Abstract
Ocean alkalinity enhancement (OAE) is a nature-based technology for CO2 removal and storage, but little is known about its environmental safety. We tested a CO2-equilibrated OAE deployment in a close-to-natural community using in situ mesocosms in the oligotrophic subtropical North Atlantic and assessed metazoan zooplankton to inform about food web stability, structure, and production. In addition, a literature review complemented experimental results by summarizing physiological responses of marine animals to decreasing proton concentrations, or increased pH. The food web studied proved resistant, and zooplankton physiologically tolerant, to the OAE tested. We observed short-term effects of OAE on zooplankton reproduction and productivity, which were likely trophically mediated. Yet, these did not affect zooplankton populations or their nutritional value as food for fish. Our study demonstrates an environmentally safe OAE application, but also stresses the risks of more intense OAE options, and the vulnerabilities of other marine ecosystems.
An oligotrophic metazoan zooplankton community remains stable after 33 days of CO2-equilibrated ocean alkalinity enhancement.
INTRODUCTION
Successfully preventing further climate change will not only require drastic reductions of CO2 emissions, but also its active removal from the atmosphere (1). The ocean is a promising candidate for CO2 removal and storage, as it has already absorbed a fourth of our emissions (2, 3). This buffering capacity emerges from the dissolution of minerals, which consumes protons and shifts carbon equilibrium toward bicarbonates and carbonates, leaving more space for CO2 to in-gas (4). Ocean alkalinity enhancement (OAE) takes inspiration from this naturally occurring process and proposes to accelerate it to permanently remove CO2 within the urgent timescales needed (5). In addition, OAE could benefit ocean health by reversing ongoing acidification (6). However, acidification and OAE can differ in their impact on pCO2, intensity, and spatial and temporal scales, calling for research on the environmental safety of OAE (7).
Different methods to implement OAE are being pursued, but what a real-world deployment will look like remains uncertain (8). OAE can use a variety of alkali and alkaline minerals, in particulate or dissolved form, and each method carries its own set of trade-offs. For instance, buffered accelerated weathering of limestone (b-AWL) dissolves minerals and/or their oxides under high pCO2, releasing a CO2-equilibrated solution (9). This makes monitoring, reporting, and verification of carbon dioxide removal (CDR) relatively simple, as the alkalized water has absorbed the intended CO2 before its dispersion. However, b-AWL has to be paired to other CDR actions, like direct air capture, raising its costs. OAE methods also differ in how and where alkalinity is released, either over offshore or coastal waters, or at the seafloor. For example, ship-based OAE proposes to use shipping routes to distribute gigatons of alkalinity over the global surface ocean (10). While this potentially avoids the accumulation of alkalinity and the generation of permanent OAE hotspots, it also raises the need to assess its impacts across ecosystems, from subtropical, oligotrophic waters to temperate, seasonal environments.
The oceans provide multiple ecosystem services of intrinsic value to humans. Fisheries, for instance, report global annual landings in the order of 67 million tons, and aquatic foods supply about 19% of our animal protein demand (11). The primary link between fish and the microalgae ultimately supporting said production are metazoan zooplankton, but they are not all equally nutritious (12). For example, copepods are more energy and nutrient dense than most gelatinous groups (13, 14). Still, some gelatinous zooplankton, like larvaceans, can also be efficient trophic links because they decrease the number of steps from picoplankton and bacteria to fish (15). Nutritional quality can also vary within a species, through adjustments in population structure (size and development) and body composition (proteins, fatty acids, and nucleic acids) (16, 17). Given their key role in fish production, it is crucial that zooplankton community- and organism-level responses to OAE are covered in environmental safety evaluations.
To date, there are no studies assessing the impacts of alkalinization on metazoan zooplankton, hereon simply zooplankton, or food webs. Still, past research on acidification, a stressor that alters the same seawater properties, may hint at potential impacts of OAE. Zooplankton physiology can be directly affected by changes in seawater proton levels and carbonate chemistry. Studies found a substantial variability in their tolerance to acidification, even among closely related species and across development (18, 19), but some recurring sensitivities could be identified. Acid-base homeostasis, calcification, and digestion were all found altered under acidification, with consequences for energy allocation and, ultimately, the organism’s fitness. For instance, copepod developmental rates were delayed and their reproductive success decreased as a result of disruptions to their acid-base balance (19, 20). Calcifiers also suffered, as costs of building and maintaining their calcium carbonate shells and skeletons increased (21). In contrast, larvaceans benefitted from the higher acidity, which eased their digestion and resulted in improved reproductive success (22, 23). These physiological functions may be equally vulnerable to the perturbations associated to OAE.
OAE may also affect zooplankton indirectly through its impacts on the availability and quality of their food. To date, few studies have investigated how phytoplankton respond to alkalinity enhancement, and they report low to moderate impacts affecting production and composition (24–26). These changes could affect zooplankton nutrition, but bottom-up regulation in the context of OAE remains to be investigated. With acidification, most community level experiments show that high pCO2 fertilized primary producers, increasing food availability and therefore zooplankton and fish biomass (27–32). Other studies showed that acidification decreased the quality of their food, hindering trophic transfer (33–36). Regardless of the direction of change, bottom-up mechanisms determined consumer responses and food web productivity under acidification and should therefore be investigated for OAE.
Here, we studied the responses of a subtropical, oligotrophic food web to OAE, with particular focus on metazoan zooplankton and their role in trophic transfer up to fish. We simulated CO2-equilibrated OAE, where associated chemical perturbations are mild: pCO2 levels remain constant and the decrease in proton levels and consequent increase in pH and carbonate saturation are relatively small (37). We used nine in situ mesocosms to test a gradient of OAE and monitored its impacts on a truncated (organisms < 3 mm), close-to-natural plankton food web. The month-long exposure was divided into three response phases to address the potential for alleviation or aggravation of OAE impacts, with an immediate period to identify possible direct effects. Throughout the experiment, we assessed the stability, trophic structure, and productivity of the food web, as well as the potential for bottom-up mediation. With this study we provide a community-level assessment of the risks and side-effects of OAE across direct and indirect response pathways.
