Abstract
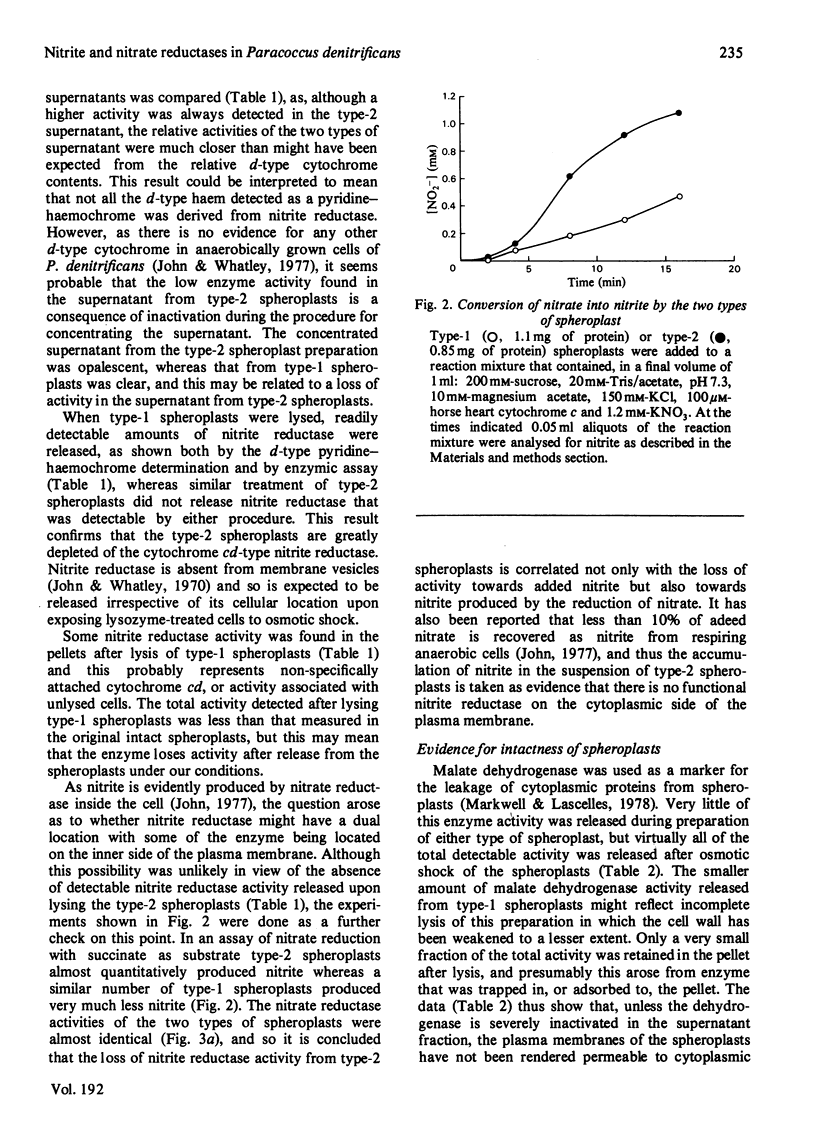
1. A method is described for preparing spheroplasts from Paracoccus denitrificans that are substantially depleted of dissimilatory nitrate reductase (cytochrome cd) activity. Treatment of cells with lysozyme + EDTA together with a mild osmotic shock, followed by centrifugation, yielded a pellet of spheroplasts and a supernatant that contained d-type cytochrome. The spheroplasts were judged to have retained an intact plasma membrane on the basis that less than 1% of the activity of a cytoplasmic marker protein, malate dehydrogenase, was released from the spheroplasts. In addition to a low activity towards added nitrite, the suspension of spheroplasts accumulated the nitrite that was produced by respiratory chain-linked reduction of nitrate. It is concluded that nitrate reduction occurs at the periplasmic side of the plasma membrane irrespective of whether nitrite is generated by nitrate reduction or is added exogenously. 2. Further evidence for the integrity of the spheroplasts was that nitrate reduction was inhibited by O2, and that chlorate was reduced at a markedly lower rate than nitrate. These data are taken as evidence for an intact plasma membrane because it was shown that cells acquire the capability to reduce nitrate under aerobic conditions after addition of low amounts of Triton X-100 which, with the same titre, also overcame the permeability barrier to chlorate reduction by intact cells. The close relationship between the appearance of chlorate reduction and the loss of the inhibitory effect of O2 on nitrate reduction also suggests that the later feature of nitrate respiration is due to a control on the accessibility of nitrate to its reductase rather than on the flow of electrons to nitrate reductase.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beacham I. R. Periplasmic enzymes in gram-negative bacteria. Int J Biochem. 1979;10(11):877–883. doi: 10.1016/0020-711x(79)90117-4. [DOI] [PubMed] [Google Scholar]
- Burnell J. N., John P., Whatley F. R. The reversibility of active sulphate transport in membrane vesicles of Paracoccus denitrificans. Biochem J. 1975 Sep;150(3):527–536. doi: 10.1042/bj1500527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobley J. G. Energy-conserving reactions in phosphorylating electron-transport particles from Nitrobacter winogradskyi. Activation of nitrite oxidation by the electrical component of the protonmotive force. Biochem J. 1976 Jun 15;156(3):481–491. doi: 10.1042/bj1560481c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman K. J., Cornish-Bowden A., Cole J. A. Purification and properties of nitrite reductase from Escherichia coli K12. Biochem J. 1978 Nov 1;175(2):483–493. doi: 10.1042/bj1750483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erecińska M., Davis J. S., Wilson D. F. Regulation of respiration in paracoccus denitrificans: the dependence on redox state of cytochrome c and [ATP]/[ADP][Pi]. Arch Biochem Biophys. 1979 Oct 15;197(2):463–469. doi: 10.1016/0003-9861(79)90268-6. [DOI] [PubMed] [Google Scholar]
