Abstract
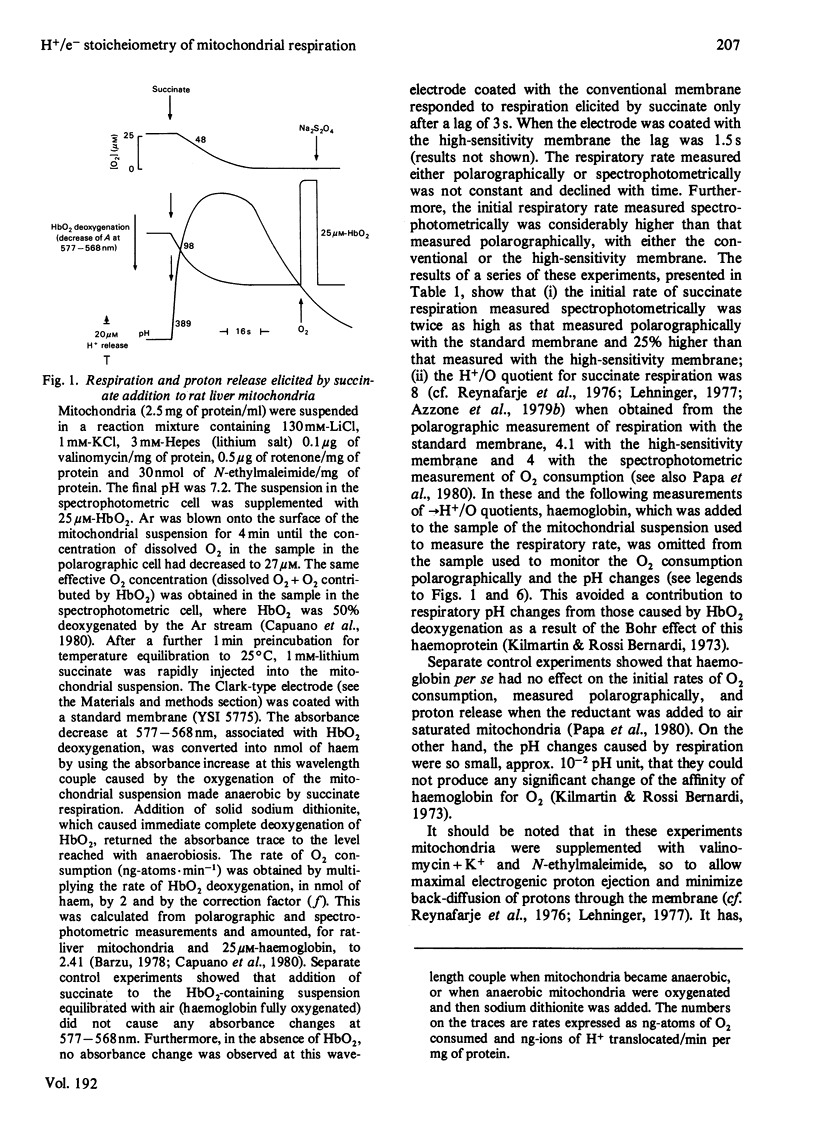
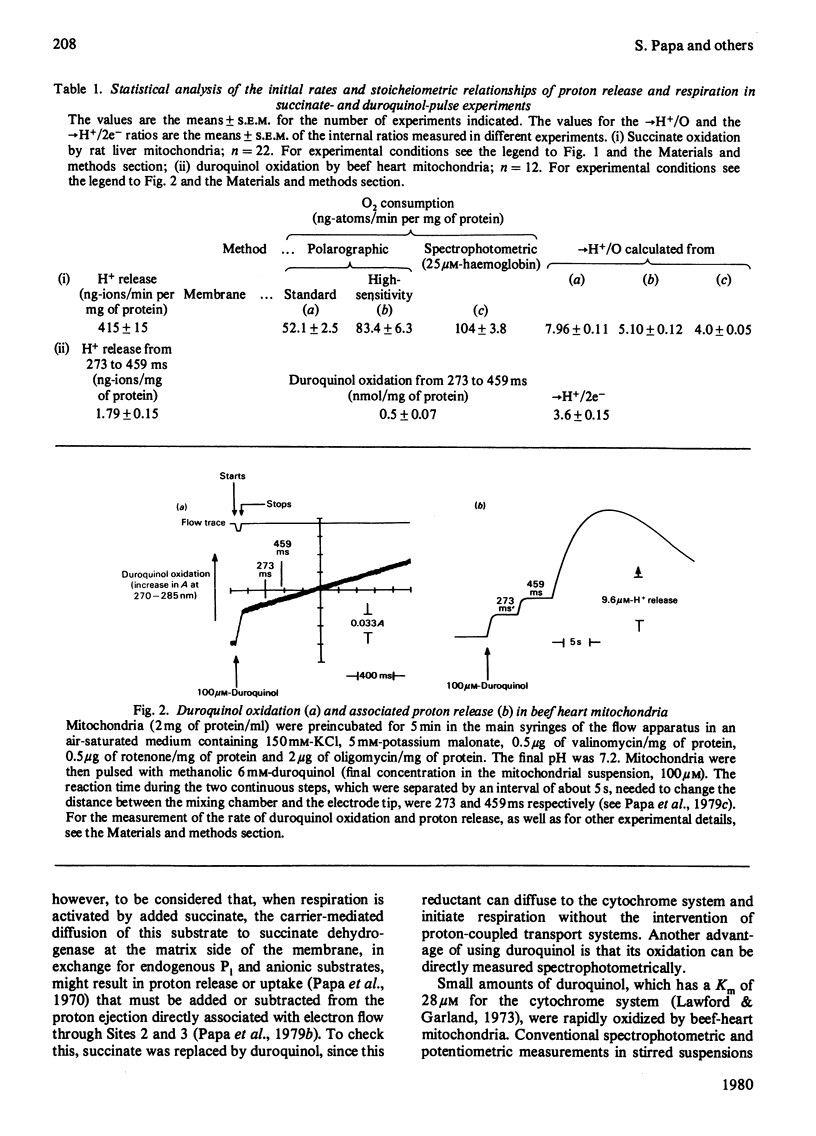
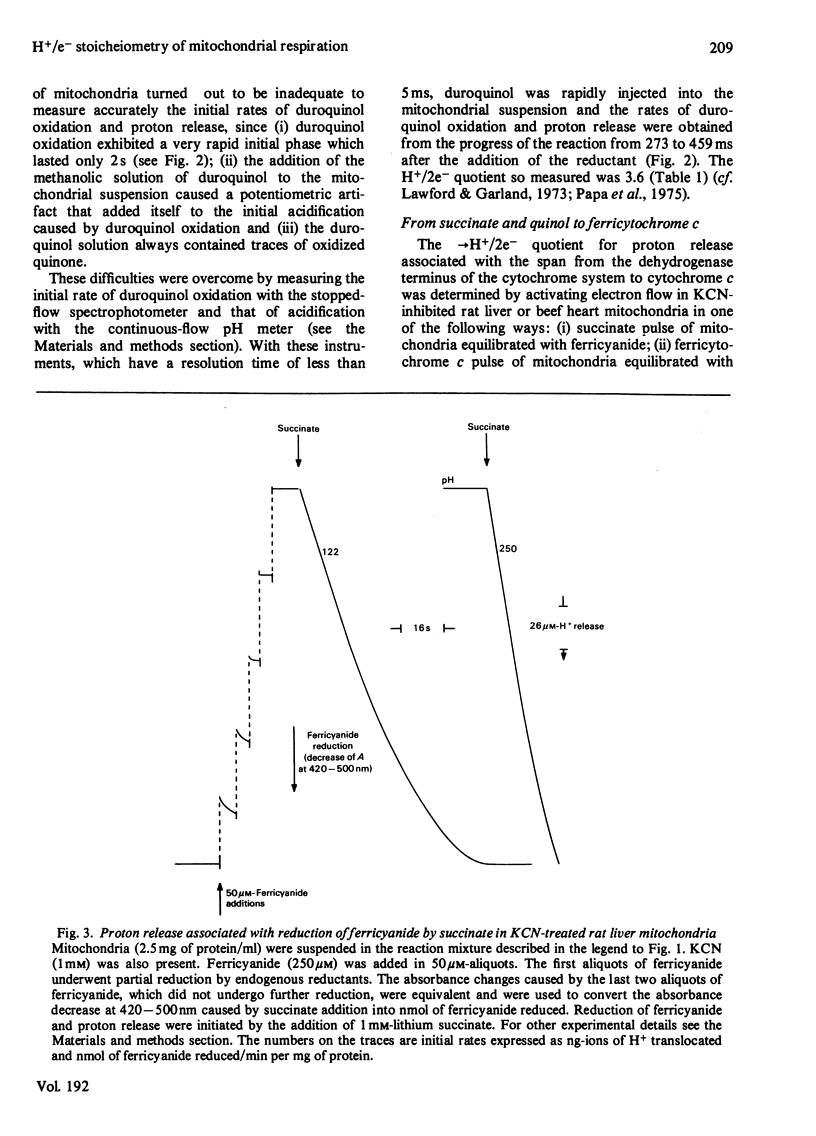
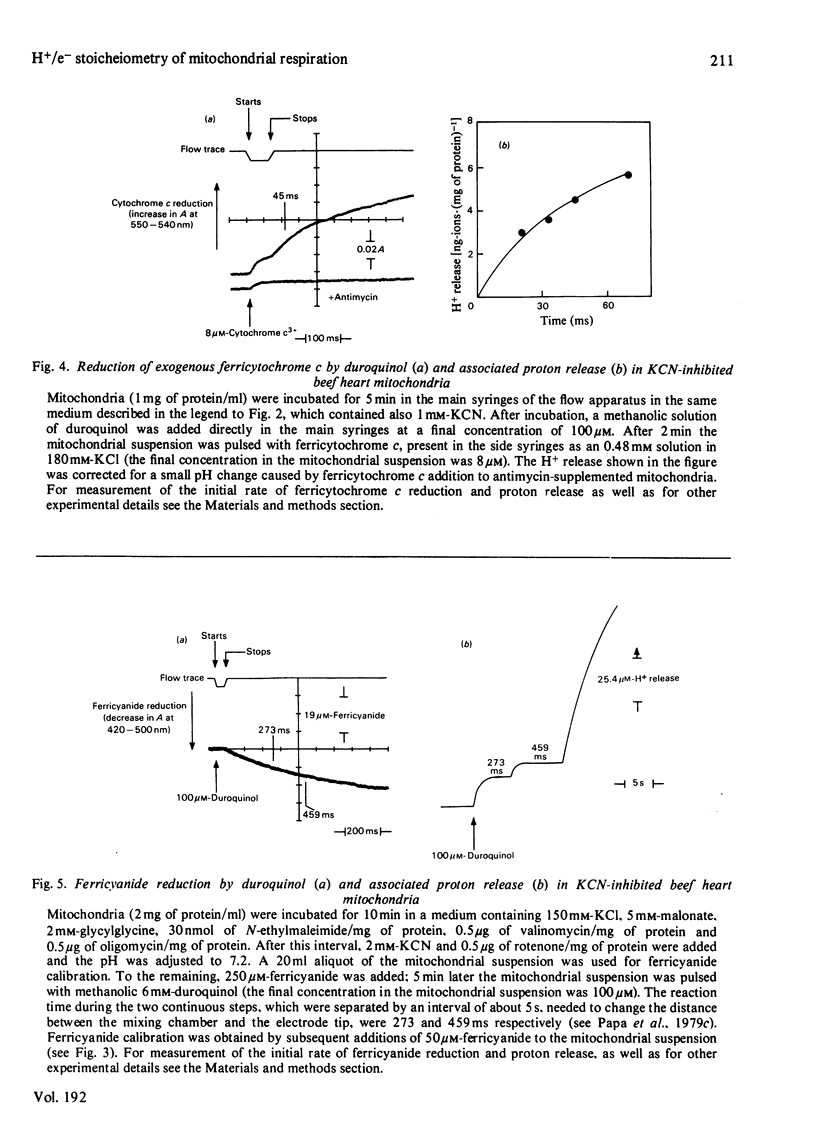
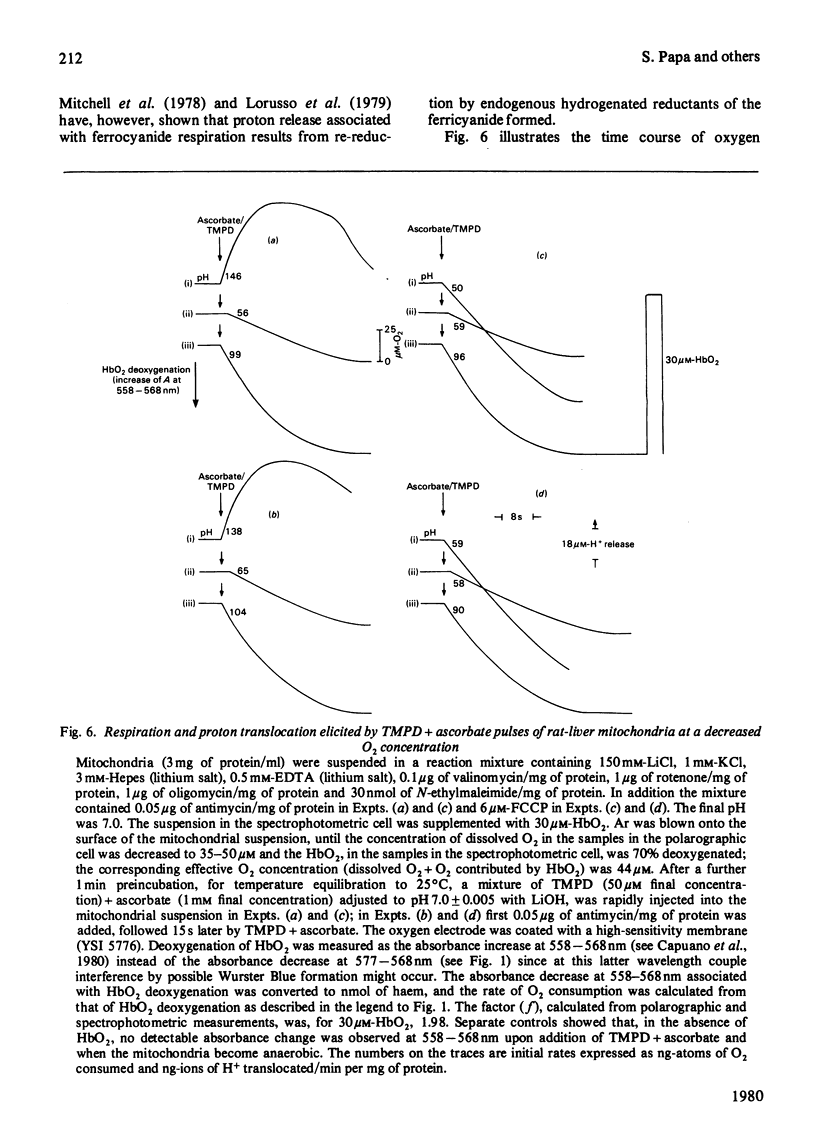
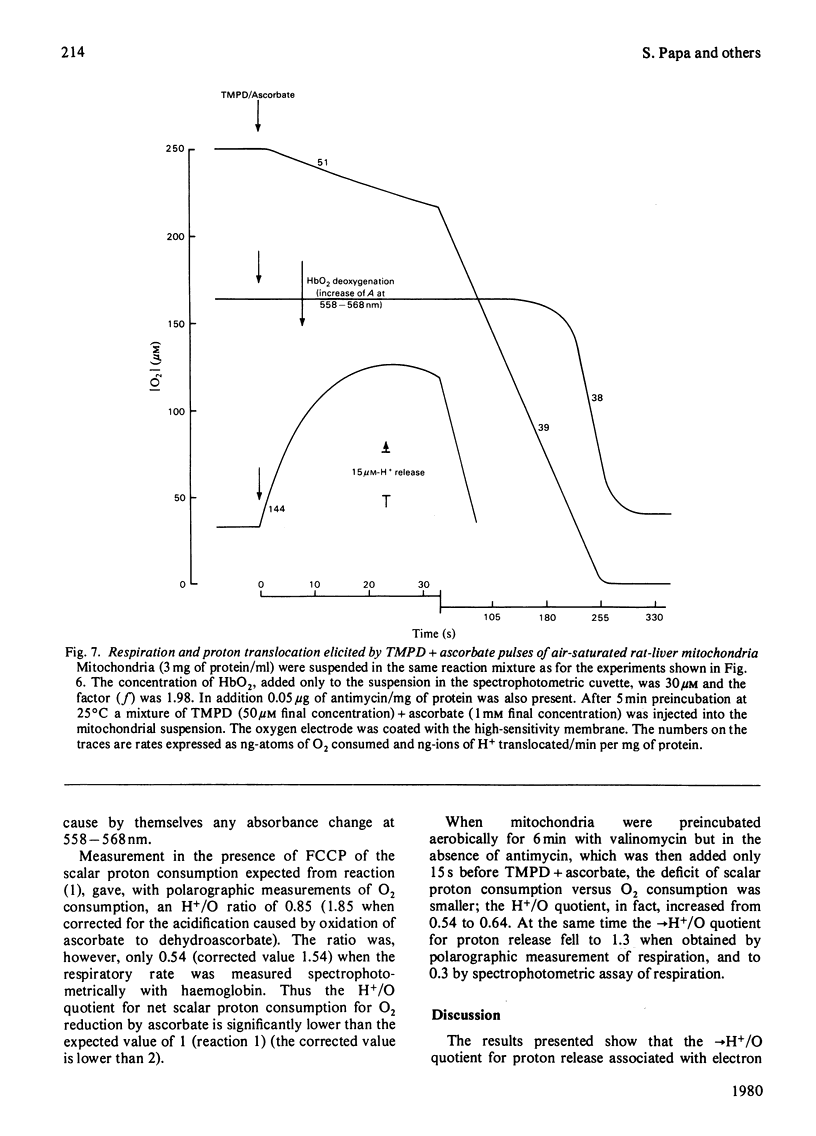
1. The →H+/e− quotients for proton release from mitochondria associated with electron flow from succinate and duroquinol to O2, ferricyanide or ferricytochrome c, and from NNN′N′-tetramethyl-p-phenylenediamine+ascorbate to O2, were determined from rate measurements of electron flow and proton translocation. 2. Care was taken to avoid, or to take into account, unrelated electron flow and proton translocation, which might take place in addition to the oxido-reductions that were the subject of our analysis. Spectrophotometric techniques were chosen to provide accurate measurement of the rate of consumption of oxidants and reductants. The rate of proton translocation was measured with fast pH meters with a precision of 10−3 pH unit. 3. The →H+/O quotient for succinate or duroquinol oxidation was, at neutral pH, 4, when computed on the basis of spectrophotometric determinations of the rate of O2 consumption or duroquinol oxidation. Higher →H+/O quotients for succinate oxidation, obtained from polarographic measurements of O2 consumption, resulted from underestimation of the respiratory rate. 4. The →H+/2e− quotient for electron flow from succinate and duroquinol to ferricyanide or ferricytochrome c ranged from 3.9 to 3.6. 