RESULTS
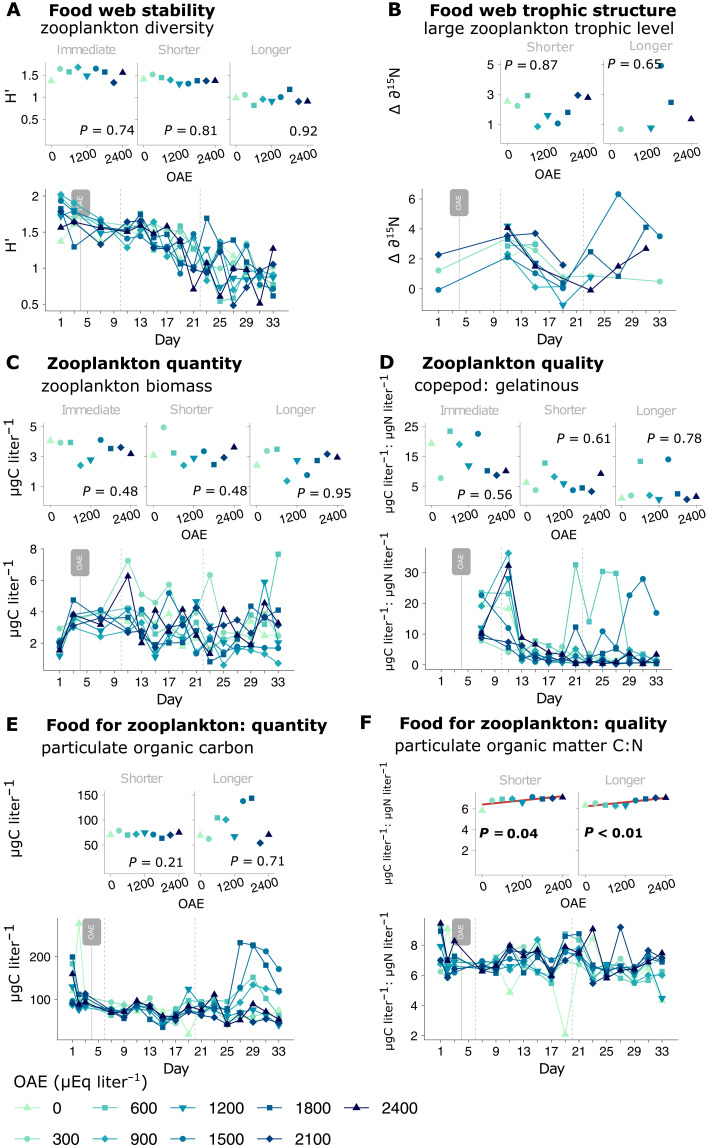
To comprehensively evaluate the safety of OAE, we investigated 12 properties in several metazoan zooplankton groups, from now referred to as zooplankton, and six in their potential food, adding up to a total of 47 responses. Figure 1 presents a selection of these responses and shows the data and linear regression models on which we base our assessment. Zooplankton diversity, trophic level of large zooplankton, zooplankton biomass, and the copepod to gelatinous zooplankton ratio were not altered (Fig. 1, A to D), suggesting the maintenance of food web stability, trophic structure, and the availability and nutritional value of food for fish, respectively. Lower trophic levels were also monitored to define the influence of bottom-up regulation. The amount of potential food for zooplankton was not affected by OAE, as informed by particulate organic carbon concentration (Fig. 1E), but its quality deteriorated based on the observed increase in the carbon-to-nitrogen stoichiometry (C:N) of particulate matter (Fig. 1F). The remaining 41 responses, which complete our assessment of food web stability, trophic structure and production, and bottom-up control, were analyzed following the same approach (fig. S1 and table S1).
Fig. 1. Metazoan zooplankton and lower trophic level responses to OAE.
We show a selection of key responses to represent each food web property (stability, structure and productivity) (A to D) and the potential for bottom-up mediation under OAE (E and F). The lower panels show the temporal development of each response, with the vertical dashed lines separating the experiment into three [(A) to (D)] and two [(E) and (F)] response phases. Response phases include an immediate (days 5 to 10), shorter- (11 to 22) and longer-term (23 to 33) phase for zooplankton [(A) to (D)], and a shorter- (days 6 to 20) and longer-term (21 to 33) phase for their potential food [(E) and (F)]. The upper panels show the phase averages, or the mean of all measurements taken within each phase, for each parameter and every treatment, on which linear regression models are tested.
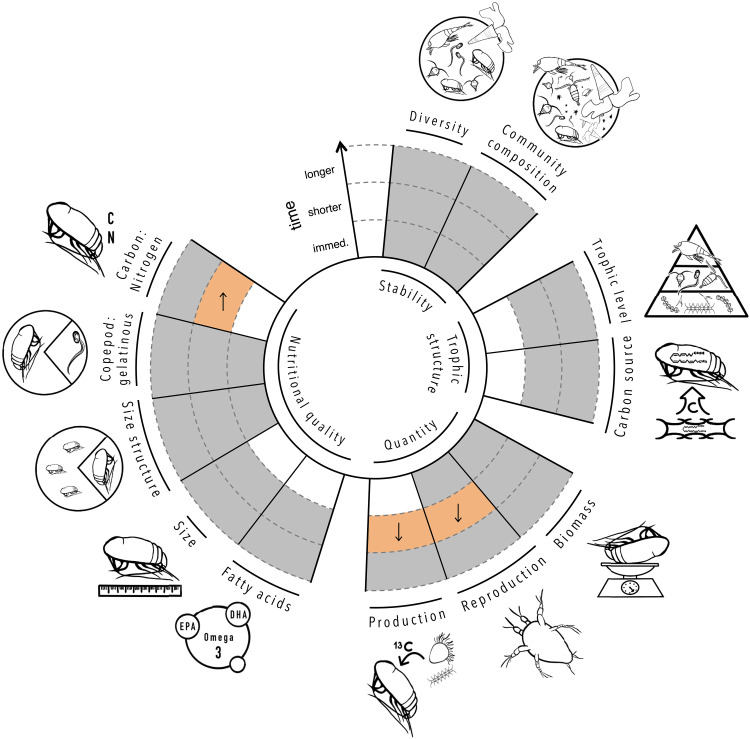
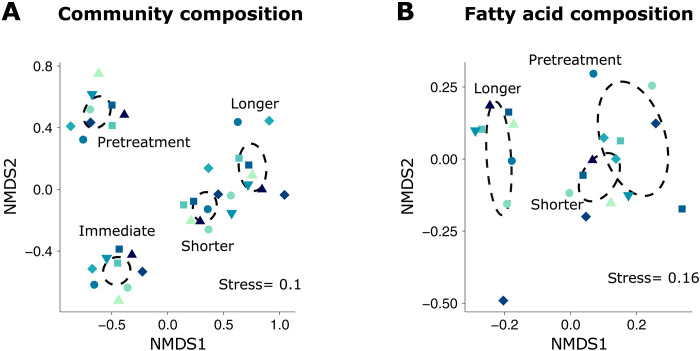
Overall, food web stability and trophic structure remained unperturbed by OAE (Fig. 2). In addition to diversity, OAE also did not affect zooplankton community composition (Fig. 3A and table S2). Instead, temporal clustering revealed successional change irrespective of alkalinity: calcifiers declined, copepods were stable, and larvaceans increased in biomass (fig. S2). Similar to large zooplankton, the trophic level of other zooplankton groups also did not change with OAE (table S1), indicating that food web length remained unchanged. Regarding the food source for zooplankton, we could not find evidence for a change with OAE based on any of the fatty acid markers investigated (fig. S1 and table S1).