- Garland P. B., Downie J. A., Haddock B. A. Proton translocation and the respiratory nitrate reductase of Escherichia coli. Biochem J. 1975 Dec;152(3):547–559. doi: 10.1042/bj1520547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrard W. T. Synthesis, assembly, and localization of periplasmic cytochrome c. J Biol Chem. 1972 Sep 25;247(18):5935–5943. [PubMed] [Google Scholar]
- Haddock B. A., Jones C. W. Bacterial respiration. Bacteriol Rev. 1977 Mar;41(1):47–99. doi: 10.1128/br.41.1.47-99.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John P. Aerobic and anaerobic bacterial respiration monitored by electrodes. J Gen Microbiol. 1977 Jan;98(1):231–238. doi: 10.1099/00221287-98-1-231. [DOI] [PubMed] [Google Scholar]
- John P., Whatley F. R. Oxidative phosphorylation coupled to oxygen uptake and nitrate reduction in Micrococcus denitrificans. Biochim Biophys Acta. 1970 Sep 1;216(2):342–352. doi: 10.1016/0005-2728(70)90225-2. [DOI] [PubMed] [Google Scholar]
- Jones R. W., Ingledew W. J., Graham A., Garland P. B. Topography of nitrate reductase of the cytoplasmic membrane of Escherichia coli: the nitrate-reducing site [proceedings]. Biochem Soc Trans. 1978;6(6):1287–1289. doi: 10.1042/bst0061287. [DOI] [PubMed] [Google Scholar]
- Kristjansson J. K., Hollocher T. C. Substrate binding site for nitrate reductase of Escherichia coli is on the inner aspect of the membrane. J Bacteriol. 1979 Mar;137(3):1227–1233. doi: 10.1128/jb.137.3.1227-1233.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristjansson J. K., Walter B., Hollocher T. C. Respiration-dependent proton translocation and the transport of nitrate and nitrite in Paracoccus denitrificans and other denitrifying bacteria. Biochemistry. 1978 Nov 14;17(23):5014–5019. doi: 10.1021/bi00616a024. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Markwell J. P., Lascelles J. Membrane-bound, pyridine nucleotide-independent L-lactate dehydrogenase of Rhodopseudomonas sphaeroides. J Bacteriol. 1978 Feb;133(2):593–600. doi: 10.1128/jb.133.2.593-600.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinkus K., Kennelly P. J., Rea T., Timkovich R. Purification and properties of Paracoccus denitrificans Azurin. Arch Biochem Biophys. 1980 Feb;199(2):465–472. doi: 10.1016/0003-9861(80)90303-3. [DOI] [PubMed] [Google Scholar]