5. Respiration elicited by NNN′N′-tetramethyl-p-phenylenediamine+ascorbate by antimycin-inhibited mitochondria resulted in extra proton release in addition to that produced for oxidation of ascorbate to dehydroascorbate. Accurate spectrophotometric measurement of respiration showed that the →H+/e− ratio was only 0.25 and not 0.7–1.0 as obtained with the inadequate polarographic assay of respiration. Proton release was practically suppressed when mitochondria were preincubated aerobically in the absence of antimycin. Furthermore, the rate of scalar proton consumption for water production was lower than that expected from the stoicheiometry. Thus the extra proton release observed during respiration elicited by NNN′N′-tetramethyl-p-phenylenediamine+ascorbate is caused by oxidation of endogenous hydrogenated reductants. 6. It is concluded that (i) the →H+/O quotient for the cytochrome system is, at neutral pH, 4 and not 6 or 8 as reported by others; (ii) all the four protons are released during electron flow from quinol to cytochrome c; (iii) the oxidase transfers electrons from cytochrome c to protons from the matrix aqueous phase and does not pump protons from the matrix to the outer aqueous phase.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexandre A., Reynafarje B., Lehninger A. L. Stoichiometry of vectorial H+ movements coupled to electron transport and to ATP synthesis in mitochondria. Proc Natl Acad Sci U S A. 1978 Nov;75(11):5296–5300. doi: 10.1073/pnas.75.11.5296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azzone G. F., Pozzan T., Bragadin M., Miconi V. Thermodynamics and kinetics of the H+ pump in mitochondrial electron transport. J Biol Chem. 1979 Oct 25;254(20):10213–10219. [PubMed] [Google Scholar]