Fig. 2. Summary of metazoan zooplankton responses to OAE, informing about food web stability, structure, and productivity.
For every response, colored cells indicate the outcome of the linear regressions fitted to average values for each phase. Gray = no effect, orange = potentially adverse effect, and blank = no data. Arrows indicate the direction of change with OAE.
Fig. 3. Metazoan zooplankton community and fatty acid composition under OAE.
Multidimensional analyses were performed on phase averages of zooplankton biomass (A) and relative fatty acid content (B). Ninety-five percent confidence ellipses (dashed circles) were computed for each response phase.
Food production also remained mostly unaffected by OAE, based on the lack of impacts on the majority of zooplankton properties informing about their availability and nutritional value to higher consumers (Fig. 2). For one, alkalinization did not affect zooplankton biomass in any taxonomic or size group (fig. S1 and table S1). Fatty acid content and composition, and all size-based quality indexes, also remained unperturbed over the course of the experiment, across all zooplankton groups investigated (Fig. 3B, fig. S1, and table S1). OAE impacts at the base of the food web were also limited (Table 1). Primary production, protozooplankton abundances, and fatty acid content in particulate matter were all unaffected (fig. S1). As expected in an oligotrophic ecosystem, primary production was low across all treatments. Yet, starting on day 25, we observed blooms of Chrysochromulina (38) unrelated to OAE, responsible for up to a threefold increase in particulate organic carbon (Fig. 1E). This type of mixoplankton (39) has also been reported to establish a symbiotic relationship with nitrogen fixer UCYN-A (40).
Table 1. Responses at the base of the food web through different stages of adjustment to OAE.
The effect of OAE was tested using linear regressions, which were fitted to the averages of each phase. Equal signs indicate no effect of OAE; arrows indicate the direction of change in those parameters significantly affected by OAE.
| Food web implication | Lower trophic level property | OAE effect | ||
|---|---|---|---|---|
| Shorter | Longer | |||
| Bottom-up mediation | Quantity | Particulate organic carbon | = | = |
| Protozooplankton abundance | = | = | ||
| Primary production | = | = | ||
| Nutritional quality | Fatty acids | = | = | |
| Particulate organic nitrogen | ↓ | = | ||
| C:N | ↑ | ↑ | ||
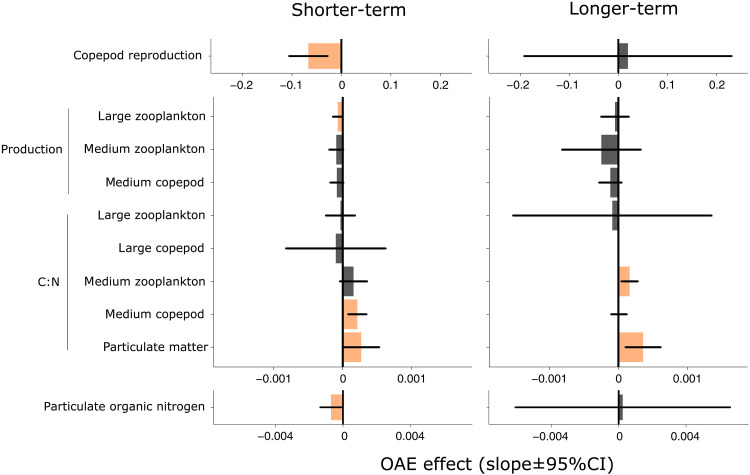
Three shorter-term impacts on zooplankton were observed (Figs. 2 and 4). With increasing OAE intensity, zooplankton production and copepod reproduction, based on nauplii biomass, decreased. The decrease in production rates was only significant in one of the three zooplankton groups where they were measured, but the other two showed a similar trend. In the longer-term, both responses disappeared. We also detected an increase in the C:N in one of the four zooplankton groups studied, and a similar trend was observed in one other group. The C:N increase persisted into the longer-term in one of the three groups studied. OAE also affected two properties of the potential food for zooplankton, both associated with organic nitrogen availability (Table 1 and Fig. 4). Particulate matter C:N increased with OAE across the shorter and longer terms, and particulate organic nitrogen decreased with alkalinization during the shorter term, but not in the longer term.
Fig. 4. Effect sizes of significant responses to OAE across the food web.
For every impact, we report all metazoan zooplankton groups that were sampled to assess the consistency of the response. Effect sizes are linear regression slopes. Gray = no effect, orange = potentially adverse effect, and blank = no data.
DISCUSSION
This study shows that a month-long exposure to CO2-equilibrated OAE had a low impact on the stability, structure, and productivity of a close-to-natural oligotrophic plankton food web. Most metazoan zooplankton properties, from now simply referred to as zooplankton, did not change throughout the experiment, and none were altered immediately after OAE implementation. Zooplankton seemed to tolerate the chemical perturbations caused by alkalinization, which aligns with findings from our literature review on the physiological impacts of decreased [H+] (Box 1). In this review, we found that most animal functions are resistant to proton perturbations, and the consequent pH increases, within the range tested in the mesocosms (from 0 to −2.91 × 10−9 mol per kg, corresponding to a pH increase from 0 to 0.225). Calcification was the only exception, but calcifiers played a minor role in our experiment, disappearing shortly after the start. In food webs of the subtropical North Atlantic, calcifiers naturally contribute little to the overall biomass of plankton communities (41), and it seems like this may not change if OAE is implemented in these waters. Our enclosed community was truncated and did not include higher trophic levels, notably fish, which are at the center of the ecosystem service of food production. Two studies investigating fish fitness under increased pH, included in the review, show that larval fish would not be directly affected by the perturbations tested in our mesocosms (42, 43). Our study also suggests few impacts of OAE on the quantity and quality of their potential food. Still, dedicated experiments including fish will be needed to comprehensively assess food web productivity under OAE.
