Abstract
In brief
Gender-affirming treatments for gender dysphoria can impact fertility. This review describes the impact of gender-affirming treatments on fertility and options to preserve fertility in transgender or gender-diverse children, adolescents, and young adults.
Abstract
Transgender individuals who pursue alignment with their gender identity through medical treatments or surgery face challenges to family building because the medical community lacks the understanding or infrastructure to serve the reproductive needs of transgender or non-binary people. Fertility preservation (FP) offers a crucial opportunity for the transgender community, enabling individuals to exercise autonomy over their reproductive choices. While fertility preservation has been extensively studied in other populations such as cancer patients, the unique biology and clinical care of transgender and gender-diverse (TGD) individuals have challenged the direct translation of what can be offered for cisgender individuals. Additionally, the FP services in transgender communities are reportedly under-utilized, despite the prevalent desire of TGD individuals to have children. This review aims to provide up-to-date information on the current standard of care and experimental FP options available to TGD individuals and their potential reproductive outcomes. We will also discuss the barriers to the success of FP utilization from both the biology/medical aspect and the perspectives of the TGD population. By recognizing the unique family-building challenges faced by TGD people and potential areas of improvement, appropriate adjustments can be made to better support fertility preservation in the TGD community.
Introduction
For transgender communities, understanding the terminology is crucial for providing effective care. According to the World Professional Association for Transgender Health (WPATH) Standard of Care version 8 (SOC8) (Coleman et al. 2022), the term transgender or gender-diverse (TGD) is used to describe individuals whose gender identities or expressions differ from the gender typically associated with the sex assigned to them at birth. Gender identity refers to an individual’s internal sense of their gender, which is distinct from sexual orientation—defined as a person’s patterns of emotional, romantic, and sexual attraction. Gender affirmation involves recognizing and validating TGD individuals in their gender identity across social, medical, legal, and behavioral domains, or a combination of these (Poteat et al. 2023). Gender affirming medical and/or surgical therapy (GAMST) is the medical and surgical intervention to align a person’s body with their gender identity (Coleman et al. 2022). GAMST may include hormonal (gender-affirming hormone therapy: GAHT) and/or gender-affirming surgery (GAS), the latter of which may include but is not limited to genital reconstruction, removal of gonads, and surgery to enhance the secondary sex characteristics that affirm gender identity (Coleman et al. 2022). The evolution of terminology and diagnostic criteria shows the efforts that have been made to remove stigma from transgender communities.
Transgender individuals represent a small yet growing segment of the global population, constituting approximately 0.6% of adults and 2.7% of children and adolescents (Scheim et al. 2024). The reported prevalence varies depending on regions, survey methodologies, and definitions used (Reisner et al. 2016). More inclusive definitions of transgender, counting non-binary, gender -diverse, and gender non-conforming persons, indicate that up to 4.5% of adults and 8.4% of children and adolescents fall within this category (Scheim et al. 2024). In the United States, according to The Williams Institute’s 2022 report, 0.5% of adults (approximately 1.3 million individuals) and 1.4% of youth aged 13–17 (around 300,000 individuals) identify as transgender. Of the 1.3 million adults identifying as transgender, 38.5% (515,200) are transgender women, 35.9% (480,000) are transgender men, and 25.6% (341,800) are gender non-conforming (Herman et al. 2022). Notably, reported numbers are often higher among younger populations and may continue to rise (Zucker 2017).
TGD people show improvement in quality of life, well-being, satisfaction in one’s body image, and sexual life after receiving gender-affirming treatments (Coleman et al. 2022). The current recommendations for GAMST by the Endocrine Society and WPATH SOC8 can be categorized into guidelines for TGD adults/adolescents with testes or ovaries (Hembree et al. 2017, Coleman et al. 2022). GAHT for adult TGD people with testes requires both anti-androgen medications, such as Cyproterone or Spironolactone, and estrogen supplements, preferably estradiol. The protocol for adult TGD people with ovaries is testosterone monotherapy. The details of dosing and regimens vary among countries, possibly due to the availability, cost, and familiarity of clinicians with drug choices (Tangpricha & Den Heijer 2017). In adolescents, the treatment usually begins by delaying puberty with GnRH agonists (GnRHa) to allow more time for the youth to explore their gender identity and ease the distress of entering puberty before GAHT is initiated. GAHT can also later encompass puberty-blocking treatment. The recommended age to initiate GAHT, using the age of majority as previously mentioned in SOC7 – at least 16 years for GAHT and 18 years for surgery – has been updated. In SOC8, to initiate GnRHa or GAHT in the youth, they must exhibit an early sign of entering puberty (Tanner stage 2). Another important consideration is that TGD individuals must be on stable GAHT treatment for at least 6 months before GAS in adults and 12 months in adolescents unless GAHT is not desired or contraindicated. Nahata et al. reported the median age at which puberty blockers and cross-sex hormone therapy were prescribed was 15.0 (range: 9–18 years) and 16.0 (range: 14–18 years), respectively. The median age at the first Endocrinology visit was 15.2 years (range: 9–18 years) (Nahata et al. 2017).
The common indications to initiate treatment across all groups (transgender adults and adolescents of both genders) include i) having marked and sustained gender incongruence, ii) having the ability to consent, iii) that the other possible causes of gender incongruence have been ruled out, and iv) that TGD individuals fully understand the effects and consequences of treatment and thus, the benefits and risks of GAHT should be discussed, including the risk of infertility.
This review is a narrative review intended to provide up-to-date and comprehensive information regarding fertility preservation (FP) options for TGD people. We will review standard of care and experimental options for FP; implications of gender-affirming treatments for FP, as well as future reproductive options. A literature search was conducted separately for each topic using the Pubmed/MEDLINE combined database and hand search from the review references.
Effects of GAHT on fertility
GAHT showed unpredictable and negative effects on fertility. Therefore, the Endocrine Society, WPATH, American Society for Reproductive Medicine (ASRM), and European Society of Human Reproduction and Embryology (ESHRE) recommended counseling on the impact of GAMST on fertility and options for fertility preservation prior to and periodically during GAMST (Hembree et al. 2017, Anderson et al. 2020, Ethics Committee of the American Society for Reproductive Medicine 2021, Coleman et al. 2022). The GAHT-prior counseling should include informing and discussing the positive and negative effects of GAHT in every aspect, not limited to reproductive health. In this section, we will discuss the effect GAHT has directly on gametogenesis and fertility.
Effect of GAHT on spermatogenesis
GAHT effects on TGD individuals with testes are pervasive (Andrews et al. 2021). The severity of spermatogenesis defects can be represented using testis histopathology classification (McLachlan et al. 2007) and semen analysis. Histopathology findings of GAHT-exposed testicular tissues with regard to the degree of spermatogenesis are summarized in Table 1. It is worth noting that androgen cessation is usually recommended before GAS-orchiectomy with a 2–6 weeks duration depending on the center. These periods of androgen cessation may or may not have a positive impact on spermatogenesis in the testicular tissue. However, the data are inconclusive, and the duration of hormonal cessation is unknown.
Table 1.
Effect of GAHT on spermatogenesis in TGD adults with testes prior to gender affirming surgery.
| Study | Adults/ testes examined, n | Age* (years) | Normal n (%) | Hypo n (%) | Maturation arrest n (%) | Presence of germ cells n (%) | GAHT regimen | Duration on GAHT* (months) | Duration of cessation before GAS |
|---|---|---|---|---|---|---|---|---|---|
| Dabel et al. (2023) | 25 | 28.1 (16–40) | 0 | 0 | SG:17 (68.0); SC: 5 (20.0); RS: 3 (12.0) | 25 (100)§ | Cyproterone acetate + estrogens | 27.6 (11–66) | 0–6 weeks |
| De Nie et al. (2022) | 19 | 19.0 ± 1.5 (TS:2–3) | 0 | 0 | 19 (100) | 19 (100) | Triptorelin or cyproterone acetate + estrogens (may include GnRHa in adolescent group, detail not specified) | 5.9 ± 1.4 | 4 weeks |
| 10 | 19.6 ± 1.9 (TS:2–3) | 0 | 0 | 10 (100) | 10 (100) | 6.8 ± 1.3 | 0 | ||
| 35 | 19.7 ± 1.2 (TS:4–5) | 0 | 2 | 33 (94.3) | 35 (100) | 4.1 ± 1.8 | 4 weeks | ||
| 14 | 19.3 ± 0.7 (TS:4–5) | 0 | 3 | 11 (78.6) | 14 (100) | 2.8 ± 0.6 | 0 | ||
| 62 | 34.5 ± 12.3 | 0 | 5 | 52 (83.9) | 57 (91.9) | 2.8 ± 1.9 | 4 weeks | ||
| 74 | 36.2 ± 12.2 | 0 | 1 | 63 (85.1) | 64 (86.5) | 2.3 ± 1.2 | 0 | ||
| Sinha et al. (2021) | 85 | 39 ± 16 | 7 (8.2) | 17 (20.0) | 24 (28.2) | 24 (28.2) | Mixed regimen | 48 (24–60)† | NS, likely continuous |
| Vereecke et al. (2021) | 97 | 31.19 (23.25–45.78)† | 0 (acrosin-negative) | 0 (acrosin-negative) | SG: 85 (87.6)‡ | 85 (87.6) | Cyproterone acetate + estrogen | 21.7 (15.2–28.4)† | 2 weeks |
| Jiang et al. (2019) | 141 testes | 39 (30–53)† | 0 | 57 (40.4) | Unspecified spermatid present | 114 (81) | Spironolactone, estrogen, progesterone | 39 (24–65)† | 2 weeks cessation of estrogen in vaginoplasty cases; the rest with continuous spironolactone or progesterone. |
| Jindarak et al. (2018) | 173 | 26.09 ± 5.37 | 19 (11) | 45 (26.0) | 63 (36.4) | 127 (73.4) | Mixed regimen | 102.2 ± 55.2 | 4 weeks |
| Kent et al. (2018) | 135 | 30 (18–76)† | 6 (4) | 0 | 17 (5.2) | 28 (21%) | Spironolactone + estradiol and/or finasteride, progesterone | 60 (12–684)† | NS |
| Matoso et al. (2018) | 99 testes | 33 (21–63) | 0 | 0 | SG:79 (80); SC:20 (20) | 99 (100%) | Estradiol and/or spironolactone, finasteride, progesterone | 6–240 | NS |
| Schneider et al. (2015) | 108 | 42 ± 12.1 | 26 (24.1)⁋ | SG: 38 (35.19); SC:26 (24.07) | 90 (83.3) | Mixed regimen | NS | Combined cohorts | |
| 22 | 10 (45.5) | 6 weeks | |||||||
| 51 | 22 (43.1) | 2 weeks | |||||||
| 35 | 14 (40.0) | 0 week |
*Mean unless stated otherwise; †values are median (IQR); ⁋complete spermatogenesis; ‡Spermatogonia (MAGEA4+) positive (among these: 22 contained spermatocytes (BOLL+) and 14 contained spermatids (CREM+); §lower SG count/mm2 seminiferous tubule compared to cisgender age-matched control.