- Azzone G. F., Pozzan T., Di Virgilio F. H+/site, charge/site, and ATP/site ratios at coupling site III in mitochondrial electron transport. J Biol Chem. 1979 Oct 25;254(20):10206–10212. [PubMed] [Google Scholar]
- Brand M. D., Harper W. G., Nicholls D. G., Ingledew W. J. Unequal charge separation by different coupling spans of the mitochondrial electron transport chain. FEBS Lett. 1978 Nov 1;95(1):125–129. doi: 10.1016/0014-5793(78)80066-0. [DOI] [PubMed] [Google Scholar]
- Brand M. D., Reynafarje B., Lehninger A. L. Re-evaluation of the H+/site ratio of mitochondrial electron transport with the oxygen pulse technique. J Biol Chem. 1976 Sep 25;251(18):5670–5679. [PubMed] [Google Scholar]
- Brand M. D., Reynafarje B., Lehninger A. L. Stoichiometric relationship between energy-dependent proton ejection and electron transport in mitochondria. Proc Natl Acad Sci U S A. 1976 Feb;73(2):437–441. doi: 10.1073/pnas.73.2.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand M. D. The stoicheiometric relationships between electron transport, proton translocation and adenosine triphosphate synthesis and hydrolysis in mitochondria. Biochem Soc Trans. 1977;5(5):1615–1620. doi: 10.1042/bst0051615. [DOI] [PubMed] [Google Scholar]
- Brandon J. R., Brocklehurst J. R., Lee C. P. Effect of antimycin A and 2-heptyl-4-hydroxyquinoline N-oxide on the respiratory chain of submitochondrial particles of beef heart. Biochemistry. 1972 Mar 28;11(7):1150–1154. doi: 10.1021/bi00757a006. [DOI] [PubMed] [Google Scholar]
- Bârzu O. Spectrophotometric assay of oxygen consumption. Methods Enzymol. 1978;54:485–498. doi: 10.1016/s0076-6879(78)54029-9. [DOI] [PubMed] [Google Scholar]