Box 1. Review of literature on direct effects of decreased [H+] on marine animals.
OAE research is at its infancy, and our knowledge about its impacts on marine communities is limited. In this systematic review, we found 16 studies that, despite being unrelated to OAE, investigated the physiological responses of marine animals to a decrease in proton levels, classically discussed in terms of increasing pH. With this, we aim to gain qualitative insight into the direct impacts of this OAE-associated stressor. Results from this review, discussed here, are presented in Fig. 5 and Table 2.
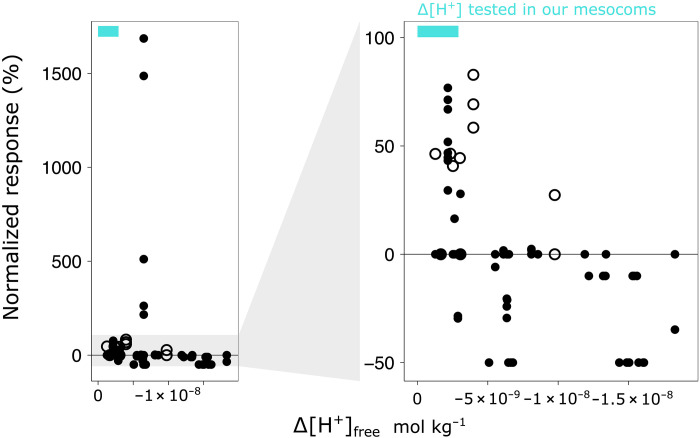
For the most part, decreased proton levels did not cause effects, with over half of the observations showing no impacts. Still, we could find evidence that both calcification and acid-base homeostasis are sensitive. Calcification increases with decreasing [H+], and in some cases, this is accompanied by other beneficial responses in calcifiers like increased growth and faster development. Signs of homeostatic stress, on the other hand, only indicate that animals compensate for changes in proton levels. The link to reduced fitness has been proposed, but not demonstrated. Last, larvaceans appear negatively affected, probably due to lower predigestion activity.
Overall, animal physiology could play an influential role in shaping individual and community responses to OAE. Much like with ocean acidification, we found that the impact of decreased [H+] or increased pH on animal fitness depends on their physiological characteristics, and that their tolerance thresholds vary, even among closely related taxa and across development. For example, fish larval survival and feeding are affected, but the pH increments at which responses are observed differs by up to 1 pH unit among species (corresponding to decreases in proton levels that differ by up to a degree of magnitude). Such variability complicates predictions and calls for understanding of how the physiological pathways identified as vulnerable function under OAE.
Fig. 5. Direct responses of marine animals to decreased [H+].
On the basis of the literature review, we show the normalized responses, across taxa and physiological functions, to decreased [H+] or increased pH. Non-significant impacts are assigned a value of zero, whereas significant effects are reported as the percent change with respect to the ambient (gradient-design experiments) or the control (replicated-design) values. Open circles highlight the effects of low [H+] on calcification, while closed circles represent all other physiological functions investigated. The blue bars indicate the range of proton perturbations that were tested in the mesocosm study. The right hand panel zooms in to the responses that ranged between −50 and 100%.
Table 2. Direct responses of marine animals to decreased [H+], or increased pH.
Shown are the results from the literature review, summarized by taxon and physiological function. The direction of the responses to increasing pH is indicated by the symbols: = means no effect, ↑ represents a significant increase, and ↓ implies a significant decrease. The last three rows, in bold, report the total count of responses evaluated.
| Taxon | Response | ∆[H+]F mol kg−1 | ∆pH | Direction | Study |
|---|---|---|---|---|---|
| Copepod | Development | −5.53 × 10−9 | 0.19 | = | (80) |
| Growth | = | ||||
| Respiration | = | ||||
| C:N | =; ↓ | ||||
| Decapod | Homeostasis | −2.87 to −8.1 × 10−9 | 0.22–0.72 | ↑; =; ↓ | (50, 51) |
| Fish | Survival | −5.1 × 10−9 to −1.61 × 10−8 | 0.55–1.42 | ↓ | (42, 43) |
| Feeding | −1.22 to −1.51 × 10−8 | 0.57–1 | ↓ | (43) | |
| Appendicularia | Survival | −6.38 to −6.35 × 10−9 | 0.4 | = | (22) |
| Growth | =; ↓ | ||||
| Reproduction | ↓; = | ||||
| Foraminifera | Calcification | −3.02 to −9.75 × 10−9 | 0.21–0.69 | ↑; = | (81, 82) |
| Respiration | −3.02 × 10−9 | 0.21 | = | (82) | |
| Photosynthesis | = | ||||
| Pteropoda | Gene Expression | −2.16 × 10−9 | 0.12 | ↑ | (52) |
| Calcification | −1.66 to −3.05 × 10−9 | 0.11–0.22 | ↑; = | (52–54) | |
| Shell Degradation | = | (53) | |||
| Survival | ↑; = | ||||
| Feeding | −2.55 × 10−9 | 0.16 | = | (54) | |
| Respiration | = | ||||
| Benthic Mollusk | Growth | −2.63 × 10−9 to −1.83 × 10−8 | 0.12–1.25 | =; ↑; ↓ | (83–85) |
| Survival | = | (84, 85) | |||
| Respiration | −8.56 × 10−9 to −1.19 × 10−8 | 0.5–1.3 | = | (85) | |
| Development | −6.5 × 10−9 | 1.05 | ↑; = | (55) | |
| Coral | Respiration | −1.27 × 10−9; −2.52 × 10−9 | 0.11; 0.41 | = | (56) |
| Calcification | ↑ | ||||
| Photosynthesis | = | ||||
| Total # no effects | 55 | ||||
| Total # increases | 28 | ||||
| Total # decreases | 25 | ||||
We did find a short-term decrease in zooplankton production and reproduction, which could have been indirectly mediated by the decrease in organic nitrogen availability observed with alkalinization. The nutritional imbalance seemed to propagate up to zooplankton, based on their increased carbon-to-nitrogen stoichiometry (C:N). Zooplankton are vulnerable to such imbalances because they commonly have stable body compositions enriched in nitrogen and phosphorus (44, 45). When the nutrient gap between zooplankton and their prey increases, functions like growth and reproduction have been observed to decrease (44, 46–48). Other studies have also shown that enhanced alkalinity could change nutritional properties of the food for zooplankton (25, 26), further indicating that trophic regulation may be important in shaping consumer responses to alkalinization. Bottom-up control in relation to a decrease in food quality has also been described under ocean acidification, where zooplankton were hindered through changes in nitrogen availability, fatty acids, and even enhanced toxicity (33, 35, 36). We cannot fully discard direct stress contributing to shape these few responses observed, especially because of the prevalent, oligotrophy-mediated food limitation. Ocean acidification research found that food limitation can aggravate physiological stress (49). Our literature search can only shed limited light on this regard, since only 7 of the 16 studies found maintained wild diets or food-limited conditions in their experiments (50–56). Among these, five investigated calcifiers and the remaining two consisted of assays that found evidence of homeostatic stress on crabs, but without a connection to organism fitness (50, 51). Further experiments will be needed to disentangle the interaction between food limitation and OAE-driven physiological responses.