BOLL, boule homologue RNA-binding protein (marker for secondary spermatocytes and round spermatids); CREM, cAMP-responsive element modulator (marker for round spermatids) and acrosin (marker for acrosome visualization); GAHT, Gender-Affirming Hormone Therapy; MAGE-A4, marker for spermatogonia and early spermatocytes); NS, not specified; TGD, Transgender and gender diverse; RS, round spermatids; SC, spermatocytes; SG, spermatogonia; TS, Tanner stage.
Testicular histology findings in TGD people with testes receiving GAHT showed evidence of complete spermatogenesis (normal/hypospermatogenesis) in 0–37% of the specimens, with 21–100% presence of germ cells. Studies found no correlations between evidence of spermatogenesis and the hormonal regimen, dosage, duration on GAHT, or time off GAHT before GAS, which may be attributed to small sample sizes. Nevertheless, these findings indicate the possibility of utilizing discarded testes at the time of GAS for fertility preservation. Utilization of tissues may include but is not limited to large-volume testicular sperm extraction (TESE) on discarded testes (Niederberger 2020), and testicular tissue cryopreservation (TTC) for utilization of experimental approaches when technologies mature (please see section: Fertility preservation options – TGD people with testes – Experimental).
Effect of GAHT on oogenesis
Regarding the ovarian histologic findings in testosterone-exposed TGD people with ovaries, some studies that reported histological findings resembling those of polycystic ovarian syndrome (PCOS) (Spinder et al. 1989, Pache et al. 1991, Grynberg et al. 2010), the disease which also involves high testosterone exposure, while other studies that found no differences in the number of primordial, early, or antral follicles compared to controls (Ikeda et al. 2013, De Roo et al. 2017, Bailie et al. 2023). Table 2 summarizes the important study designs from each report.
Table 2.
Effects of GAHT on the ovarian tissues of TGD with ovaries.
| Effect/study | Donors, n | Age (years) | Testosterone exposure duration | Control | Summary of findings |
|---|---|---|---|---|---|
| Consistent with ovarian syndrome-like change | |||||
| Pache et al. (1991) | 17 | 25 (18–35) | 21 months average | 13 (Age: 29 (27–39)) |
|
| Grynberg et al. (2010) | 112 | 28.9 ± 0.9 | 2–9 years (3.7 ± 0.6) | None |
|
| Spinder et al. (1989) | 26 | 26 ± 6 | 9–36 months | 9 age-matched patients |
|
| Comparable oocyte distribution to control, no PCOS features | |||||
| Ikeda et al. (2013) | 11 | 27–38 | 17 months–14 years (median: 38 months) | 10 age and BMI-matched oncology patients |
|
| De Roo et al. (2017) | 40 | 24.30 ± 6.15 | 14.5 ± 6.6 months |
|
|
| Bailie et al. (2023) | 8 | 27.6 ± 1.7 | 18 months–10 years | 31.8 ± 1.5 healthy donors |
|
BMI, body mass index; PCOS, Polycystic Ovarian Syndrome; GAHT, Gender-Affirming Hormone Therapy; SD = standard deviation; TGD = Transgender and gender diverse.
Fertility preservation options
There are still no standard guidelines regarding FP choices for TGD individuals. This may be due to limited evidence to make the recommendations. We will review standard-of-care fertility preservation options that have been offered to TGD individuals and experimental options that are offered at very few centers with Institutional Review Board (IRB) approval. It is very important to note that, unlike in cancer patients, FP interventions are not usually offered until Tanner stage 2 (approximately 11 years old in females and 11.5 years old in males) is reached as this stage of development is required for GnRHa/GAHT initiation. Therefore, we will focus our review on findings from the peripubertal period and older.
Fertility preservation options for TGD people with testes
Two fertility preservation options are possible for TGD people with testes. The established and standard of care option is to cryopreserve a semen sample with sperm. Cryopreserved sperm can be thawed in the future to fertilize partner or donor eggs and establish a pregnancy. This method has extensive evidence supporting its use in adult cisgender males and is the only recommended standard protocol for adults facing gonadal threats, such as chemotherapy or total body radiation (Gassei et al. 2017, Martinez 2017, Oktay et al. 2018, Practice Committee of the American Society for Reproductive Medicine 2019). The second option is TTC, which is typically reserved for prepubertal patients who are not producing sperm. TTC is experimental both for cisgender patients with a cancer diagnosis or TGD patients with a gender dysphoria diagnosis because there is no evidence yet that those tissues can be matured in the future to produce sperm. While many centers around the world provide TTC to cancer or bone marrow transplantation patients who are at risk of infertility, very few provide this service to TGD individuals who cannot or will not interrupt GAHT to collect and freeze a semen sample with sperm. TESE can be offered to TGD people with testes who are going to gender-affirming surgery, as the testes are typically removed during the GAS process and would otherwise be discarded. However, the long-term impact of GAHT prior to GAS is not known. Table 3 summarizes fertility preservation outcomes by semen collection and TESE based on age groups and history of GnRHa/GAHT exposure.
Table 3.
Fertility preservation (semen analysis or TESE outcomes) in TGD people with testes.
| Age group | GAHT exposure status | Technique used | Results | References |
|---|---|---|---|---|
| Adult | No prior GnRHa and GAHT exposure | Semen collection | Poor semen parameters compared to referenced cisgender samples | Adeleye et al. (2019b); Rodriguez-Wallberg et al. (2021a); Hamada et al. (2015); Barda et al. (2023); de Nie et al. (2020); Li et al. (2018) |
| Poor semen parameter in post-thawed samples | de Nie et al. (2020); Hamada et al. (2015) | |||
| With continued GnRHa/GAHT at collection | Semen collection | Low semen parameters compared to previously-used GAHT and GAHT-naïve | Adeleye et al. (2019b) | |
| Stop GnRHa/GAHT at collection | Semen collection | Semen parameters poorer than GAHT-naïve TGD samples | Rodriguez-Wallberg et al. (2021a) | |
| Semen parameters comparable with GAHT-naïve TGNB samples. | Adeleye et al. (2019b); Barda et al. (2023) | |||
| Semen parameters higher than continuously-used GAHT | Adeleye et al. (2019b) | |||
| Semen collection or testicular sperm extraction | Natural conceptions reported in 3/9 cases; Viable sperm retrieved from all 9 cases by semen collection or testicular sperm extraction. | de Nie et al. (2023) | ||
| Peripubertal | No prior GnRHa and GAHT exposure | Semen collection (16-24-year-old TGDs) | Normal semen parameters except for low percentage (3%) of normal morphology compared to normal reference per Modified Kruger criteria (>13%) in group with mean age 19.5 | Barnard et al. (2019) |
| Testicular sperm extraction (13-17-year-old TGDs) | Successful sperm retrieval (68%, 17/25) | Peri et al. (2021) | ||
| With continued GnRHa/GAHT at collection | No data | No data | No data | |
| Stop GnRHa/GAHT at collection | Semen collection (age 17.5 at GnRHa initiation, age 18 at retrieval, n=1) | 12 sperm (2 motile) found 3 months after suspending GnRHa; Normal semen sample 5 months after suspending GnRHa | Barnard et al. (2019) | |
| Semen collection (age 18 at initiation, age 19 at retrieval, n=1) | Azoospermic at 4 months after suspending GAHT | Barnard et al. (2019) |
GnRHa, Gonadotropin releasing hormone agonist; GAHT, gender-affirming hormone therapy
Standard of care FP options for TGD people with testes
Adult
Before the initiation of GnRHa or GAHT
Although sperm cryopreservation is recommended in adults who can produce sperm, the collection of semen via masturbation may cause psychological distress and exacerbate gender dysphoria in some cases (Reckhow et al. 2023). Also, there is a high prevalence (47%) of orgasmic dysfunction in TGD people with testes, even before GAHT (Kerckhof et al. 2019). In such cases, alternative ways to obtain sperm, such as Electro- or vibratory stimulation, TESE, Testicular Sperm Aspiration (TESA), or Epididymal Sperm Aspiration (PESA), among others, may be offered (Esteves et al. 2011). Adult TGD people with testes also had poorer semen parameters (sperm concentration, total motile sperm count, and/or morphology) compared to the WHO-referenced male or healthy cisgender male control group even before GAHT (Li et al. 2018, De Nie et al. 2020, Rodriguez-Wallberg et al. 2021a) (Table 3). Although not directly evaluated in these reports, poor sperm parameters before GAHT were thought to be attributed to lifestyle or environmental factors such as the tucking of the testicles (Trussler and Carrasquillo 2020). Additionally, cryopreserved semen from TGD individuals before GAHT showed that only 26% of the post-thawed samples were of adequate quality for intrauterine insemination (IUI), the cheapest and simplest assisted reproductive technology (ART) (De Nie et al. 2020, Hamada et al. 2015). Therefore, even when pursuing FP before GAHT, TGD patients with testes may need to plan for more expensive ARTs in the future, such as in vitro fertilization (IVF) with intracytoplasmic sperm injection (ICSI). However, Hamada and colleagues did report a case of fertilization and pregnancy using a single transwoman’s cryopreserved sperm for IUI in a surrogate mother (Hamada et al. 2015).
After the initiation of GnRHa or GAHT
TGD people with testes whose GAHT treatment has been initiated without prior fertility preservation can collect sperm via the same means as the GAHT-naive group, opening up more flexibility to those who were undecided, prioritized initiation of GAHT, or simply changed their plan on family building. There is histologic evidence of complete spermatogenesis (Table 1) and evidence to suggest that sperm can be recovered in the semen or by TESE after temporary cessation of gender-affirming treatments in some cases (Table 3). Therefore, the state of GnRHa or GAHT should not preclude fertility preservation.
Adolescent
Recommendations for FP choice in adolescent TGD people with testes still sperm cryopreservation. However, this may not be feasible in adolescents under 15 years old due to the high prevalence of azoospermia (no sperm in the ejaculate). A recent study in peripubertal cancer patients reported azoospermia in 66.7% of 12-year-olds, 31.3% of 13-year-olds, and approximately 10% of 14–17-year-olds, decreasing to 0% in 18–19-year-olds (Halpern et al. 2019). Even if no sperm are found in the ejaculate, it is sometimes possible to retrieve sperm directly from the testis by TESE. Peri and colleagues reported that sperm recovery via TESE was successful in 68% of patients in the 13–17 year-old range with no prior gender-affirming treatments (Peri et al. 2021) (Table 3).