- CHANCE B., WILLIAMS G. R. The respiratory chain and oxidative phosphorylation. Adv Enzymol Relat Subj Biochem. 1956;17:65–134. doi: 10.1002/9780470122624.ch2. [DOI] [PubMed] [Google Scholar]
- Capuano F., Izzo G., Altamura N., Papa S. Spectrophotometric determination with hemoglobin of the rate of oxygen consumption in mitochondria. FEBS Lett. 1980 Feb 25;111(1):249–254. doi: 10.1016/0014-5793(80)80804-0. [DOI] [PubMed] [Google Scholar]
- Casey R. P., Chappell J. B., Azzi A. Limited-turnover studies on proton translocation in reconstituted cytochrome c oxidase-containing vesicles. Biochem J. 1979 Jul 15;182(1):149–156. doi: 10.1042/bj1820149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddock B. A., Jones C. W. Bacterial respiration. Bacteriol Rev. 1977 Mar;41(1):47–99. doi: 10.1128/br.41.1.47-99.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hultborn R. A sensitive method for measuring oxygen consumption. Anal Biochem. 1972 Jun;47(2):442–450. doi: 10.1016/0003-2697(72)90137-6. [DOI] [PubMed] [Google Scholar]
- Izzo G., Guerrieri F., Papa S. On the mechanism of inhibition of the respiratory chain by 2-heptyl-4-hydroxyquinoline-N-oxide. FEBS Lett. 1978 Sep 15;93(2):320–322. doi: 10.1016/0014-5793(78)81130-2. [DOI] [PubMed] [Google Scholar]
- Kilmartin J. V., Rossi-Bernardi L. Interaction of hemoglobin with hydrogen ions, carbon dioxide, and organic phosphates. Physiol Rev. 1973 Oct;53(4):836–890. doi: 10.1152/physrev.1973.53.4.836. [DOI] [PubMed] [Google Scholar]