- Newton N. The two-haem nitrite reductase of Micrococcus denitrificans. Biochim Biophys Acta. 1969;185(2):316–331. doi: 10.1016/0005-2744(69)90425-2. [DOI] [PubMed] [Google Scholar]
- Prince R. C., Baccarini-Melandri A., Hauska G. A., Melandri B. A., Crofts A. R. Asymmetry of an energy transducing membrane the location of cytochrome c2 in Rhodopseudomonas spheroides and Rhodopseudomonas capsulata. Biochim Biophys Acta. 1975 May 15;387(2):212–227. doi: 10.1016/0005-2728(75)90104-8. [DOI] [PubMed] [Google Scholar]
- Robinson M. K., Martinkus K., Kennelly P. J., Timkovich R. Implications of the integrated rate law for the reactions of Paracoccus denitrificans nitrite reductase. Biochemistry. 1979 Sep 4;18(18):3921–3926. doi: 10.1021/bi00585a012. [DOI] [PubMed] [Google Scholar]
- Saraste M., Kuronen T. Interaction of Pseudomonas cytochrome cd1 with the cytoplasmic membrane. Biochim Biophys Acta. 1978 Oct 19;513(1):117–131. doi: 10.1016/0005-2736(78)90117-7. [DOI] [PubMed] [Google Scholar]
- Scholes P. B., Smith L. The isolation and properties of the cytoplasmic membrane of Micrococcus denitrificans. Biochim Biophys Acta. 1968 Feb 12;153(2):350–362. doi: 10.1016/0005-2728(68)90080-7. [DOI] [PubMed] [Google Scholar]
- Sias S. R., Ingraham J. L. Isolation and analysis of mutants of Pseudomonas aeruginosa unable to assimilate nitrate. Arch Microbiol. 1979 Sep;122(3):263–270. doi: 10.1007/BF00411289. [DOI] [PubMed] [Google Scholar]
- Sorgato M. C., Ferguson S. J. Evidence that in submitochondrial particles cytochrome oxidase translocates protons [proceedings]. Biochem Soc Trans. 1979 Feb;7(1):219–221. doi: 10.1042/bst0070219. [DOI] [PubMed] [Google Scholar]
- Wientjes F. B., Kolk A. H., Nanninga N., Van T'Riet J. Respiratory nitrate reductase: its localization in the cytoplasmic membrane of Klebsiella aerogenes and Bacillus licheniformis. Eur J Biochem. 1979 Mar 15;95(1):61–67. doi: 10.1111/j.1432-1033.1979.tb12939.x. [DOI] [PubMed] [Google Scholar]
- Witholt B., Boekhout M., Brock M., Kingma J., Heerikhuizen H. V., Leij L. D. An efficient and reproducible procedure for the formation of spheroplasts from variously grown Escherichia coli. Anal Biochem. 1976 Jul;74(1):160–170. doi: 10.1016/0003-2697(76)90320-1. [DOI] [PubMed] [Google Scholar]
- Wood P. M. Periplasmic location of the terminal reductase in nitrite respiration. FEBS Lett. 1978 Aug 15;92(2):214–218. doi: 10.1016/0014-5793(78)80757-1. [DOI] [PubMed] [Google Scholar]
- Zumft W. G., Sherr B. F., Payne W. J. A reappraisal of the nitric oxide-binding protein of denitrifying Pseudomonas. Biochem Biophys Res Commun. 1979 Jun 27;88(4):1230–1236. doi: 10.1016/0006-291x(79)91111-2. [DOI] [PubMed] [Google Scholar]