Despite these few responses, no change in the availability and quality of zooplankton as food for fish was observed under OAE. The decrease in reproduction did not alter population dynamics and biomass of any zooplankton groups, which developed irrespective of alkalinization. In oligotrophic ecosystems, food limitation can be a strong structural force and, in our experiment, it potentially buffered the indirect impacts that OAE had on zooplankton. For example, organic phosphorus remained low throughout the experiment irrespective of OAE (<0.1 μM, on average). This possibly limited zooplankton growth and development to some extent, as phosphorus is needed for growth, and can be especially important during early life stages (57, 58). With acidification, mesocosm studies carried out in nutrient-deplete systems also reported low impacts, and zooplankton responses only emerged or were amplified after relief from food limitation (31, 59). Thus, the role of trophic pathways in mediating responses to OAE should be further investigated in food webs where nutrients, and therefore food, are not limiting. Moreover, microalgae blooms can also modify seawater carbonate chemistry (decreasing pCO2 and increasing pH), possibly aggravating OAE perturbations in these ecosystems. Yet, bloom seasonality entails that such environments naturally undergo carbonate chemistry fluctuations (60), raising the question of whether they could be more resilient to OAE compared to oligotrophic ones.
We further found that the responses by zooplankton dissipated overtime. Throughout the experiment, we enclosed at least three copepod cohorts, two of which entirely developed under OAE. In theory, physiological adjustments made by individuals to withstand a change in the environment can be transmitted to their offspring. This has been observed, in as few as two generations, in relation to acidification (20, 61). It has also been described with regards to dietary stress (62), which appeared to be the path through which OAE affected zooplankton in our study. The dissipation of effects could also have resulted from an increase in background variability, meaning our power to detect (weak) responses may have decreased. Communities progressively diverge during mesocosm and laboratory experiments, a pattern referred to as the “bottle effect.” Although the duration of the experiment was adjusted to the characteristics of the mesocosms used and of the plankton community enclosed (63), we could observe this phenomenon in some properties. For example, zooplankton reproduction and trophic level become more variable with time, which could have been facilitated by the unexplained blooms observed in the long term. Despite the difficulties associated to longer-term experiments, it should be noted that these will be further needed to assess the welfare of larger, longer-lived zooplankton and nekton, also key players in natural plankton food webs.
Our study provides evidence of a safe implementation of OAE in an oligotrophic, close-to-natural plankton food web, making a first promising step toward defining a framework of environmentally responsible deployments. The food web was resistant, and zooplankton physiologically tolerant, to the moderate chemical perturbations that come with CO2-equilibrated OAE. In parallel, we also identified potential vulnerabilities that warrant further research. Subtropical plankton communities like the one we investigated only have small contributions of calcifiers, the only group in our literature review that was sensitive to [H+] perturbations within the range tested. Calcifiers can play key roles in other marine ecosystems (64, 65), and studies need to investigate those food webs under OAE. The literature review also showed that greater decreases in proton levels can be detrimental to marine animals, including fish. This may not be relevant for a CO2-equilibrated approach, but unequilibrated deployments could reach those thresholds. In unequilibrated OAE, the particulate and/or dissolved alkalinity that is released into the environment causes the pCO2 in the seawater to decrease, leading to more severe seawater chemistry perturbations (37) that need to be specifically evaluated. In this context, the role that food limitation can play in aggravating physiological stress makes further investigations in oligotrophic food webs of particular importance. Likewise, the interaction between OAE perturbations and the trophic propagation of its impacts makes investigating environments with wider nutrient availability and higher productivity, like upwelling areas or temperate seas, essential. Overall, it is unlikely that a single OAE approach will be safely applied everywhere. To continue building the framework of responsible OAE application, community-level experiments are needed to investigate the vulnerability of different ocean food webs to this and other OAE deployment options.
MATERIALS AND METHODS
Study system
Gran Canaria is an offshore island belonging to the Canary archipelago, on the edge of the North Atlantic subtropical gyre. It is surrounded by open ocean with mostly year-round stratified, nutrient-poor surface waters. The experiment took place from September to October 2021 in Taliarte harbor, on its northeastern coast. Here, we deployed 8-m3 mesocosms, enclosing a natural plankton community formed by microalgae, heterotrophic protists, and zooplankton, for 33 days. The community was truncated at 3 mm, excluding larger organisms from the enclosures.
Experimental design
We tested CO2-equilibrated OAE ranging from no alkalinity enhancement up to a doubling of natural alkalinity. Using nine mesocosm units, we established an alkalinity gradient with intervals of 300 μmol kg−1 (Table 3). We achieved this on day 4 by dissolving precise weights of sodium carbonate and bicarbonate and evenly distributing the alkaline solutions into the correspondent mesocosms to reach target alkalinity values. The upper alkalinity threshold tested in our experimental design was defined on the basis of the saturation state of calcium carbonate. Beyond the omega reached in our highest alkalinity level, one can expect spontaneous carbonate precipitation and associated alkalinity loss (66). As this would impair the CDR efficiency of the technology, a real-world deployment beyond the precipitation point would not be advisable (67).
Table 3. Experimental design to simulate CO2-equilibrated OAE.