Experimental: TTC
TTC has been offered and studied as an experimental FP approach in prepubertal cancer patients worldwide with the expectation that these tissues can be matured in the future to produce sperm from resident spermatogonial stem cells (SSCs) (Tran et al. 2022). Our center has extended this experimental FP option to young TGD patients (NCT05829928). This protocol is separate from our cancer patient TTC protocol because the risks and benefits for TGD patients are different than those for cancer patients. Our center is approved to cryopreserve testicular tissues for patients who have a diagnosis of gender dysphoria and are referred by their physician for fertility preservation. Patients must be ≥9 years old, getting ready to start or already on gender-affirming treatments, and unwilling or unable to delay or interrupt GnRHa or GAHT to collect sperm. If patients are 12 years or older, we provide the option to search a portion of the tissue for sperm, similar to TESE. However, the majority of the tissue is cryopreserved with the expectation that SSCs in the tissue have the potential to produce sperm in the future. Peri and colleagues reported retrieval of sperm from the testicular tissues of young TGD patients who were Tanner stage 3 or higher and when testis volumes were greater than 10–12 mL. Age, hormone levels, and previous gender-affirming treatments were not reliable determinants of whether sperm could be retrieved from testicular tissues (Peri et al. 2021). Therefore, Tanner staging and testis volume data may be useful in counseling young TGD patients about the potential future uses of their cryopreserved testicular tissue. Several studies showed the presence of undifferentiated germ cells (stem and progenitor spermatogonia) in TGD testicular tissue regardless of GAHT history, showing the potential utility of cryopreserved testicular tissues in this group (Table 1). This may suggest that suspension of gender-affirming treatments is not necessary prior to cryopreservation of testicular tissue with SSCs. TTC may also be possible when testes are being removed for GAS. However, there is no data on the function of germ cells that may remain in that tissue after long-term GAHT treatment. Studies in animal models have shown different ways to utilize the cryopreserved testicular tissue in both tissue-based and cell-based approaches (reviewed in (Tran et al. 2022)). Future utilization of tissues requires different considerations than in cisgender cancer survivors because TGD people may not want the tissue or cells transplanted back into their bodies or want to go through puberty in the gender that would be required to mature their tissues/cells inside their bodies. Methods to mature testicular tissue or cells outside the body to produce sperm (see below) may be required but are in very early stages of development.
Potential uses of cryopreserved testicular tissues in reproduction: considerations for TGD individuals
Testicular tissue or cell transplantation
Brinster and colleagues pioneered the method of spermatogonial stem cell transplantation more than three decades ago. Testicular cells (including SSCs) were injected into the seminiferous tubules of the testes where they regenerated spermatogenesis with sperm that were competent to fertilize and produce offspring (Brinster and Zimmermann 1994, Brinster and Avarbock 1994). Donor SSCs of any age are competent to regenerate spermatogenesis. In addition, cells that were thawed after 14 years of cryostorage could regenerate spermatogenesis (Wu et al. 2012), which is relevant in the context of fertility preservation in young patients. Testicular tissue grafting is an alternative approach that involves transplanting intact pieces of testicular tissue under the skin. Fresh or cryopreserved immature testicular tissue can be matured over several months in vivo and then recovered and dissected to release sperm that are competent to fertilize by IVF with ICSI and produce offspring (Honaramooz et al. 2002, Schlatt et al. 2003, Shinohara et al. 2002, Fayomi et al. 2019). Testicular tissue grafting is usually performed in castrated recipients, which may be germane to TGD patients after GAS. This approach works only with immature (prepubertal) testicular tissues and not adult tissues (Arregui and Dobrinski 2014). It is not known whether testicular tissues from TGD patients where spermatogenesis is suppressed by gender-affirming treatments would function more like adult tissues or immature prepubertal tissues in this context. However, it is noteworthy that when spermatogenesis was suppressed in mice with acyline (GnRH antagonist) prior to transplantation, grafts survived and produced spermatogenesis (Arregui et al. 2012).
Spermatogonial stem cell transplantation and testicular tissue grafting are mature technologies that have been replicated in numerous animal models, including nonhuman primates (reviewed in (Tran et al. 2022)) and may be ready for translation to the human clinic. However, as indicated above, TGD patients may not want their testicular tissues or cells transplanted back into their body or to go through male puberty with testosterone production, which is necessary for spermatogenesis to occur from transplanted testicular cells or tissues. Below, we review ex vivo approaches to mature testicular tissues or cells and produce sperm. These methods are at a much earlier stage than the transplant approaches described above but may have valuable applications for TGD patients who have cryopreserved their testicular tissues.
Xenotransplant into SCID/Nude mice or other animal hosts
An alternative to autologous transplantation is testicular tissue grafting into an animal host. Testicular tissue from several species (reviewed in (Tran et al. 2022)) can be transplanted under the dorsal skin or scrotal skin of immune-deficient SCID or nude mice and matured to produce sperm as well as offspring in rabbits (Shinohara et al. 2002), pigs (Nakai et al. 2010) and monkey (Liu et al. 2016). In humans, the most advanced germ cells produced by this technique were premeiotic spermatocytes, which have been reported for both immature and adult as well as fresh or frozen human testicular grafts (References can be reviewed in Table 4). It is unclear why prepubertal monkey testicular tissues can be matured to produce sperm in a mouse host, while human testicular tissues cannot. Perhaps other animal hosts, such as immune-deficient pigs (Boettcher et al. 2018) will support better development of human tissues. The risk of transmitting viruses or other xenobiotics from the animal host to the patient must be carefully considered (Kimsa et al. 2014, 2017). However, it is noteworthy that pigs are actively being developed as organ donors for human patients (Kozlov 2024).
Table 4.
Developing technologies for maturing patient testicular tissues/cells and producing sperm outside the patient’s body. Evidence in human studies.
| Tissue source | Technique | Methods | Results | Reference |
|---|---|---|---|---|
| Cisgender prepubertal tissue | Tissue- based | Xenotransplant into SCID or nude mice | ||
| Fresh tissue into dorsal skin | ||||
| From 10-11-year-old donors, n=3 | Spermatogonia | Goossens et al. (2008) | ||
| From 3-9-year-old donors, n=3 | BOLL+ spermatocytes | Ntemou et al. (2019) | ||
| Fresh tissue into scrotum | ||||
| From 5-year-old donor, n=1 | Spermatogonia | Van Saen et al. (2011) | ||
| From 12-13-year-old donors, n=2 | Spermatocyte | Van Saen et al. (2011) | ||
| From 2-12-year-old donors, n=10 | Spermatocyte | Poels et al. (2013) | ||
| From 3-9 -ear-old donors, n=3 | Spermatocyte | Ntemou et al. (2019) | ||
| Frozen prepubertal tissue into scrotum | ||||
| From 3-13-year-old donors, n=3 | Spermatogonia | Van Saen et al. (2011) | ||
| From 2-12-year-old donors, n=11 | Spermatogonia | Wyns et al. (2007) | ||
| From 2-15-year-old donors, n=6 | Spermatogonia | Poels et al. (2014) | ||
| From 7-14-year-old donors, n=5 | Spermatocyte | Wyns et al. (2008) | ||
| From 2-12-year-old donors, n=10 | Spermatocyte | Poels et al. (2013) | ||
| Cisgender adult tissue | ||||
| Fresh tissue into dorsal skin | Degenerated tissue | Schlatt et al. (2006) | ||
| Spermatogonia | Geens et al. (2006) | |||
| Fresh adult tissue into scrotum | Spermatocyte | Van Saen et al. (2011) | ||
| Frozen adult tissue into scrotum | Spermatocyte | Van Saen et al. (2011) | ||
| Cisgender immature tissues (age 6-14) | Tissue- based | IVM with testicular tissue culture | ||
| Used fresh | Spermatogonia | Portela et al. (2019) | ||
| Used frozen | Spermatogonia | Portela et al. (2019), de Michele et al. (2017) | ||
| SYCP3+ primary spermatocytes | Medrano et al. (2018), Younis et al. (2023) | |||
| Round spermatid | de Michele et al. (2018) | |||
| Cisgender adult tissue | ||||
| Used Fresh | Spermatogonia | Jorgensen et al. (2014) | ||
| Transgender adult tissue | ||||
| Fresh and cryopreserved adult GAHT-exposed testicular tissue | No progression of spermatogenesis after 2 weeks in culture | Komeya et al. (2021) | ||
| Cisgender prepubertal and adult cells | Cell-based | De novo testicular morphogenesis (organoid culture) | ||
| Fresh pubertal (age 15) and adult testicular cells | SC- based, or SC-free transwell | Mitotically-active germ cells, normal somatic cells function and arrangement | Baert et al. (2017) | |
| Frozen prepubertal testicular cells | Matrigel | Inverted organization of spermatogonia and somatic cells | Sakib et al. (2019) | |
| Fresh and frozen adult testicular cells | ECM | Spermatogonia clusters, normal somatic cells function and arrangement | Baert et al. (2015) | |
| PRM2+ elongated spermatids | Pendergraft et al. (2017), Nikmahzar et al. (2023) |
TGNB, transgender and non-binary; GnRHa, gonadotropin-releasing hormone agonist; GAHT, gender-affirming hormone therapy, SYCP3 +, synaptonemal complex protein 3 (marker for primary spermatocytes); SCID, severe combined immunodeficiency;, BOLL , boule homologue RNA-binding protein (marker for secondary spermatocytes and round spermatids); ECM, extracellular matrix; IVM, in vitro maturation; SC, scaffold.
In vitro maturation with testicular tissue organ culture
Sato and colleagues pioneered a method for culturing immature mouse testicular tissues at the air-liquid interface. Tissues matured over several weeks in culture and produced sperm that were competent to fertilize and produce offspring (Sato et al. 2011). Like testicular tissue grafting, this approach only works with immature testicular tissues; and it is not yet known whether it would work with testicular tissues where spermatogenesis is suppressed by gender-affirming treatments. Several groups have reported culturing human testicular tissues at the air-liquid interface. Tissues could be maintained for weeks to months with the maintenance of spermatogonia and occasional differentiation to produce spermatocytes or spermatids but not sperm (Medrano et al. 2018, De Michele et al. 2018, Portela et al. 2019, Younis et al. 2023). Komeya and colleagues reported that GAHT-exposed testicular tissues could be maintained for 2 weeks in culture, but the number of germ cells declined over that time (Komeya et al. 2021). Testing the fertilization potential of experimentally derived human sperm, using this approach or others, is necessary to demonstrate safety and feasibility, but raises ethical concerns and is challenged by restrictive funding or laws in some states and countries.
De novo testicular morphogenesis in an animal host or organoid culture
Heterogeneous testis cell suspensions have the remarkable ability to reform seminiferous tubules, both in vivo and ex vivo. Testis cells from mice, sheep, and pigs can be pelleted and transplanted under the skin of mouse recipients, where they reform into seminiferous tubules, which sometimes contain spermatids and/or sperm (Honaramooz et al. 2007, Kita et al. 2007, Arregui et al. 2008). The fertilization potential of those sperm has not been tested, and to our knowledge, in vivo de novo testicular morphogenesis has not been reported with human testis cells. Many groups have described methods for de novo testicular morphogenesis ex vivo, but none have yet produced sperm or offspring. Sakib and colleagues reported a microwell aggregation approach to produce 3D testicular organoids from neonatal or prepubertal testicular cell suspensions of mice, pigs, monkeys, and humans. The tubules formed inside out and contained spermatogonia but did not support complete spermatogenesis (Sakib et al. 2019). Two studies reported human testicular organoids from adults (15+ years) formed in the human testicular extracellular matrix (htECM). Baert and colleagues seeded heterogeneous prepubertal or adult human testis cell suspensions onto a 3-dimensional htECM scaffold that was shaped in the form of a tubule (Baert et al. 2017). Pendergraft and colleagues used a hanging-drop method to induce organoid formation from cultured adult human spermatogonia mixed with immortalized human Sertoli and Leydig cells suspended in a hydrogel of htECM (Pendergraft et al. 2017). Both approaches led to the production of organoids including germ cells and somatic cells, but neither approach produced seminiferous tubule-like structures (Pendergraft et al. 2017, Baert et al. 2017). The Pendergraft study reported elongated spermatids, but since the starting point was adult tissues, it is impossible to determine whether those post-meiotic spermatids arose in culture or were already present in the original cell suspension (Pendergraft et al. 2017) (Table 4).