- Krab K., Wikström M. Effect of 2-n-heptyl-4-hydroxyquinoline N-oxide on proton permeability of the mitochondrial membrane. Biochem J. 1980 Feb 15;186(2):637–639. doi: 10.1042/bj1860637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krab K., Wikström M. On the stoichiometry and thermodynamics of proton-pumping cytochrome c oxidase in mitochondria. Biochim Biophys Acta. 1979 Oct 10;548(1):1–15. doi: 10.1016/0005-2728(79)90182-8. [DOI] [PubMed] [Google Scholar]
- Kröger A., Klingenberg M. On the role of ubiquinone in mitochondria. II. Redox reactions of ubiquinone under the control of oxidative phosphorylation. Biochem Z. 1966 Jun 7;344(4):317–336. [PubMed] [Google Scholar]
- Lawford H. G., Garland P. B. Proton translocation coupled to quinol oxidation in ox heart mitochondria. Biochem J. 1973 Nov;136(3):711–720. doi: 10.1042/bj1360711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorusso M., Capuano F., Boffoli D., Stefanelli R., Papa S. The mechanism of transmembrane delta muH+ generation in mitochondria by cytochrome c oxidase. Biochem J. 1979 Jul 15;182(1):133–147. doi: 10.1042/bj1820133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MYERS D. K., SLATER E. C. The enzymic hydrolysis of adenosine triphosphate by liver mitochondria. I. Activities at different pH values. Biochem J. 1957 Dec;67(4):558–572. doi: 10.1042/bj0670558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller M., Petersen L. C., Hansen F. B., Nicholls P. Effect of ionophores on carrier-mediated electron translocation in ferricyanide-containing liposomes. Biochem J. 1979 Oct 15;184(1):125–131. doi: 10.1042/bj1840125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell P. Chemiosmotic coupling in oxidative and photosynthetic phosphorylation. Biol Rev Camb Philos Soc. 1966 Aug;41(3):445–502. doi: 10.1111/j.1469-185x.1966.tb01501.x. [DOI] [PubMed] [Google Scholar]