Shown are total alkalinity (TA) and dissolved inorganic carbon (DIC) measured on day 7 following Dickson et al. (86). Other carbonate system parameters were calculated with TA and DIC as input values using CO2SYS version 2.5 Excel sheet as detailed in Pierrot et al. (87). Free protons ([H+]F) are computed from calculated total pH (79), at a salinity of 36.4 and a temperature of 24°C.
| OAE [∆TA (Eq liter−1)] | 0 | 300 | 600 | 900 | 1200 | 1500 | 1800 | 2100 | 2400 |
|---|---|---|---|---|---|---|---|---|---|
| TA (Eq liter−1) | 2402 | 2690 | 2968 | 3272 | 3576 | 3838 | 4107 | 4418 | 4689 |
| DIC (μM) | 2097 | 2328 | 2559 | 2795 | 3051 | 3254 | 3463 | 3699 | 3912 |
| pH | 8.03 | 8.07 | 8.1 | 8.14 | 8.15 | 8.18 | 8.21 | 8.24 | 8.25 |
| [H+]F (mol kg−1) | 7.32 × 10−9 | 6.68 × 10−9 | 6.23 × 10−9 | 5.68 × 10−9 | 5.55 × 10−9 | 5.18 × 10−9 | 4.84 × 10−9 | 4.51 × 10−9 | 4.41 × 10−9 |
| pCO2 (μatm) | 422 | 418 | 430 | 422 | 443 | 439 | 438 | 434 | 438 |
| HCO3− (μM) | 1865 | 2049 | 2237 | 2415 | 2625 | 2774 | 2929 | 3099 | 3255 |
| CO3−2 (μM) | 219 | 267 | 309 | 368 | 414 | 521 | 567 | 588 | 644 |
| Ωaragonite | 3.4 | 4.2 | 4.8 | 5.8 | 6.5 | 7.3 | 8.1 | 9.2 | 10.1 |
| Ωcalcite | 5.2 | 6.3 | 7.3 | 8.7 | 9.8 | 11.1 | 12.4 | 14 | 15.3 |
Metazoan zooplankton measurements
In our study, metazoan zooplankton, from hereon simply zooplankton, were defined to include all drifting animals larger than 55 μm (and smaller than 3 mm). We sampled them regularly using Apstein nets (ø 17 cm; mesh size of 55 μm; and 62 liters per net). One net was taken for abundances approximately every 2 days, a second net was taken for carbon (C) and nitrogen (N) mass (not to be confused with zooplankton biomass discussed later on, which is derived from abundances) and 13C and 15N isotopes every 4 days, and a third net was taken for fatty acids roughly every 8 days (see fig. S3). Zooplankton were then fractioned into three size classes, and further processed therein: 55 to 200 μm (small), 200 to 500 μm (medium), and 500 μm to 3 mm (large). The only exception were zooplankton sampled for fatty acids.
Zooplankton sampled for fatty acids were incubated live for an hour in filtered seawater (0.7 μm) at 4° to 7°C to empty their guts. Afterward, all organisms larger than 200 μm were picked into a cryotube (>100 μg C) and immediately frozen at −80°C until extraction and measurement via gas chromatography (68). Given the high C mass requirements of this measurement, one replicate per treatment was taken at each sampling time.
Zooplankton sampled for C and N mass, and stable isotopes, were also incubated for gut clearance, after which they were picked in groups (>2.5 μg N for the small and medium size classes, and >15 μg N for the large) into tin capsules, oven dried at 60°C, and measured in an elemental analyzer coupled to a mass spectrometer. Functional groups investigated were bulk zooplankton, copepod zooplankton, and copepod nauplii. Bulk zooplankton consisted of a random mix across all zooplankton taxa, and it was taken in each size fraction. Copepod zooplankton consisted of a random mix across all copepod taxa and developmental stages (excluding nauplii), and it was measured in the medium and large sizes. Copepod nauplii targeted this developmental stage across all copepod taxa, and it was only measured in the small size fraction. Replicates were taken for each functional group in accordance to zooplankton availability (ideally duplicates, but toward the end of the experiment, we were mostly restricted to one replicate). Before being transferred into the tin capsules, samples from the medium and large size classes were photographed to measure individual sizes and derive per capita biovolumes and C mass with high taxonomic resolution (table S3-1).
For abundances, zooplankton were preserved in 70% EtOH and all organisms were counted and identified to the lowest possible taxonomic level and developmental stage. These data were then combined with the most appropriate C mass conversion factors available, largely derived from our elemental analyzer and image-based measurements, to calculate zooplankton biomass (table S4).
We assessed food web stability and trophic structure under OAE based on four zooplankton responses (see fig. S4 for information on which samples were taken for each response). (i) Shannon diversity informed about functional redundancy within the zooplankton community and was calculated from the relative biomass contribution of each taxon (table S3-2). (ii) To calculate community composition, we further distinguished zooplankton based on size and development. We excluded taxa found on less than two time points and that contributed on average less than 0.1% to the total biomass for both diversity and community composition calculations. (iii) Food web length was estimated from the trophic level of zooplankton, which we calculated from their delta15N stable isotope values and that of their potential food (table S3-3). Bulk 15N isotopes carry limitations (69). For one, the isotopic signal of particulate matter is not purely autotrophic (it includes bacteria, mixoplankton and protozooplankton) and is also informed by taxa that zooplankton may not feed on. With bulk 15N, we cannot disentangle the various contributions, and changes in particulate matter composition may hide or fabricate shifts in zooplankton trophic level. These limitations become particularly apparent when assessing food webs with different community structures or varying nutrient conditions (70). While this did not apply to our study, focused on one plankton community under persistent oligotrophy, we would recommend future studies focused on trophic structure to use compound specific stable isotopes (69). (iv) Last, three fatty acid trophic markers were selected to track the sources of food for zooplankton: 22:6ω3/20:5ω3 [docosahexaenoic acid (DHA)/eicosapentaenoic acid (EPA), a flagellate versus diatom marker], the ratio of polyunsaturated to saturated fatty acids (PUFA/SFA, a carnivory marker), and 18:1ω7 (a bacterial marker) (71, 72). These markers were calculated from percent fatty acid data.