Standard of care FP options for TGD people with ovaries
Adult
Before the initiation of GAHT
Ovarian stimulation and oocyte cryopreservation can be done the same way as for cisgender females. Maxwell and colleagues reported four successful four live births in two couples utilizing cryopreserved oocytes from GAHT-naive adult TGD with ovaries, followed by fertilization with donor sperm and embryo transfer into cisgender, sexually intimate, female partners (Maxwell et al. 2017, Adeleye et al. 2019a). TGD people with ovaries (with and without prior testosterone exposure) produced a similar number of oocytes, with a similar maturity rate as age/BMI-matched cisgender women (Adeleye et al. 2019a, Leung et al. 2019) (Table 5).
Table 5.
Oocyte cryopreservation fertility outcome in TGD with ovaries
| Age group | GAHT exposure status | Technique used | Results | References |
|---|---|---|---|---|
| Adults | No prior GAHT exposure | Cryopreserved oocytes and/or embryos | Live births | Maxwell et al. (2017) |
| Fresh oocytes | 3 pregnancies | Adeleye et al. (2019a) | ||
| With continued GAHT at collection | Fresh transfer with reciprocal IVF | Live birth | Greenwald et al. (2022) | |
| Fresh transfer | Live birth | White et al. (2024) | ||
| Oocyte retrieval | Successful oocyte retrieval | Stark & Mok-Lin (2022); Gale et al. (2021); Cho et al. (2020) | ||
| Stop GAHT at collection | Fresh transfer and embryo cryopreservation) | 2 pregnancies | Adeleye et al. (2019a) | |
| IUI with donor sperm, IVF and reciprocal IVF, no freezing | 5 live births | Ghofranian et al. (2023) | ||
| Fresh or frozen transfer | 7 live births | Leung et al. (2019) | ||
| Pubertal/ adolescent | No prior GAHT exposure | Oocyte retrieval | Successful oocyte retrieval | Chen et al. (2018); Barrett et al. (2022); Insogna et al. (2020) |
| With continued GAHT at collection | No data | No data | No data | |
| Stop GAHT at collection | Oocyte retrieval in GnRHa-only, and who had history of prior testosterone use | Successful oocyte retrieval | Insogna et al. (2020) |
GAHT, gender-affirming hormone therapy; IUI, intrauterine insemination
After the initiation of GAHT
Oocyte cryopreservation and embryo cryopreservation can be offered even after the initiation of GAHT. However, Adeleye et al. reported that the number of oocytes retrieved from GAHT naive TGD with ovaries was higher than in the group with prior GAHT who had suspended testosterone treatment for a median time of 6 months (Adeleye et al. 2019a). The main question in this scenario is whether or not to discontinue testosterone supplements before oocyte retrieval. Testosterone cessation has traditionally been encouraged to ensure a good oocyte retrieval outcome, and the duration recommended is at least 3 months or until the return of menstruation (De Roo et al. 2016, Armuand et al. 2017). However, the necessity to suspend GAHT and resume menstruation requires further investigation because GAHT interruption can cause distress in TGD people (Armuand et al. 2017, Greenwald et al. 2022) (Table 5).
Ovarian tissue cryopreservation (OTC) is no longer considered experimental by the ASRM (Practice Committee of the American Society for Reproductive Medicine 2019), based in part on the evidence of more than 130 live births from transplanted ovarian tissues (Donnez and Dolmans 2017). However, that guidance was based almost entirely on data from survivors of cancer or bone marrow transplantation who were adults at the time of OTC. Data on the transplantation potential of ovarian tissues that were cryopreserved during childhood or from TGD individuals on GAHT are limited or absent, respectively. Thus, it is reasonable to offer OTC as an experimental option until more transplantation and live birth data can be accumulated for those populations.
Adolescent
Before GAHT initiation
Oocyte cryopreservation is the standard fertility preservation option for hormone-naive adolescent TGD people with ovaries. OTC could be offered at the time of GAS, but those patients have usually already initiated GAHT according to WPATH SOC 8 recommendations. According to WPATH SOC 8, GAS is usually recommended after 6 months of stable GAHT in adults and 12 months in youth unless the GAHT is not desired or contraindicated. This means that, in most cases, OTC with simultaneous GAS is generally not possible unless GAHT has begun (Amir et al. 2020a). Embryo cryopreservation, which requires partner sperm, is not usually offered in adolescents. OTC for fertility preservation is not generally offered as a stand-alone option to adolescent TGD patients with ovaries, although it is offered at our center as an experimental protocol (NCT05863676).
After initiation of gender affirming treatments
Two studies have shown successful oocyte retrieval in adolescent TGD with ovaries who had GnRHa only and who had prior testosterone use (Insogna et al. 2020, Barrett et al. 2022). Considerations for embryo freezing and OTC are the same as described above. Our center does not require the cessation of GnRHa or GAHT prior to OTC. This may be a consideration for TGD people who do not want to interrupt their gender-affirming treatments for fertility preservation.
Potential uses of cryopreserved ovarian tissues in reproduction: Considerations for TGD individuals
Autologous transplantation
Cryopreserved ovarian tissue can be transplanted back to the donor at the ovary or pelvic site. Transplanted ovarian tissues can restore hormonal and reproductive function, including the possibility of in vivo conception and pregnancy. There have been more than 180 live births after transplantation of cryopreserved ovarian tissues using in vivo conception or IVF (Donnez and Dolmans 2017, Gellert et al. 2018, Practice Committee of the American Society for Reproductive Medicine 2019, Khattak et al. 2022) Transplantation of ovarian tissues that were cryopreserved in prepuberty, adolescence, or adulthood has resulted in live births (Table 6). To our knowledge, there are no reports of ovarian tissue transplantation in TGD individuals. While ovarian tissue transplantation is a robust technology, it probably requires GAHT cessation and the production of estrogen from developing follicles. However, we note that ovulation appears to be possible while still on testosterone treatment (Asseler et al. 2024, Stark and Mok-Lin 2022, Gale et al. 2021, White et al. 2024, Greenwald et al. 2022). Additional research may reveal protocols that enable follicle development in transplanted ovarian tissues without compromising gender-affirming medical treatments.
Table 6.
Technology maturity of potential experimental fertility preservation approach for transgender men
| Patient population | Method | Technique | GAHT cessation* | Results | References |
|---|---|---|---|---|---|
| Cisgender | ALT | Tissue-based | Yes | ||
| Adult tissue | Live births | Reviewed in Gellert et al. (2018), Donnez & Dolmans (2017), Khattak et al. (2022) | |||
| Cisgender prepubertal and adolescent tissue | Live births | Demeestere et al. (2015), Matthews et al. (2018), Rodriguez-Wallberg et al. (2021b) | |||
| Transgender | No data | No data | |||
| Cisgender | OTO/IVM | Cell-based | No | ||
| Patients with cancer or ovarian neoplasm | Live births | Segers et al. (2020) | |||
| Live birth† | Kedem et al. (2018), Uzelac et al. (2015), Prasath et al. (2014) | ||||
| 50-76.9% fertilization rate; Pregnancy rate not reported due to no utilization | Reviewed in Mohd Faizal et al. (2022) | ||||
| Successful oocyte aspiration during cesarean section | Hwang et al. (1997) | ||||
| Benign pelvic AVM | Pregnancy | Segers et al. (2015) | |||
| TGD with ovaries | |||||
| Adult | Normal spindle after thawing | Lierman et al. (2017) | |||
| Poor embryonic progression after fertilization | Lierman et al. (2021) | ||||
| Poor embryonic progression overcome by spindle transfer | Christodoulaki et al. (2023) |
ALT, autologous transplantation; AVM, arteriovenous malformation; TGD, transgender and gender diverse individuals; OTO/IVM,ovarian tissue oocyte/in vitro maturation.
*GAHT cessation at the time of fertility restoration; †Live birth rate after embryo transfer = 43%
Ovarian tissue oocyte followed by in vitro maturation (OTO/IVM)
OTC can be performed prior to the initiation of GAHT, during GAHT, or concomitantly with ovariectomy as a part of the GAS. During ovarian tissue processing, the outer cortex of the ovary, which contains primordial follicles, is dissected away from the inner medulla and then cut into strips for cryopreservation. Small antral follicles that are present in the medulla are released into the dissection media and are usually discarded. Cumulus-oocyte complexes (COCs) retrieved from these medullary antral follicles can potentially be matured to produce MII oocytes or embryos that can be cryopreserved in parallel with the ovarian tissues (Cadenas et al. 2023). This approach does not require stimulation with exogenous hormones because the final steps of egg maturation occur in vitro. The birth of five healthy infants has been reported using this approach (Prasath et al. 2014, Uzelac et al. 2015, Segers et al. 2020) (Table 6). While data are limited in TGD with ovaries, studies showed normal oocyte distribution across all layers of ovarian tissue (De Michele et al. 2017, Bailie et al. 2023), though one study indicated higher yH2AX staining, a marker for DNA breaks, in primordial germ cells compared to cisgender control (Bailie et al. 2023). The COCs that were extracted from the medulla resulted in MII oocytes after IVM with 87% normal spindle structure, also indicating the possibility of using ovarian tissue oocytes with IVM (OTO-IVM) in TGD people with ovaries and a history of GAHT. However, poor embryo development was noted in GAHT-exposed in vitro-matured ovarian tissue oocytes recovered at the time of GAS during ovarian tissue processing (Lierman et al. 2021, Christodoulaki et al. 2023) and may be improved by spindle transfer (Christodoulaki et al. 2023). Thus, OTC earlier in transition before exposure to GAHT may be beneficial.
In vitro growth of primordial follicles followed by IVM in multistep culture
Cortical strips contain primordial follicles that can be extracted for in vitro development of primordial follicles (primordial follicle to antral follicle) and IVM (immature antral follicle to MII oocytes). The resulting MII can then be used for cryopreservation or fertilized for embryo transfer/cryopreservation. This approach has been studied as an alternative for cancer patients where the chance of reintroducing cancer is high. It shows promise in TGD with ovaries whose primordial follicles are retained in the cortical strip, and the reversal of GAHT is not required at the time of fertility restoration. While in vitro maturation from primordial follicles to mature MII oocytes and preimplantation embryos was described more than a decade ago in mice (Jin et al. 2010), IVM to mature MII oocytes has only been achieved when starting from growing primary and secondary follicles in primates and humans (Xu et al. 2013) (reviewed in (Hu et al. 2023)). An artificial ovary that reconstitutes the ovarian microenvironment ex vivo may provide a path forward (Amorim and Shikanov 2016, Laronda et al. 2017).
Barriers to successful fertility restoration in TGD communities
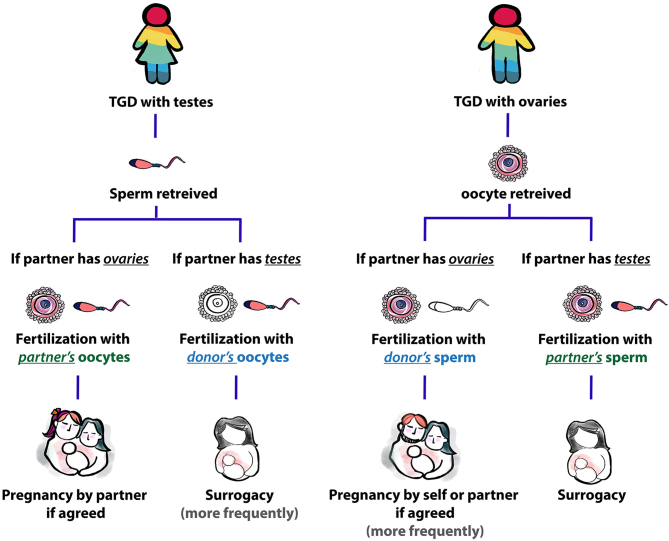
Reproductive desire and/or interest in family building is high among transgender people, both adults and youth, but FP services are reportedly under-utilized in many countries around the world. A meta-analysis using 76 studies showed 48.7–67.0% of transgender adolescents and 18.4–82.1% of transgender adults desired children, but FP utilization rates were 2–4% (Stolk et al. 2023). It is noteworthy that successful sperm/oocyte/gonadal tissue cryopreservation is only the beginning of the journey to successful family building. Multidisciplinary teams are required to ensure that TGD people have access to fertility preservation care and develop technologies that will enable them to use their cryopreserved cells or tissues for family building with minimal disruption to gender-affirming care. Figure 1 shows the journey of a TGD person to have a biological child. For TGD people with testes, ejaculated sperm or sperm from testicular tissues can be used to fertilize partner or donor eggs using standard Assisted Reproductive Technologies (ARTs). If the partner has testes, egg donation for same-sex couples and surrogacy is often required. For TGD people with ovaries, fertility seems to be less affected by hormonal treatment compared to TGD people with testes. Once oocytes are collected by hormonal stimulation or from ovarian tissues, partner or donor sperm and ART are required for fertilization and conception. If the partner has ovaries, sperm donation for same-sex couples will be needed. Surrogacy is possible but may not be needed if the partner is a biological female who will carry the pregnancy.
Figure 1.
Journey of TGD people to have a biological child.
Conclusion
The impacts of gender-affirming treatments on fertility and family building should be discussed before and throughout treatment. Explaining options for fertility preservation and restoration provides a sense of reproductive autonomy, even if the patient is unsure of their family-building goals. Like FP for cancer patients, it is important to start these discussions early while the medical and research communities are still learning the impacts of gender-affirming treatments on the ovaries, testes, eggs, and sperm. Early intervention for FP may be important in some cases. For fertility preservation to accomplish its purpose (which is to allow TGD people to have biological children if they want to), it takes multidisciplinary teams, ranging from pediatric and adult endocrinologists, mental health professionals, reproductive medicine experts and scientists. Laws that support same-sex parenting, egg/sperm donation for same-sex couples, and surrogacy will help ensure that TGD people have the same access to reproductive care as cisgender people. There is an unmet need for counseling and education to cisgender and TGD communities about the availability, accessibility, and feasibility of fertility preservation and fertility restoration options for all people as well as the specific challenges and opportunities for TGD people.
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the study reported.
Funding
This work was supported by anonymous donor funds to Magee-Womens Research Institute and Foundation.
Author contribution statement
CA wrote the initial draft of the manuscript and produced the figure and tables. KEO edited and revised the manuscript, figure, and tables. Both authors approved the manuscript for submission.
References
- 2017. CDC: diseases directly transmitted by rodents (Online). Centers for Disease Control. Available at: https://www.cdc.gov/rodents/diseases/direct.html. [Google Scholar]
- Adeleye AJ Cedars MI Smith J & Mok-Lin E. 2019a. Ovarian stimulation for fertility preservation or family building in a cohort of transgender men. Journal of Assisted Reproduction and Genetics 36 2155–2161. ( 10.1007/s10815-019-01558-y) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adeleye AJ Reid G Kao C-N Mok-Lin E & Smith JF. 2019b. Semen parameters among transgender women with a history of hormonal treatment. Urology 124 136–141. ( 10.1016/j.urology.2018.10.005) [DOI] [PubMed] [Google Scholar]
- Ainsworth AJ Allyse M & Khan Z. 2020. Fertility preservation for transgender individuals: a review. Mayo Clinic Proceedings 95 784–792. ( 10.1016/j.mayocp.2019.10.040) [DOI] [PubMed] [Google Scholar]
- Amir H Oren A Klochendler Frishman E Sapir O Shufaro Y Segev Becker A Azem F & Ben-Haroush A. 2020a. Oocyte retrieval outcomes among adolescent transgender males. Journal of Assisted Reproduction and Genetics 37 1737–1744. ( 10.1007/s10815-020-01815-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amir H Yaish I Samara N Hasson J Groutz A & Azem F. 2020b. Ovarian stimulation outcomes among transgender men compared with fertile cisgender women. Journal of Assisted Reproduction and Genetics 37 2463–2472. ( 10.1007/s10815-020-01902-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amorim CA & Shikanov A. 2016. The artificial ovary: current status and future perspectives. Future Oncology 12 2323–2332. ( 10.2217/fon-2016-0202) [DOI] [PubMed] [Google Scholar]
- Anderson RA, Amant F, Braat D, D’Angelo A, Chuva de Sousa Lopes SM, Demeestere I, Dwek S, Frith L, Lambertini M, Maslin C. et al. 2020. ESHRE guideline: female fertility preservation. Human Reproduction Open 2020 hoaa052. ( 10.1093/hropen/hoaa052) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews AR Kakadekar A Greene DN Khalifa MA Santiago V & Schmidt RL. 2021. Histologic findings in surgical pathology specimens from individuals taking masculinizing hormone therapy for the purpose of gender transition: a systematic scoping review. Archives of Pathology and Laboratory Medicine 146 766–779. ( 10.5858/arpa.2020-0774-RA) [DOI] [PubMed] [Google Scholar]
- Armuand G Dhejne C Olofsson JI & Rodriguez-Wallberg KA. 2017. Transgender men's experiences of fertility preservation: a qualitative study. Human Reproduction 32 383–390. ( 10.1093/humrep/dew323) [DOI] [PubMed] [Google Scholar]
- Arregui L & Dobrinski I. 2014. Xenografting of testicular tissue pieces: 12 years of an in vivo spermatogenesis system. Reproduction 148 R71–R84. ( 10.1530/REP-14-0249) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arregui L Rathi R Megee SO Honaramooz A Gomendio M Roldan ERS & Dobrinski I. 2008. Xenografting of sheep testis tissue and isolated cells as a model for preservation of genetic material from endangered ungulates. Reproduction 136 85–93. ( 10.1530/REP-07-0433) [DOI] [PubMed] [Google Scholar]
- Arregui L Rathi R Modelski M Zeng W Roldan ERS & Dobrinski I. 2012. Suppression of spermatogenesis before grafting increases survival and supports resurgence of spermatogenesis in adult mouse testis. Fertility and Sterility 97 1422–1429. ( 10.1016/j.fertnstert.2012.03.009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asseler JD Del Valle JS Chuva De Sousa Lopes SM Verhoeven MO Goddijn M Huirne JAF & van Mello NM. 2024. One-third of amenorrheic transmasculine people on testosterone ovulate. Cell Reports. Medicine 5 101440. ( 10.1016/j.xcrm.2024.101440) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baert Y Stukenborg JB Landreh M De Kock J Jornvall H Soder O & Goossens E. 2015. Derivation and characterization of a cytocompatible scaffold from human testis. Human Reproduction 30 256–267. ( 10.1093/humrep/deu330) [DOI] [PubMed] [Google Scholar]
- Baert Y De Kock J Alves-Lopes JP Söder O Stukenborg J-B & Goossens E. 2017. Primary human testicular cells self-organize into organoids with testicular properties. Stem Cell Reports 8 30–38. ( 10.1016/j.stemcr.2016.11.012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailie E Maidarti M Jack S Hawthorn R Watson N Telfer E & Anderson RA. 2023. The ovaries of transgender men indicate effects of high dose testosterone on the primordial and early growing follicle pool. Reproduction and Fertility 4 e220102. ( 10.1530/RAF-22-0102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barda S Amir H Mizrachi Y Dviri M Yaish I Greenman Y Sofer Y Azem F Hauser R & Lantsberg D. 2023. Sperm parameters in Israeli transgender women before and after cryopreservation. Andrology 11 1050–1056. ( 10.1111/andr.13369) [DOI] [PubMed] [Google Scholar]
- Barnard EP Dhar CP Rothenberg SS Menke MN Witchel SF Montano GT Orwig KE & Valli-Pulaski H. 2019. Fertility preservation outcomes in adolescent and young adult feminizing transgender patients. Pediatrics 144 e20183943. ( 10.1542/peds.2018-3943) [DOI] [PubMed] [Google Scholar]
- Barrett F Shaw J Blakemore JK & Fino ME. 2022. Fertility preservation for adolescent and Young adult transmen: a case series and insights on oocyte cryopreservation. Frontiers in Endocrinology 13. ( 10.3389/fendo.2022.873508) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boettcher AN Loving CL Cunnick JE & Tuggle CK. 2018. Development of severe combined immunodeficient (SCID) pig models for translational cancer modeling: future insights on how humanized SCID pigs can improve preclinical cancer research. Frontiers in Oncology 8 559. ( 10.3389/fonc.2018.00559) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinster RL & Avarbock MR. 1994. Germline transmission of donor haplotype following spermatogonial transplantation. Proceedings of the National Academy of Sciences of the United States of America 91 11303–11307. ( 10.1073/pnas.91.24.11303) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinster RL & Zimmermann JW. 1994. Spermatogenesis following male germ-cell transplantation. Proceedings of the National Academy of Sciences of the United States of America 91 11298–11302. ( 10.1073/pnas.91.24.11298) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadenas J La Cour Poulsen L Mamsen LS & Andersen CY. 2023. Future potential of in vitro maturation including fertility preservation. Fertility and Sterility 119 550–559. ( 10.1016/j.fertnstert.2023.01.027) [DOI] [PubMed] [Google Scholar]
- Chen D Bernardi LA Pavone ME Feinberg EC & Moravek MB. 2018. Oocyte cryopreservation among transmasculine youth: a case series. Journal of Assisted Reproduction and Genetics 35 2057–2061. ( 10.1007/s10815-018-1292-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho K Harjee R Roberts J & Dunne C. 2020. Fertility preservation in a transgender man without prolonged discontinuation of testosterone: a case report and literature review. F&S Reports 1 43–47. ( 10.1016/j.xfre.2020.03.003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christodoulaki A, He H, Zhou M, Cardona Barberán A, De Roo C, Chuva De Sousa Lopes SM, Baetens M, Menten B, Van Soom A, De Sutter P, et al. 2023. Characterization of ovarian tissue oocytes from transgender men reveals poor calcium release and embryo development, which might be overcome by spindle transfer. Human Reproduction 38 1135–1150. ( 10.1093/humrep/dead068) [DOI] [PubMed] [Google Scholar]
- Coleman E, Radix AE, Bouman WP, Brown GR, De Vries ALC, Deutsch MB, Ettner R, Fraser L, Goodman M, Green J, et al. 2022. Standards of care for the health of transgender and gender diverse people, version 8. International Journal of Transgender Health 23 S1–S259. ( 10.1080/26895269.2022.2100644) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dabel J Schneider F Wistuba J Kliesch S Schlatt S & Neuhaus N. 2023. New perspectives on fertility in transwomen with regard to spermatogonial stem cells. Reproduction and Fertility 4 e220022. ( 10.1530/RAF-22-0022) [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Michele F Poels J Weerens L Petit C Evrard Z Ambroise J Gruson D & Wyns C. 2017. Preserved seminiferous tubule integrity with spermatogonial survival and induction of Sertoli and Leydig cell maturation after long-term organotypic culture of prepubertal human testicular tissue. Human Reproduction 32 32–45. ( 10.1093/humrep/dew300) [DOI] [PubMed] [Google Scholar]
- De Michele F Poels J Vermeulen M Ambroise J Gruson D Guiot Y & Wyns C. 2018. Haploid germ cells generated in organotypic culture of testicular tissue from prepubertal boys. Frontiers in Physiology 9 1413. ( 10.3389/fphys.2018.01413) [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Nie I Meißner A Kostelijk EH Soufan AT Voorn-de Warem IAC den Heijer M Huirne J & van Mello NM. 2020. Impaired semen quality in trans women: prevalence and determinants. Human Reproduction 35 1529–1536. ( 10.1093/humrep/deaa133) [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Nie I, Mulder CL, Meißner A, Schut Y, Holleman EM, Van Der Sluis WB, Hannema SE, Den Heijer M, Huirne J, Van Pelt AMM, et al. 2022. Histological study on the influence of puberty suppression and hormonal treatment on developing germ cells in transgender women. Human Reproduction 37 297–308. ( 10.1093/humrep/deab240) [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Nie I Van Mello NM Vlahakis E Cooper C Peri A Den Heijer M Meißner A Huirne J & Pang KC. 2023. Successful restoration of spermatogenesis following gender-affirming hormone therapy in transgender women. Cell Reports. Medicine 4 100858. ( 10.1016/j.xcrm.2022.100858) [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Roo C Tilleman K T’sjoen G & De Sutter P. 2016. Fertility options in transgender people. International Review of Psychiatry 28 112–119. ( 10.3109/09540261.2015.1084275) [DOI] [PubMed] [Google Scholar]
- De Roo C, Lierman S, Tilleman K, Peynshaert K, Braeckmans K, Caanen M, Lambalk CB, Weyers S, T'sjoen G, Cornelissen R, et al. 2017. Ovarian tissue cryopreservation in female-to-male transgender people: insights into ovarian histology and physiology after prolonged androgen treatment. Reproductive Biomedicine Online 34 557–566. ( 10.1016/j.rbmo.2017.03.008) [DOI] [PubMed] [Google Scholar]
- Demeestere I Simon P Dedeken L Moffa F Tsépélidis S Brachet C Delbaere A Devreker F & Ferster A. 2015. Live birth after autograft of ovarian tissue cryopreserved during childhood. Human Reproduction 30 2107–2109. ( 10.1093/humrep/dev128) [DOI] [PubMed] [Google Scholar]
- Donnez J & Dolmans M-M. 2017. Fertility preservation in women. New England Journal of Medicine 377 1657–1665. ( 10.1056/NEJMra1614676) [DOI] [PubMed] [Google Scholar]
- Esteves SC Miyaoka R & Agarwal A. 2011. Sperm retrieval techniques for assisted reproduction. International Brazilian Journal of Urology 37 570–583. ( 10.1590/s1677-55382011000500002) [DOI] [PubMed] [Google Scholar]
- Ethics Committee of the American Society for Reproductive Medicine. 2021. Access to fertility services by transgender and nonbinary persons: an Ethics Committee opinion. Fertility and Sterility 115 874–878. ( 10.1016/j.fertnstert.2021.01.049) [DOI] [PubMed] [Google Scholar]
- Fayomi AP, Peters K, Sukhwani M, Valli-Pulaski H, Shetty G, Meistrich ML, Houser L, Robertson N, Roberts V, Ramsey C, et al. 2019. Autologous grafting of cryopreserved prepubertal rhesus testis produces sperm and offspring. Science 363 1314–1319. ( 10.1126/science.aav2914) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gale J Magee B Forsyth-Greig A Visram H & Jackson A. 2021. Oocyte cryopreservation in a transgender man on long-term testosterone therapy: a case report. F&S Reports 2 249–251. ( 10.1016/j.xfre.2021.02.006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gassei K Shaw PH Cannon GM Meacham LR & Orwig KE. 2017. Male fertility preservation: current options and advances in research. In Pediatric and Adolescent Oncofertility: Best Practices and Emerging Technologies. Woodruff TK & Gosiengfiao YC Eds. Cham: Springer International Publishing. ( 10.1007/978-3-319-32973-4_8) [DOI] [Google Scholar]
- Geens M De Block G Goossens E Frederickx V Van Steirteghem A & Tournaye H. 2006. Spermatogonial survival after grafting human testicular tissue to immunodeficient mice. Human Reproduction 21 390–396. ( 10.1093/humrep/dei412) [DOI] [PubMed] [Google Scholar]
- Gellert SE Pors SE Kristensen SG Bay-Bjørn AM Ernst E & Andersen CY. 2018. Transplantation of frozen-thawed ovarian tissue: an update on worldwide activity published in peer-reviewed papers and on the Danish cohort. Journal of Assisted Reproduction and Genetics 35 561–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghofranian A Estevez SL Gellman C Gounko D Lee JA Thornton K & Copperman AB. 2023. Fertility treatment outcomes in transgender men with a history of testosterone therapy. F&S Reports 4 367–374. ( 10.1016/j.xfre.2023.10.006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goossens E Geens M De Block G & Tournaye H. 2008. Spermatogonial survival in long-term human prepubertal xenografts. Fertility and Sterility 90 2019–2022. ( 10.1016/j.fertnstert.2007.09.044) [DOI] [PubMed] [Google Scholar]
- Greenwald P Dubois B Lekovich J Pang JH & Safer J. 2022. Successful in vitro fertilization in a cisgender female carrier using oocytes retrieved from a transgender man maintained on testosterone. AACE Clinical Case Reports 8 19–21. ( 10.1016/j.aace.2021.06.007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grynberg M Fanchin R Dubost G Colau JC Brémont-Weil C Frydman R & Ayoubi JM. 2010. Histology of genital tract and breast tissue after long-term testosterone administration in a female-to-male transsexual population. Reproductive Biomedicine Online 20 553–558. ( 10.1016/j.rbmo.2009.12.021) [DOI] [PubMed] [Google Scholar]
- Halpern JA, Thirumavalavan N, Kohn TP, Patel AS, Leong JY, Cervellione RM, Keene DJB, Ibrahim E, Brackett NL, Lamb DJ, et al. 2019. Distribution of semen parameters among adolescent males undergoing fertility preservation in a multicenter international cohort. Urology 127 119–123. ( 10.1016/j.urology.2019.01.027) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamada A Kingsberg S Wierckx K T'sjoen G De Sutter P Knudson G & Agarwal A. 2015. Semen characteristics of transwomen referred for sperm banking before sex transition: a case series. Andrologia 47 832–838. ( 10.1111/and.12330) [DOI] [PubMed] [Google Scholar]
- Hembree WC Cohen-Kettenis PT Gooren L Hannema SE Meyer WJ Murad MH Rosenthal SM Safer JD Tangpricha V & T’sjoen GG. 2017. Endocrine treatment of gender-dysphoric/gender-incongruent persons: an endocrine society* clinical practice guideline. Journal of Clinical Endocrinology and Metabolism 102 3869–3903. ( 10.1210/jc.2017-01658) [DOI] [PubMed] [Google Scholar]
- Herman JL Flores AR & O'neill KK. 2022. How Many Adults and Youth Identify as Transgender in the United States ? T; he Williams Institute, UCLA School of Law. (https://williamsinstitute.law.ucla.edu/wp-content/uploads/Trans-Pop-Update-Jun-2022.pdf) [Google Scholar]
- Honaramooz A Snedaker A Boiani M Scholer H Dobrinski I & Schlatt S. 2002. Sperm from neonatal mammalian testes grafted in mice. Nature 418 778–781. ( 10.1038/nature00918) [DOI] [PubMed] [Google Scholar]
- Honaramooz A Megee SO Rathi R & Dobrinski I. 2007. Building a testis: formation of functional testis tissue after transplantation of isolated porcine (Sus scrofa) testis cells. Biology of Reproduction 76 43–47. ( 10.1095/biolreprod.106.054999) [DOI] [PubMed] [Google Scholar]
- Hu B Wang R Wu D Long R Ruan J Jin L Ma D Sun C & Liao S. 2023. Prospects for fertility preservation: the ovarian organ function reconstruction techniques for oogenesis, growth and maturation in vitro. Frontiers in Physiology 14 1177443. ( 10.3389/fphys.2023.1177443) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang JL Lin YH & Tsai YL. 1997. Pregnancy after immature oocyte donation and intracytoplasmic sperm injection. Fertility and Sterility 68 1139–1140. ( 10.1016/s0015-0282(97)00398-1) [DOI] [PubMed] [Google Scholar]
- Ikeda K Baba T Noguchi H Nagasawa K Endo T Kiya T & Saito T. 2013. Excessive androgen exposure in female-to-male transsexual persons of reproductive age induces hyperplasia of the ovarian cortex and stroma but not polycystic ovary morphology. Human Reproduction 28 453–461. ( 10.1093/humrep/des385) [DOI] [PubMed] [Google Scholar]
- Insogna IG Ginsburg E & Srouji S. 2020. Fertility preservation for adolescent transgender male patients: a case series. Journal of Adolescent Health 66 750–753. ( 10.1016/j.jadohealth.2019.12.004) [DOI] [PubMed] [Google Scholar]
- Jiang DD Swenson E Mason M Turner KR Dugi DD Hedges JC & Hecht SL. 2019. Effects of estrogen on spermatogenesis in transgender women. Urology 132 117–122. ( 10.1016/j.urology.2019.06.034) [DOI] [PubMed] [Google Scholar]
- Jin SY Lei L Shikanov A Shea LD & Woodruff TK. 2010. A novel two-step strategy for in vitro culture of early-stage ovarian follicles in the mouse. Fertility and Sterility 93 2633–2639. ( 10.1016/j.fertnstert.2009.10.027) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jindarak S Nilprapha K Atikankul T Angspatt A Pungrasmi P Iamphongsai S Promniyom P Suwajo P Selvaggi G & Tiewtranon P. 2018. Spermatogenesis abnormalities following hormonal therapy in transwomen. BioMed Research International 2018 7919481. ( 10.1155/2018/7919481) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorgensen A Young J Nielsen JE Joensen UN Toft BG Rajpert-De Meyts E & Loveland KL. 2014. Hanging drop cultures of human testis and testis cancer samples: a model used to investigate activin treatment effects in a preserved niche. British Journal of Cancer 110 2604–2614. ( 10.1038/bjc.2014.160) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kedem A Yerushalmi GM Brengauz M Raanani H Orvieto R Hourvitz A & Meirow D. 2018. Outcome of immature oocytes collection of 119 cancer patients during ovarian tissue harvesting for fertility preservation. Journal of Assisted Reproduction and Genetics 35 851–856. ( 10.1007/s10815-018-1153-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kent MA Winoker JS & Grotas AB. 2018. Effects of feminizing hormones on sperm production and malignant changes: microscopic examination of post orchiectomy specimens in transwomen. Urology 121 93–96. ( 10.1016/j.urology.2018.07.023) [DOI] [PubMed] [Google Scholar]
- Kerckhof ME Kreukels BPC Nieder TO Becker-Hébly I Van De Grift TC Staphorsius AS Köhler A Heylens G & Elaut E. 2019. Prevalence of sexual dysfunctions in transgender persons: results from the ENIGI follow-up study. Journal of Sexual Medicine 16 2018–2029. ( 10.1016/j.jsxm.2019.09.003) [DOI] [PubMed] [Google Scholar]
- Khattak H, Malhas R, Craciunas L, Afifi Y, Amorim CA, Fishel S, Silber S, Gook D, Demeestere I, Bystrova O, et al. 2022. Fresh and cryopreserved ovarian tissue transplantation for preserving reproductive and endocrine function: a systematic review and individual patient data meta-analysis. Human Reproduction Update 28 400–416. ( 10.1093/humupd/dmac003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimsa MC Strzalka-Mrozik B Kimsa MW Gola J Nicholson P Lopata K & Mazurek U. 2014. Porcine endogenous retroviruses in xenotransplantation--molecular aspects. Viruses 6 2062–2083. ( 10.3390/v6052062) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kita K, Watanabe T, Ohsaka K, Hayashi H, Kubota Y, Nagashima Y, Aoki I, Taniguchi H, Noce T, Inoue K, et al. 2007. Production of functional spermatids from mouse germline stem cells in ectopically reconstituted seminiferous tubules. Biology of Reproduction 76 211–217. ( 10.1095/biolreprod.106.056895) [DOI] [PubMed] [Google Scholar]
- Komeya M Odaka H Matsumura T Yamanaka H Sato T Yao M Masumori N & Ogawa T. 2021. P–017 The maintenance of testicular architecture and germ cell in adult testis tissue under organ culture condition based on the gas-liquid interface method. Human Reproduction 36(Supplement 1). ( 10.1093/humrep/deab130.016) [DOI] [Google Scholar]
- Kozlov M. 2024. Pig-organ transplants: what three human recipients have taught scientists. Nature 629 980–981. ( 10.1038/d41586-024-01453-2) [DOI] [PubMed] [Google Scholar]
- Laronda MM Rutz AL Xiao S Whelan KA Duncan FE Roth EW Woodruff TK & Shah RN. 2017. A bioprosthetic ovary created using 3D printed microporous scaffolds restores ovarian function in sterilized mice. Nature Communications 8 15261. ( 10.1038/ncomms15261) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung A Sakkas D Pang S Thornton K & Resetkova N. 2019. Assisted reproductive technology outcomes in female-to-male transgender patients compared with cisgender patients: a new frontier in reproductive medicine. Fertility and Sterility 112 858–865. ( 10.1016/j.fertnstert.2019.07.014) [DOI] [PubMed] [Google Scholar]
- Li K Rodriguez D Gabrielsen JS Centola GM & Tanrikut C. 2018. Sperm cryopreservation of transgender individuals: trends and findings in the past decade. Andrology 6 860–864. ( 10.1111/andr.12527) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lierman S Tilleman K Braeckmans K Peynshaert K Weyers S T’sjoen G & De Sutter P. 2017. Fertility preservation for trans men: frozen-thawed in vitro matured oocytes collected at the time of ovarian tissue processing exhibit normal meiotic spindles. Journal of Assisted Reproduction and Genetics 34 1449–1456. ( 10.1007/s10815-017-0976-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lierman S, Tolpe A, De Croo I, De Gheselle S, Defreyne J, Baetens M, Dheedene A, Colman R, Menten B, T’sjoen G, et al. 2021. Low feasibility of in vitro matured oocytes originating from cumulus complexes found during ovarian tissue preparation at the moment of gender confirmation surgery and during testosterone treatment for fertility preservation in transgender men. Fertility and Sterility 116 1068–1076. ( 10.1016/j.fertnstert.2021.03.009) [DOI] [PubMed] [Google Scholar]
- Liu Z Nie Y-H Zhang C-C Cai Y-J Wang Y Lu H-P Li Y-Z Cheng C Qiu Z-L & Sun Q. 2016. Generation of macaques with sperm derived from juvenile monkey testicular xenografts. Cell Research 26 139–142. ( 10.1038/cr.2015.112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez F. 2017. Update on fertility preservation from the Barcelona International Society for Fertility Preservation-ESHRE-ASRM 2015 expert meeting: indications, results and future perspectives. Fertility and Sterility 108 407–415.e11. ( 10.1016/j.fertnstert.2017.05.024) [DOI] [PubMed] [Google Scholar]
- Matoso A Khandakar B Yuan S Wu T Wang LJ Lombardo KA Mangray S Mannan AASR & Yakirevich E. 2018. Spectrum of findings in orchiectomy specimens of persons undergoing gender confirmation surgery. Human Pathology 76 91–99. ( 10.1016/j.humpath.2018.03.007) [DOI] [PubMed] [Google Scholar]
- Matthews SJ Picton H Ernst E & Andersen CY. 2018. Successful pregnancy in a woman previously suffering from β-thalassemia following transplantation of ovarian tissue cryopreserved before puberty. Minerva Ginecologica 70 432–435. ( 10.23736/S0026-4784.18.04240-5) [DOI] [PubMed] [Google Scholar]
- Maxwell S Noyes N Keefe D Berkeley AS & Goldman KN. 2017. Pregnancy outcomes after fertility preservation in transgender men. Obstetrics and Gynecology 129 1031–1034. ( 10.1097/AOG.0000000000002036) [DOI] [PubMed] [Google Scholar]
- Mclachlan RI Rajpert-De Meyts E Hoei-Hansen CE De Kretser DM & Skakkebaek NE. 2007. Histological evaluation of the human testis—approaches to optimizing the clinical value of the assessment: mini review. Human Reproduction 22 2–16. ( 10.1093/humrep/del279) [DOI] [PubMed] [Google Scholar]
- Medrano JV, Vilanova-Pérez T, Fornés-Ferrer V, Navarro-Gomezlechon A, Martínez-Triguero ML, García S, Gómez-Chacón J, Povo I, Pellicer A, Andrés MM, et al. 2018. Influence of temperature, serum, and gonadotropin supplementation in short- and long-term organotypic culture of human immature testicular tissue. Fertility and Sterility 110 1045–1057.e3. ( 10.1016/j.fertnstert.2018.07.018) [DOI] [PubMed] [Google Scholar]
- Mohd Faizal A Sugishita Y Suzuki-Takahashi Y Iwahata H Takae S Horage-Okutsu Y & Suzuki N. 2022. Twenty-first century oocyte cryopreservation—in vitro maturation of immature oocytes from ovarian tissue cryopreservation in cancer patients: a systematic review. Women's Health 18 17455057221114269. ( 10.1177/17455057221114269) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moravek MB Dixon M Pena SM & Obedin-Maliver J. 2023. Management of testosterone around ovarian stimulation in transmasculine patients: challenging common practices to meet patient needs—2 case reports. Human Reproduction 38 482–488. ( 10.1093/humrep/dead003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nahata L Tishelman AC Caltabellotta NM & Quinn GP. 2017. Low fertility preservation utilization among transgender youth. Journal of Adolescent Health 61 40–44. ( 10.1016/j.jadohealth.2016.12.012) [DOI] [PubMed] [Google Scholar]
- Nakai M Kaneko H Somfai T Maedomari N Ozawa M Noguchi J Ito J Kashiwazaki N & Kikuchi K. 2010. Production of viable piglets for the first time using sperm derived from ectopic testicular xenografts. Reproduction 139 331–335. ( 10.1530/REP-09-0509) [DOI] [PubMed] [Google Scholar]
- Niederberger C. 2020. Re: effects of estrogen on spermatogenesis in transgender women. Journal of Urology 203 1048. ( 10.1097/JU.0000000000000992.03) [DOI] [PubMed] [Google Scholar]
- Nikmahzar A, Koruji M, Jahanshahi M, Khadivi F, Shabani M, Dehghani S, Forouzesh M, Jabari A, Feizollahi N, Salem M, et al. 2023. Differentiation of human primary testicular cells in the presence of SCF using the organoid culture system. Artificial Organs 47 1818–1830. ( 10.1111/aor.14643) [DOI] [PubMed] [Google Scholar]
- Ntemou E Kadam P Van Laere S Van Saen D Vicini E & Goossens E. 2019. Effect of recombinant human vascular endothelial growth factor on testis tissue xenotransplants from prepubertal boys: a three-case study. Reproductive Biomedicine Online 39 119–133. ( 10.1016/j.rbmo.2019.02.012) [DOI] [PubMed] [Google Scholar]
- Oktay K Harvey BE Partridge AH Quinn GP Reinecke J Taylor HS Wallace WH Wang ET & Loren AW. 2018. Fertility preservation in patients with cancer: ASCO clinical practice guideline update. Journal of Clinical Oncology 36 1994–2001. ( 10.1200/JCO.2018.78.1914) [DOI] [PubMed] [Google Scholar]
- Pache TD Chadha S Gooren LJG Hop WCJ Jaarsma KW Dommerholt HBR & Fauser BCJM. 1991. Ovarian morphology in long-term androgen-treated female to male transsexuals. A human model for the study of polycystic ovarian syndrome? Histopathology 19 445–452. ( 10.1111/j.1365-2559.1991.tb00235.x) [DOI] [PubMed] [Google Scholar]
- Pendergraft SS Sadri-Ardekani H Atala A & Bishop CE. 2017. Three-dimensional testicular organoid: a novel tool for the study of human spermatogenesis and gonadotoxicity in vitro. Biology of Reproduction 96 720–732. ( 10.1095/biolreprod.116.143446) [DOI] [PubMed] [Google Scholar]
- Peri A Ahler A Gook D O’connell MA Bourne H Nightingale M Telfer M Jayasinghe Y & Pang KC. 2021. Predicting successful sperm retrieval in transfeminine adolescents after testicular biopsy. Journal of Assisted Reproduction and Genetics 38 2735–2743. ( 10.1007/s10815-021-02293-z) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poels J Van Langendonckt A Many MC Wese FX & Wyns C. 2013. Vitrification preserves proliferation capacity in human spermatogonia. Human Reproduction 28 578–589. ( 10.1093/humrep/des455) [DOI] [PubMed] [Google Scholar]
- Poels J Abou-Ghannam G Herman S Van Langendonckt A Wese F-X & Wyns C. 2014. In search of better spermatogonial preservation by supplementation of cryopreserved human immature testicular tissue xenografts with N-acetylcysteine and testosterone. Frontiers in Surgery 1 47. ( 10.3389/fsurg.2014.00047) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portela JMD De Winter-Korver CM Van Daalen SKM Meißner A De Melker AA Repping S & Van Pelt AMM. 2019. Assessment of fresh and cryopreserved testicular tissues from (pre)pubertal boys during organ culture as a strategy for in vitro spermatogenesis. Human Reproduction 34 2443–2455. ( 10.1093/humrep/dez180) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poteat T Davis AM & Gonzalez A. 2023. Standards of care for transgender and gender diverse people. JAMA 329 1872–1874. ( 10.1001/jama.2023.8121) [DOI] [PubMed] [Google Scholar]
- Practice Committee of the American Society for Reproductive Medicine 2019. Fertility preservation in patients undergoing gonadotoxic therapy or gonadectomy: a committee opinion. Fertility and Sterility 112 1022–1033. ( 10.1016/j.fertnstert.2019.09.013) [DOI] [PubMed] [Google Scholar]
- Prasath EB Chan ML Wong WH Lim CJ Tharmalingam MD Hendricks M Loh SF & Chia YN. 2014. First pregnancy and live birth resulting from cryopreserved embryos obtained from in vitro matured oocytes after oophorectomy in an ovarian cancer patient. Human Reproduction 29 276–278. ( 10.1093/humrep/det420) [DOI] [PubMed] [Google Scholar]
- Reckhow J Kula H & Babayev S. 2023. Fertility preservation options for transgender and nonbinary individuals. Therapeutic Advances in Endocrinology and Metabolism 14 20420188231178371. ( 10.1177/20420188231178371) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisner SL Poteat T Keatley J Cabral M Mothopeng T Dunham E Holland CE Max R & Baral SD. 2016. Global health burden and needs of transgender populations: a review. Lancet 388 412–436. ( 10.1016/S0140-6736(16)00684-X) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Wallberg KA Häljestig J Arver S Johansson ALV & Lundberg FE. 2021. a Sperm quality in transgender women before or after gender affirming hormone therapy—A prospective cohort study. Andrology 9 1773–1780. ( 10.1111/andr.12999) [DOI] [PubMed] [Google Scholar]
- Rodriguez-Wallberg KA, Milenkovic M, Papaikonomou K, Keros V, Gustafsson B, Sergouniotis F, Wikander I, Perot R, Borgström B, Ljungman P, et al. 2021. b Successful pregnancies after transplantation of ovarian tissue retrieved and cryopreserved at time of childhood acute lymphoblastic leukemia - A case report. Haematologica 106 2783–2787. ( 10.3324/haematol.2021.278828) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakib S Uchida A Valenzuela-Leon P Yu Y Valli-Pulaski H Orwig K Ungrin M & Dobrinski I. 2019. Formation of organotypic testicular organoids in microwell culture†. Biology of Reproduction 100 1648–1660. ( 10.1093/biolre/ioz053) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato T Katagiri K Gohbara A Inoue K Ogonuki N Ogura A Kubota Y & Ogawa T. 2011. In vitro production of functional sperm in cultured neonatal mouse testes. Nature 471 504–507. ( 10.1038/nature09850) [DOI] [PubMed] [Google Scholar]
- Schagen SEE Cohen-Kettenis PT Delemarre-Van De Waal HA & Hannema SE. 2016. Efficacy and safety of gonadotropin-releasing hormone agonist treatment to suppress puberty in gender dysphoric adolescents. Journal of Sexual Medicine 13 1125–1132. ( 10.1016/j.jsxm.2016.05.004) [DOI] [PubMed] [Google Scholar]
- Scheim AI, Rich AJ, Zubizarreta D, Malik M, Baker KE, Restar AJ, Van Der Merwe LA, Wang J, Beebe B, Ridgeway K, et al. 2024. Health status of transgender people globally: a systematic review of research on disease burden and correlates. PLOS ONE 19 e0299373. ( 10.1371/journal.pone.0299373) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlatt S Honaramooz A Boiani M Scholer HR & Dobrinski I. 2003. Progeny from sperm obtained after ectopic grafting of neonatal mouse testes. Biology of Reproduction 68 2331–2335. ( 10.1095/biolreprod.102.014894) [DOI] [PubMed] [Google Scholar]
- Schlatt S Honaramooz A Ehmcke J Goebell PJ Rubben H Dhir R Dobrinski I & Patrizio P. 2006. Limited survival of adult human testicular tissue as ectopic xenograft. Human Reproduction 21 384–389. ( 10.1093/humrep/dei352) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider F Neuhaus N Wistuba J Zitzmann M Heß J Mahler D Van Ahlen H Schlatt S & Kliesch S. 2015. Testicular functions and clinical characterization of patients with gender dysphoria (GD) undergoing sex reassignment surgery (SRS). Journal of Sexual Medicine 12 2190–2200. ( 10.1111/jsm.13022) [DOI] [PubMed] [Google Scholar]
- Segers I Mateizel I Van Moer E Smitz J Tournaye H Verheyen G & De Vos M. 2015. In vitro maturation (IVM) of oocytes recovered from ovariectomy specimens in the laboratory: a promising “ex vivo” method of oocyte cryopreservation resulting in the first report of an ongoing pregnancy in Europe. Journal of Assisted Reproduction and Genetics 32 1221–1231. ( 10.1007/s10815-015-0528-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segers I Bardhi E Mateizel I Van Moer E Schots R Verheyen G Tournaye H & De Vos M. 2020. Live births following fertility preservation using in-vitro maturation of ovarian tissue oocytes. Human Reproduction 35 2026–2036. ( 10.1093/humrep/deaa175) [DOI] [PubMed] [Google Scholar]
- Shinohara T, Inoue K, Ogonuki N, Kanatsu-Shinohara M, Miki H, Nakata K, Kurome M, Nagashima H, Toyokuni S, Kogishi K, et al. 2002. Birth of offspring following transplantation of cryopreserved immature testicular pieces and in-vitro microinsemination. Human Reproduction 17 3039–3045. ( 10.1093/humrep/17.12.3039) [DOI] [PubMed] [Google Scholar]
- Sinha A Mei L & Ferrando C. 2021. The effect of estrogen therapy on spermatogenesis in transgender women. F&S Reports 2 347–351. ( 10.1016/j.xfre.2021.06.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spinder T Spijkstra JJ Van Den Tweel JG Burger CW Van Kessel H Hompes PG & Gooren LJ. 1989. The effects of long term testosterone administration on pulsatile luteinizing hormone secretion and on ovarian histology in eugonadal female to male transsexual subjects. Journal of Clinical Endocrinology and Metabolism 69 151–157. ( 10.1210/jcem-69-1-151) [DOI] [PubMed] [Google Scholar]
- Stark BA & Mok-Lin E. 2022. Fertility preservation in transgender men without discontinuation of testosterone. F&S Reports 3 153–156. ( 10.1016/j.xfre.2022.02.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolk THR Asseler JD Huirne JAF van den Boogaard E & van Mello NM. 2023. Desire for children and fertility preservation in transgender and gender-diverse people: a systematic review. Best Practice and Research. Clinical Obstetrics and Gynaecology 87 102312. ( 10.1016/j.bpobgyn.2023.102312) [DOI] [PubMed] [Google Scholar]
- Tangpricha V & Den Heijer M. 2017. Oestrogen and anti-androgen therapy for transgender women. Lancet. Diabetes and Endocrinology 5 291–300. ( 10.1016/S2213-8587(16)30319-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran KTD Valli-Pulaski H Colvin A & Orwig KE. 2022. Male fertility preservation and restoration strategies for patients undergoing gonadotoxic therapies. Biology of Reproduction 107 382–405. ( 10.1093/biolre/ioac072) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trussler JT & Carrasquillo RJ. 2020. Cryptozoospermia associated with genital tucking behavior in a transwoman. Reviews in Urology 22 170–173. [PMC free article] [PubMed] [Google Scholar]
- Uzelac PS Delaney AA Christensen GL Bohler HCL & Nakajima ST. 2015. Live birth following in vitro maturation of oocytes retrieved from extracorporeal ovarian tissue aspiration and embryo cryopreservation for 5 years. Fertility and Sterility 104 1258–1260. ( 10.1016/j.fertnstert.2015.07.1148) [DOI] [PubMed] [Google Scholar]
- Van Saen D Goossens E Bourgain C Ferster A & Tournaye H. 2011. Meiotic activity in orthotopic xenografts derived from human postpubertal testicular tissue. Human Reproduction 26 282–293. ( 10.1093/humrep/deq321) [DOI] [PubMed] [Google Scholar]
- Vereecke G Defreyne J Van Saen D Collet S Van Dorpe J T'sjoen G & Goossens E. 2021. Characterisation of testicular function and spermatogenesis in transgender women. Human Reproduction 36 5–15. ( 10.1093/humrep/deaa254) [DOI] [PubMed] [Google Scholar]
- White J Jackson A Druce I & Gale J. 2024. Oocyte cryopreservation and reciprocal in vitro fertilization in a transgender man on long term testosterone gender-affirming hormone therapy: a case report. F&S Reports 5 111–113. ( 10.1016/j.xfre.2023.11.004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X Goodyear SM Abramowitz LK Bartolomei MS Tobias JW Avarbock MR & Brinster RL. 2012. Fertile offspring derived from mouse spermatogonial stem cells cryopreserved for more than 14 years. Human Reproduction 27 1249–1259. ( 10.1093/humrep/des077) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyns C Curaba M Martinez-Madrid B Van Langendonckt A Francois-Xavier W & Donnez J. 2007. Spermatogonial survival after cryopreservation and short-term orthotopic immature human cryptorchid testicular tissue grafting to immunodeficient mice. Human Reproduction 22 1603–1611. ( 10.1093/humrep/dem062) [DOI] [PubMed] [Google Scholar]
- Wyns C Van Langendonckt A Wese FX Donnez J & Curaba M. 2008. Long-term spermatogonial survival in cryopreserved and xenografted immature human testicular tissue. Human Reproduction 23 2402–2414. ( 10.1093/humrep/den272) [DOI] [PubMed] [Google Scholar]
- Xu J Xu M Bernuci MP Fisher TE Shea LD Woodruff TK Zelinski MB & Stouffer RL. 2013. Primate follicular development and oocyte maturation in vitro. Advances in Experimental Medicine and Biology 761 43–67. ( 10.1007/978-1-4614-8214-7_5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Younis N Caldeira-Brant AL Chu T Abdalla S & Orwig KE. 2023. Human immature testicular tissue organ culture: a step towards fertility preservation and restoration. Frontiers in Endocrinology 14 1242263. ( 10.3389/fendo.2023.1242263) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucker KJ. 2017. Epidemiology of gender dysphoria and transgender identity. Sexual Health 14 404–411. ( 10.1071/SH17067) [DOI] [PubMed] [Google Scholar]



 This work is licensed under a
This work is licensed under a