- Mitchell P., Moyle J., Mitchell R. Measurement of translocation of H+/O in mitochondria and submitochondrial vesicles. Methods Enzymol. 1979;55:627–640. doi: 10.1016/0076-6879(79)55071-x. [DOI] [PubMed] [Google Scholar]
- Mitchell P., Moyle J. Respiration-driven proton translocation in rat liver mitochondria. Biochem J. 1967 Dec;105(3):1147–1162. doi: 10.1042/bj1051147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell P. The Ninth Sir Hans Krebs Lecture. Compartmentation and communication in living systems. Ligand conduction: a general catalytic principle in chemical, osmotic and chemiosmotic reaction systems. Eur J Biochem. 1979 Mar 15;95(1):1–20. doi: 10.1111/j.1432-1033.1979.tb12934.x. [DOI] [PubMed] [Google Scholar]
- Mitchell P. Vectorial chemistry and the molecular mechanics of chemiosmotic coupling: power transmission by proticity. Biochem Soc Trans. 1976;4(3):399–430. doi: 10.1042/bst0040399. [DOI] [PubMed] [Google Scholar]
- Moyle J., Mitchell P. Measurements of mitochondrial comes from H+/O quotients: effects of phosphate and N-ethylmaleimide. FEBS Lett. 1978 Jun 15;90(2):361–365. doi: 10.1016/0014-5793(78)80405-0. [DOI] [PubMed] [Google Scholar]
- Papa S., Capuano F., Markert M., Altamura N. The H+/O stoicheiometry of mitochondrial respiration. FEBS Lett. 1980 Feb 25;111(1):243–248. doi: 10.1016/0014-5793(80)80803-9. [DOI] [PubMed] [Google Scholar]
- Papa S., Guerrieri F., Izzo G. Redox Bohr-effects in the cytochrome system of mitochondria. FEBS Lett. 1979 Sep 15;105(2):213–216. doi: 10.1016/0014-5793(79)80614-6. [DOI] [PubMed] [Google Scholar]
- Papa S., Guerrieri F., Lorusso M., Simone S. Proton translocation and energy transduction in mitochondria. Biochimie. 1973;55(6):703–716. doi: 10.1016/s0300-9084(73)80024-0. [DOI] [PubMed] [Google Scholar]
- Papa S., Lofrumento N. E., Quagliariello E., Meijer A. J., Tager J. M. Coupling mechanisms in anionic substrate transport across the inner membrane of rat-liver mitochondria. J Bioenerg. 1971 Sep;1(3):287–307. doi: 10.1007/BF01516289. [DOI] [PubMed] [Google Scholar]
- Papa S., Lorusso M., Guerrieri F. Mechanism of respiration-driven proton translocation in the inner mitochondrial membrane. Analysis of proton translocation associated with oxidation of endogenous ubiquinol. Biochim Biophys Acta. 1975 Jun 17;387(3):425–440. doi: 10.1016/0005-2728(75)90083-3. [DOI] [PubMed] [Google Scholar]
- Papa S. Proton translocation reactions in the respiratory chains. Biochim Biophys Acta. 1976 Apr 30;456(1):39–84. doi: 10.1016/0304-4173(76)90008-2. [DOI] [PubMed] [Google Scholar]
- Reynafarje B., Brand M. D., Lehninger A. L. Evaluation of the H+/site ratio of mitochondrial electron transport from rate measurements. J Biol Chem. 1976 Dec 10;251(23):7442–7451. [PubMed] [Google Scholar]
- SZARKOWSKA L., KLINGENBERG M. ON THE ROLE OF UBIQUINONE IN MITOCHONDRIA. SPECTROPHOTOMETRIC AND CHEMICAL MEASUREMENTS OF ITS REDOX REACTIONS. Biochem Z. 1963;338:674–697. [PubMed] [Google Scholar]
- Sigel E., Carafoli E. The charge stoichiometry of cytochrome c oxidase in the reconstituted system. J Biol Chem. 1979 Nov 10;254(21):10572–10574. [PubMed] [Google Scholar]
- Sigel E., Carafoli E. The proton pump of cytochrome c oxidase and its stoichiometry. Eur J Biochem. 1978 Aug 15;89(1):119–123. doi: 10.1111/j.1432-1033.1978.tb20903.x. [DOI] [PubMed] [Google Scholar]
- Slater E. C. Mechanism of oxidative phosphorylation. Annu Rev Biochem. 1977;46:1015–1026. doi: 10.1146/annurev.bi.46.070177.005055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorgato M. C., Ferguson S. J. Measurements of the components of the protonmotive force generated by cytochrome oxidase in submitochondrial particles. FEBS Lett. 1978 Jun 1;90(1):178–182. doi: 10.1016/0014-5793(78)80324-x. [DOI] [PubMed] [Google Scholar]
- Wikstrom M. K. Proton pump coupled to cytochrome c oxidase in mitochondria. Nature. 1977 Mar 17;266(5599):271–273. doi: 10.1038/266271a0. [DOI] [PubMed] [Google Scholar]
- Wikström M. K., Saari H. T. The mechanism of energy conservation and transduction by mitochondrial cytochrome c oxidase. Biochim Biophys Acta. 1977 Nov 17;462(2):347–361. doi: 10.1016/0005-2728(77)90133-5. [DOI] [PubMed] [Google Scholar]
- Wikström M., Krab K. Cytochrome c oxidase is a proton pump: a rejoinder to recent criticism. FEBS Lett. 1978 Jul 1;91(1):8–14. doi: 10.1016/0014-5793(78)80006-4. [DOI] [PubMed] [Google Scholar]
- Wikström M., Krab K. Proton-pumping cytochrome c oxidase. Biochim Biophys Acta. 1979 Aug 17;549(2):177–122. doi: 10.1016/0304-4173(79)90014-4. [DOI] [PubMed] [Google Scholar]
- Wrigglesworth J. M., Nicholls P. Turnover and vectorial properties of cytochrome c oxidase in reconstituted vesicles. Biochim Biophys Acta. 1979 Jul 10;547(1):36–46. doi: 10.1016/0005-2728(79)90093-8. [DOI] [PubMed] [Google Scholar]