We addressed the availability of zooplankton as food for fish based on three responses (fig. S4). (i) Biomass was studied in each zooplankton taxa, size class and stage of development, with a focus on copepods. (ii) Copepod nauplii biomass in particular was approached as a proxy for their reproductive potential. (iii) Secondary production was assessed by tracking 13C through the food web as described in de Kluijver et al. (73) and calculating its incorporation in zooplankton (table S3-4). To do so, we added 10 g of 13C-bicarbonate on day 11, enriching the isotopic signature of the inorganic carbon pool by 100‰ (fig. S3).
We also evaluated the nutritional quality of zooplankton as prey based on six responses (fig. S4). (i) Three fatty acid nutritional indexes were quantified in zooplankton: total fatty acids, PUFAs, and DHA/EPA (68,72). Indexes were calculated from fatty acid concentrations. (ii) Fatty acid composition was also investigated, in this case based on percent fatty acid data and excluding rare fatty acids (<0.1% of total) (table S5). (iii) In terms of zooplankton composition, we explored the copepod to gelatinous zooplankton ratio, which assumes that copepods are more nutritious than gelatinous prey (14). We calculated the ratio based on the biomass of the pertinent zooplankton groups. For this metric, we assessed and included all gelatinous taxa found, but we would like to highlight that larvaceans were the only abundant group in our mesocosm plankton communities (see fig. S2). Size also is a measure of nutritional quality, and larger preys have been associated with more efficient energy transfer and higher potential fish yield (74). (iv) On the basis of this, we measured copepod community size structure by comparing the biomass of the large copepod population to the medium one. (v) We also assessed the per capita size of copepods from image-based measurements. Temora and Labidocera were assessed separately from other copepods in the medium and large size classes, respectively, as they were larger than the average copepod. (vi) Last, we calculated the carbon-to-nitrogen stoichiometry (C:N) of bulk zooplankton and copepod zooplankton, and in the medium and the large size classes, as an indication of protein availability. This was calculated from the C and N mass obtained by mass spectrometry.
Phyto-, mixo-, and protist zooplankton measurements
We assessed the potential for indirect, trophic-mediated OAE impacts by studying its effects at the base of the food web. To do so, integrated water samples were taken every 2 days throughout the experiment (fig. S3). From these, duplicate subsamples (1 to 1.5 liters) were filtered (>0.7 μm) for particulate matter C, N, and stable isotopes. One replicate set was acidified to isolate the organic fraction, but since inorganic C contributed little to total particulate C, values for particulate organic C (POC), N (PON), 13C, and 15N were averaged across acidified and nonacidified filters. Although acidification is known to affect the 15N signal, we did not find a consistent offset and averaged across acidified and nonacidified samples to calculate zooplankton trophic level. All filters were oven dried at 60°C for 24 hours and measured in an elemental analyzer coupled to a mass spectrometer. We also took one replicate set of filters (1 to 1.5 liters, >0.7 μm) for particulate matter fatty acids. Because of low C mass filtered, and the high C mass demands for fatty acid measurements, we pooled samples on selected time points (fig. S3). Fatty acid filters were immediately frozen at −80°C and further processed as indicated for zooplankton fatty acids. Fatty acid mass was normalized by POC to calculate the fatty acid concentration in suspended particulate matter.
We also took integrated water samples every 4 days for primary production measurements. Triplicates (70 ml) were taken and prefiltered through 280 μm to exclude some zooplankton (trade-off between excluding grazers while keeping larger microphytoplankton). Then, primary production was measured via 14C uptake in 24-hour incubations (38).
Last, integrated water samples were taken every 4 days to assess the abundances of protist zooplankton comprised between 10 and 200 μm, mainly ciliates and dinoflagellates. Because of their large size and low abundances, foraminifera and radiolarians were assessed with Apstein nets, together with metazoan zooplankton, despite operationally belonging to protozooplankton. On each sampling point, one 50-ml subsample was sedimented, and all protozooplankton were counted and identified by Ütermohl light microscopy (75).
Experimental phases
We analyzed zooplankton responses to OAE across three phases after treatment implementation (on day 4): immediate (days 5 to 10), shorter term (days 11 to 22), and longer term (days 23 to 33). To do so, we averaged measurements taken throughout each phase. During the immediate phase, measurements were taken only at one time point, day 7, and they were restricted to biomass-based parameters. This phase aimed to assess any potential direct stress of OAE, based on the assumption that not enough time had passed for indirect responses to emerge. We defined the shorter- and longer-term phases to assess the potential for acclimation to OAE or, on the contrary, the aggravation of its impacts. Throughout the study, we observed three “peaks” in copepod nauplii biomass (fig. S1C): The first occurred before treatment and was possibly a result of release from grazing (due to the exclusion of larger predators), the second took place halfway through the shorter-term phase, and the third was observed toward the end of the longer-term phase. Given the approximate 2-week developmental times from egg to mature copepod at these temperatures (76), we can assume that the longer-term copepod community will be composed to a greater extent of individuals born into OAE conditions, compared to the shorter-term community, which possibly has more individuals that experienced OAE perturbations in their lifetime. Lower trophic level parameters, informing about the potential for bottom-up mediation in OAE effects on zooplankton, were consequently analyzed across two phases after treatment: shorter term (days 6 to 20) and longer term (days 21 to 33).
Statistical analyses
We tested all food web responses in linear regressions with OAE as the continuous explanatory variable. We used normal Q-Q plots to check the normality of residuals, and residual versus fitted plots to check the homogeneity of variance.
Multivariate zooplankton community and fatty acid composition were also assessed based on phase average values. Community composition was transformed to account for different scales of biomass across size classes and zooplankton groups (tables S4 and S5). First, we visually explored whether OAE influenced zooplankton community and/or fatty acid composition by performing nondimensional multivariate scaling. Next, we tested these same questions quantitatively with linear regression models based on the ecological distances (the composition data transformed based on Bray-Curtis dissimilarity) and environmental distances (the OAE gradient transformed using Euclidean distances). We also correlated the ecological and environmental distances by performing Mantel tests. Analyses were carried out at α = 0.05 with R version 4.0.4 and multivariate analyses with the vegan package (77).
Literature review
To complement our experiment on ecosystem impacts of OAE, we revised the literature for direct, physiological responses of marine animals to decreased [H+], and the consequent increase in pH. This research question has received limited attention thus far, so we explored different fields including ocean acidification, where studies occasionally test for pre-industrial conditions. In total, we reviewed 16 studies.
Using Web of Science, we carried out three searches between March and June 2021:
(i)TS = (“high alkalinity” OR “enhance* alkalinity” OR “increase* alkalinity” OR “high pH” OR “enhance* pH” OR “increase* pH”) AND TS = (“zooplankton” OR “copepod*” OR “calcifi*” OR “radiolaria*” OR “foram*” OR “fish*” OR “gelatinous zooplankton” OR “jell*” OR “tunicat*” OR “thaliacea” OR “salp*” OR “doliolid*” OR “appendicuria*” OR “hydro*”) AND TS = (“marine” OR “seawater” OR “ocean*”) AND TS = (“direct effect*” OR “physiolog*” OR “fitness” OR “response”) NOT TI = (“phytoplankton” OR “diatom*” OR “dinoflagellate*” OR “coccolithophore*” OR “alga*”)
(ii)TS = (“ocean acidification”) AND TS = (“copepod*”) AND TS = (“marine” OR “sea” OR “seawater” OR “ocean*”) AND TS = (“physiology*” OR “fitness” OR “metaboli*”)
(iii)TS = (“ocean acidification”) AND TS = (“foraminifer*”) AND TS = (“marine” OR “sea” OR “seawater” OR “ocean*”) AND TS = (“calcification” OR “physiology*” OR “fitness” OR “metaboli*”)
For every search, we selected papers based on whether they tested in any of their treatments for increased pH (which is the reason why the largest portion of OA literature could not be used). We selected seven studies from search (i), two from (ii) and one from (iii). Searches (ii) and (iii) were done because we found that copepods and Foraminifera were underrepresented in search (i). Six additional studies were further found cited in the selected publications. We also included one study that we did not come across through any of the previously described ways, but knew to fit the criteria of the review.
In each selected study, we first checked the significance of the response to elevated pH and assigned a 0 to nonsignificant responses. When significant, we compared treatment and control averages, for replicated experiments, and the model output at the highest and ambient pH levels, for gradient experiments. Responses were then normalized by ambient values and transformed to percentages. When the experimental design included independent variables other than pH, we checked for the significance of each driver independently and their interaction, and assessed pH accordingly. If the other drivers and/or the interaction had a significant impact, the effect of pH was analyzed within each level of the other manipulated variables, as if these were separate experiments.
Last, we show physiological responses to decreased free [H+], which we calculated from the reported treatment and ambient pH (78, 79). Conversions were specific to the pH scale used in each study, as well as to temperature and salinity. In the three studies where the pH scale was not specified, and authors were not responsive, we assumed the NBS scale, which was found to be more commonly used.
Acknowledgments
We thank the Plataforma Oceánica de Canarias (PLOCAN) and the research group of J. Arístegui at the University of Las Palmas de Gran Canaria (ULPGC) for providing laboratory facilities and logistical and technical support. Further, we thank the KOSMOS team on site and our technical support at GEOMAR, especially T. Hansen and K. Nachtigall. We also acknowledge S. Dorssers and M. Weichler, a fundamental part of the Food Web team without whom this work would not have been possible. We thank X. Xin, L. Marín-Samper, and N. Hernández-Hernández for contributing the microscopy and primary production data discussed. We thank M. Hill-Cruz, who was so generous to co-design the symbols for the zooplankton properties.
Funding: This work was supported by GEOMAR Helmholtz-Zentrum für Ozeanforschung through the projects OceanNETS (“Ocean-based Negative Emissions Technologies – analysing the feasibility, risks and co-benefits of ocean-based negative emission technologies for stabilizing the climate,” EU Horizon 2020 Research and Innovation Program grant agreement no.: 869357), Ocean-CDR (“Ocean-based carbon dioxide removal strategies,” Helmholtz European Partnering project no.: PIE-0021), and AQUACOSM-plus (“AQUACOSM-plus: Network of Leading European AQUAtic MesoCOSM Facilities Connecting Mountains to Oceans from the Arctic to the Mediterranean,” EU H2020-INFRAIA project no.: 731065). We acknowledge financial support by Land Schleswig-Holstein within the funding program Open Access Publikationsfonds.
Author contributions: Conceptualization: N.S., S.U.G., C.J., J.T., and U.R. Methodology: N.S., S.U.G., D.B., J.T., and U.R. Investigation: N.S., D.B., S.G., and U.R. Visualization: N.S. Supervision: S.U.G., C.J., and U.R. Writing–original draft: NS. Writing–review and editing: N.S., S.U.G., D.B., C.J., J.T., and U.R.
Competing interests: The authors declare that they have no competing interests.
Data and materials availability: All data are available in the main text and/or the Supplementary Materials, and are also archived on the PANGAEA database: (https://doi.pangaea.de/10.1594/PANGAEA.971765; https://doi.pangaea.de/10.1594/PANGAEA.971776; https://doi.pangaea.de/10.1594/PANGAEA.971777; https://doi.pangaea.de/10.1594/PANGAEA.971778; https://doi.pangaea.de/10.1594/PANGAEA.971779; https://doi.pangaea.de/10.1594/PANGAEA.971764; and https://doi.pangaea.de/10.1594/PANGAEA.971762).
Supplementary Materials
This PDF file includes:
Figs. S1 to S4
Tables S1 to S5
REFERENCES AND NOTES
- 1.IPCC, “Mitigation pathways compatible with 1.5°C in the context of sustainable development” in Global Warming of 1.5°C (Cambridge Univ. Press, 2022) pp. 93–174. [Google Scholar]
- 2.National Academy of Sciences, Engineering and Medicine (NASEM), A research strategy for ocean-based carbon dioxide removal and sequestration (NASEM, Washington, DC, 2022). [Google Scholar]
- 3.Sabine C. L., Feely R. A., Gruber N., Key R. M., Lee K., Bullister J. L., Wanninkhof R., Wong C. S., Wallace D. W. R., Tilbrook B., Millero F. J., Peng T.-H., Kozyr A., Ono T., Rios A. F., The oceanic sink for anthropogenic CO2. Science 305, 367–371 (2004). [DOI] [PubMed] [Google Scholar]
- 4.IPCC, “Carbon and other biogeochemical cycles” in Climate change 2013: The physical science basis (Cambridge Univ. Press, 2014) pp. 465–570. [Google Scholar]
- 5.Kheshgi H. S., Sequestering atmospheric carbon dioxide by increasing ocean alkalinity. Energy 20, 915–922 (1995). [Google Scholar]
- 6.Köhler P., Hartmann J., Wolf-Gladrow D. A., Geoengineering potential of artificially enhanced silicate weathering of olivine. