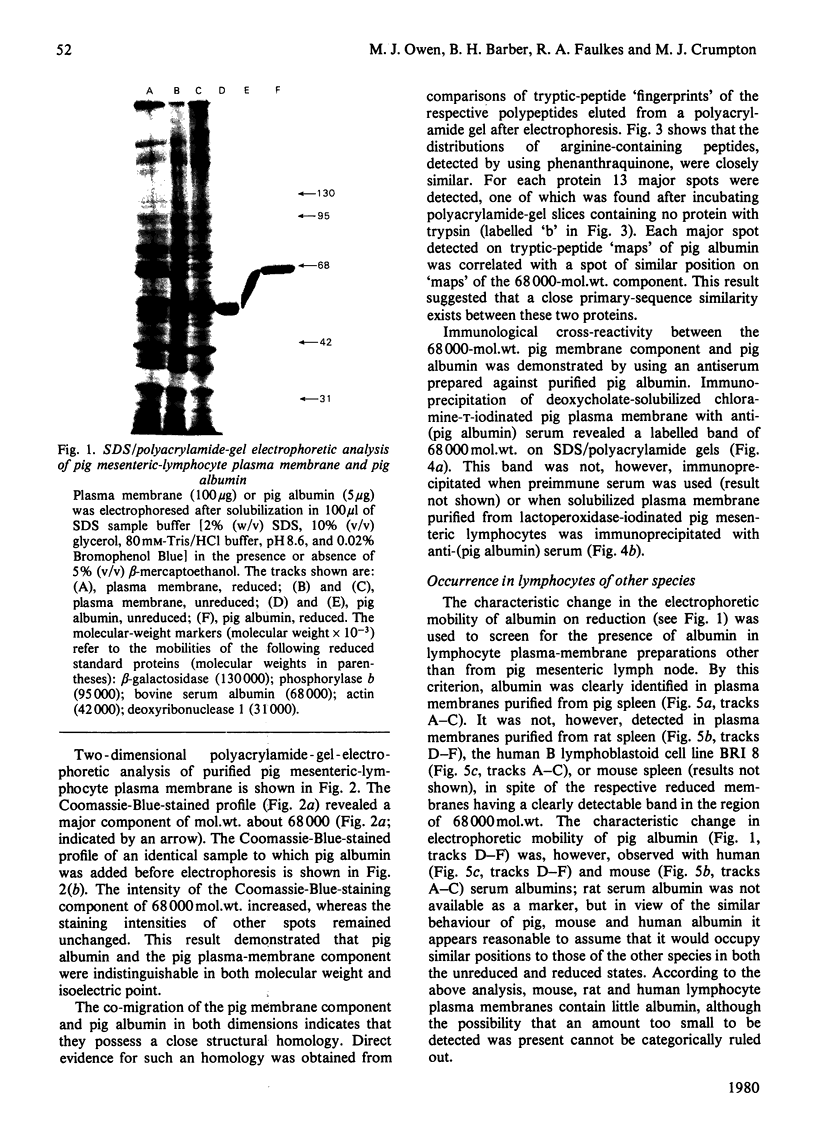
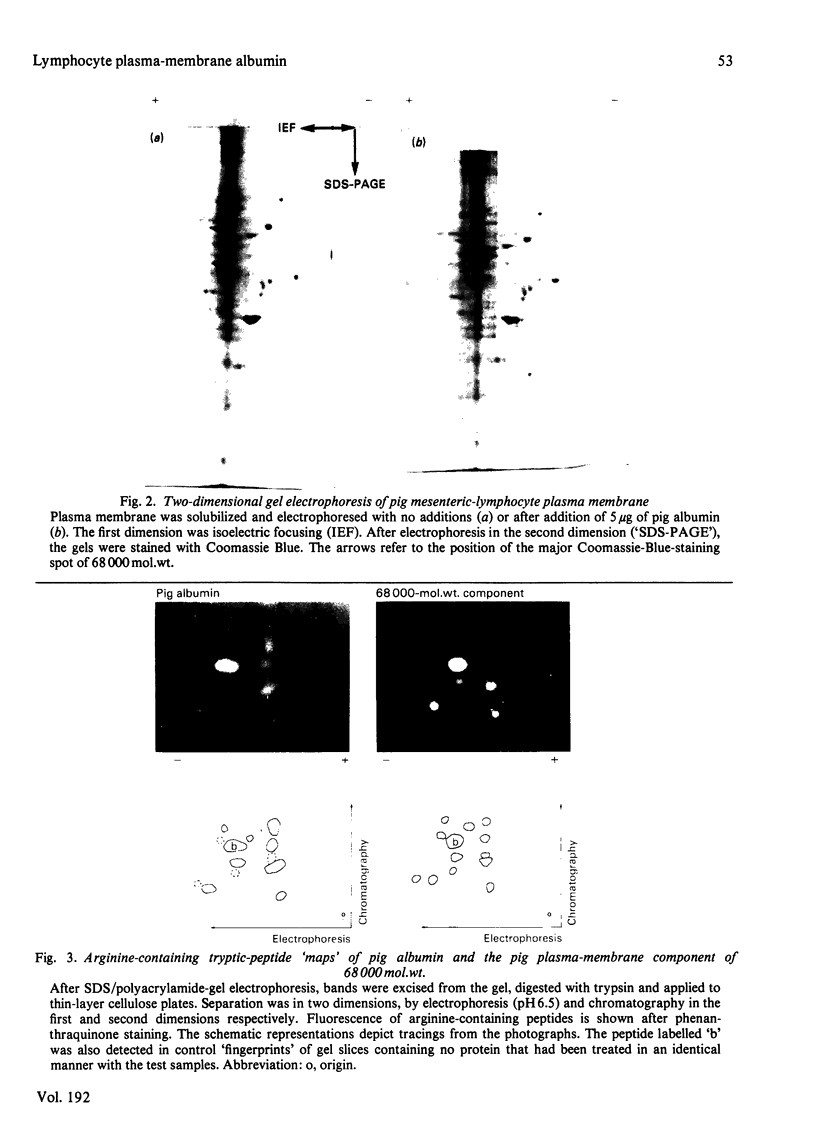
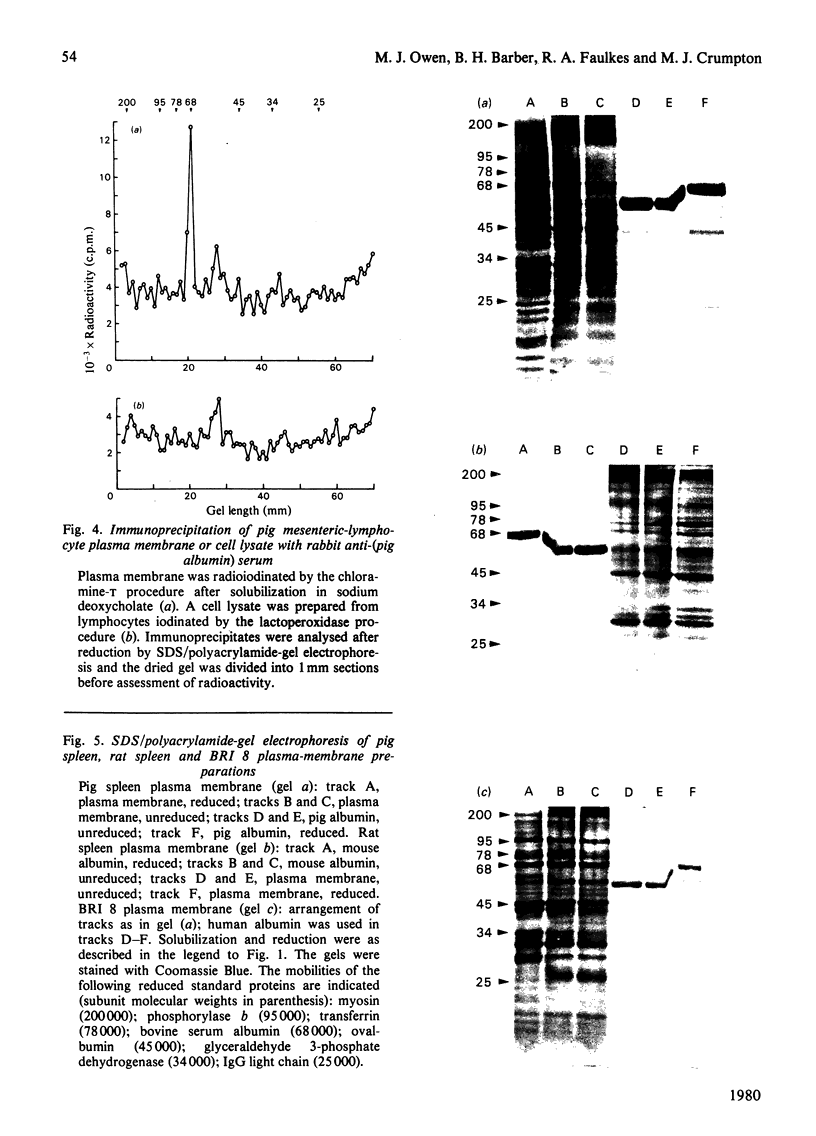
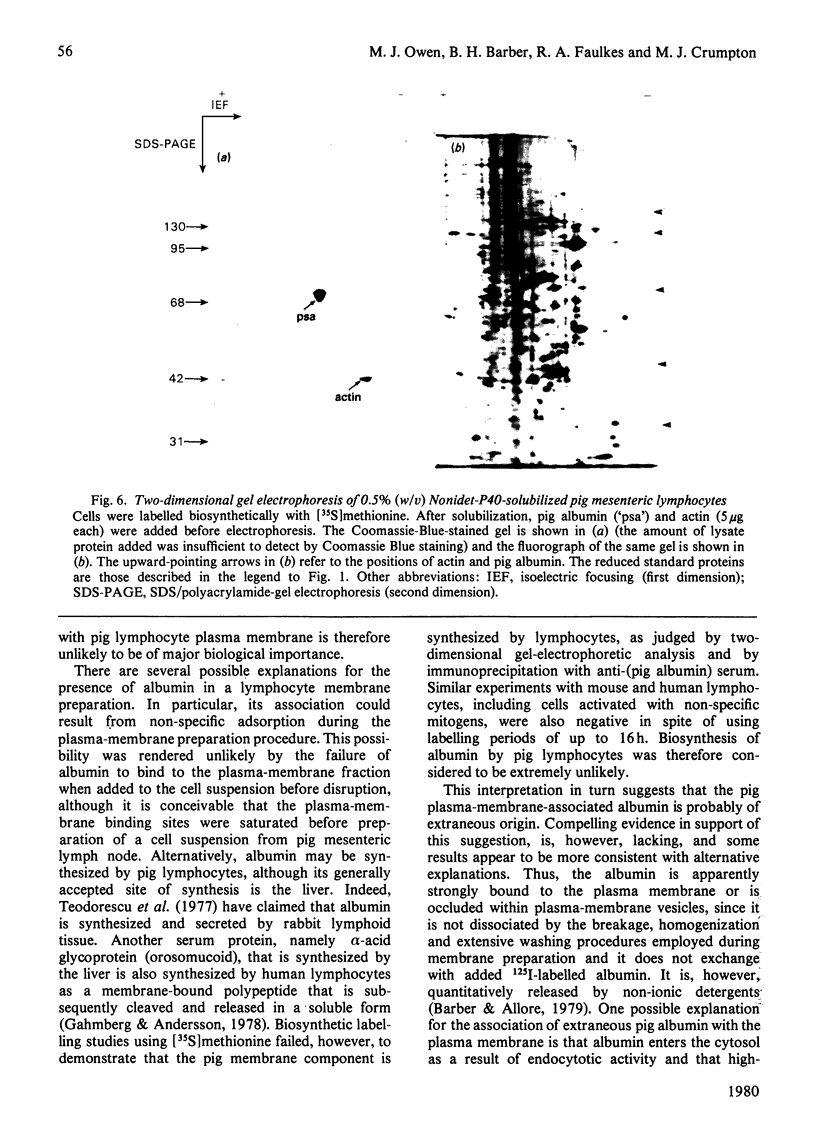
Plasma-membrane preparations purified from pig lymphocytes contained a major polypeptide component of mol.wt. about 68 000. This component was identified as pig albumin by the following comparisons with authentic pig serum albumin: (a) co-migration when analysed by sodium dodecyl sulphate/polyacrylamide-gel electrophoresis under reducing and non-reducing conditions; (b) identical isoelectric points; (c) similar "fingerprints" of arginine-containing tryptic peptides; (d) reactivity with anti-(pig albumin) serum. The albumin was bound tightly to the plasma membrane. Biosynthetic labelling of pig lymphocytes under a variety of conditions failed to provide evidence that albumin was synthesized by lymphocytes, suggesting that the plasma-membrane-associated albumin was of extraneous origin. Radiolabelled pig serum albumin, however, failed to bind to the plasma-membrane fraction when added before cell disruption. Although lymphocyte plasma membrane preparations from other species possessed a polypeptide of about 68 000 mol.wt., this was judged not to be albumin on the basis of electrophoretic mobility under non-reducing conditions; also, no polypeptide was precipitated by anti-albumin sera. It is concluded that pig lymphocyte plasma-membrane preparations possess albumin which, although firmly attached, was probably of extraneous origin. This association appeared not to be common to lymphocytes from other species.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allan D., Crumpton M. J. Isolation and composition of human thymocyte plasma membrane. Biochim Biophys Acta. 1972 Jul 3;274(1):22–27. doi: 10.1016/0005-2736(72)90276-3. [DOI] [PubMed] [Google Scholar]
- Allan D., Crumpton M. J. Solubilization of pig lymphocyte plasma membrane and fractionation of some of the components. Biochem J. 1971 Aug;123(5):967–975. doi: 10.1042/bj1230967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ames G. F., Nikaido K. Two-dimensional gel electrophoresis of membrane proteins. Biochemistry. 1976 Feb 10;15(3):616–623. doi: 10.1021/bi00648a026. [DOI] [PubMed] [Google Scholar]
- Barber B. H., Crumpton M. J. Actin associated with purified lymphocyte plasma membrane. FEBS Lett. 1976 Jul 15;66(2):215–220. doi: 10.1016/0014-5793(76)80507-8. [DOI] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Chavin S. I., Holliman A. Reconstitution of pig lymphocyte plasma membranes from solubilized components, with particular reference to membrane-associated immunoglobulins. Biochem J. 1975 Nov;152(2):267–270. doi: 10.1042/bj1520267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crumpton M. J., Snary D. Preparation and properties of lymphocyte plasma membrane. Contemp Top Mol Immunol. 1974;3:27–56. doi: 10.1007/978-1-4684-2838-4_2. [DOI] [PubMed] [Google Scholar]
- Dumonde D. C., Wolstencroft R. A., Panayi G. S., Matthew M., Morley J., Howson W. T. "Lymphokines": non-antibody mediators of cellular immunity generated by lymphocyte activation. Nature. 1969 Oct 4;224(5214):38–42. doi: 10.1038/224038a0. [DOI] [PubMed] [Google Scholar]
- GREENWOOD F. C., HUNTER W. M., GLOVER J. S. THE PREPARATION OF I-131-LABELLED HUMAN GROWTH HORMONE OF HIGH SPECIFIC RADIOACTIVITY. Biochem J. 1963 Oct;89:114–123. doi: 10.1042/bj0890114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gahmberg C. G., Andersson L. C. Leukocyte surface origin of human alpha1-acid glycoprotein (orosomucoid). J Exp Med. 1978 Aug 1;148(2):507–521. doi: 10.1084/jem.148.2.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kekwick R. A. The serum proteins in multiple myelomatosis. Biochem J. 1940 Sep;34(8-9):1248–1257. doi: 10.1042/bj0341248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler S. W. Rapid isolation of antigens from cells with a staphylococcal protein A-antibody adsorbent: parameters of the interaction of antibody-antigen complexes with protein A. J Immunol. 1975 Dec;115(6):1617–1624. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Ladoulis C. T., Gill T. J., 3rd, Chen S. H., Misra D. N. The structure and metabolism of lymphocyte membranes. Prog Allergy. 1975;18:205–288. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Monneron A., d'Alayer J. Isolation of plasma and nuclear membranes of thymocytes. II. Biochemical composition. J Cell Biol. 1978 Apr;77(1):232–245. doi: 10.1083/jcb.77.1.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Owen M. J., Barber B. H., Faulkes R. A., Crumpton M. J. Albumin is associated with the inner surface of the lymphocyte plasma membrane. Biochem Soc Trans. 1978;6(5):920–922. doi: 10.1042/bst0060920. [DOI] [PubMed] [Google Scholar]
- Perlés B., Flanagan M. T., Auger J., Crumpton M. J. Mechanism of lymphocyte activation: the binding of phytohemagglutinin to the lymphocyte surface. Eur J Immunol. 1977 Sep;7(9):613–619. doi: 10.1002/eji.1830070907. [DOI] [PubMed] [Google Scholar]
- RUDMAN D., KENDALL F. E. Bile acid content of human serum. II. The binding of cholanic acids by human plasma proteins. J Clin Invest. 1957 Apr;36(4):538–542. doi: 10.1172/JCI103451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocklin R. E. Partial characterization of leukocyte inhibitory factor by concanavalin A-stimulated human lymphocytes (LIF Con A). J Immunol. 1975 Apr;114(4):1161–1165. [PubMed] [Google Scholar]
- Snary D., Woods F. R., Crumpton M. J. Disruption of solid tissue for plasma membrane preparation. Anal Biochem. 1976 Aug;74(2):457–465. doi: 10.1016/0003-2697(76)90226-8. [DOI] [PubMed] [Google Scholar]
- Walsh F. S., Barber B. H., Crumpton M. J. Preparation of inside-out vesicles of pig lymphocyte plasma membrane. Biochemistry. 1976 Aug 10;15(16):3557–3563. doi: 10.1021/bi00661a025. [DOI] [PubMed] [Google Scholar]
- Yamada S., Itano H. Phenanthrenequinone as an analytical reagent for arginine and other monosubstituted guanidines. Biochim Biophys Acta. 1966 Dec 28;130(2):538–540. doi: 10.1016/0304-4165(66)90256-x. [DOI] [PubMed] [Google Scholar]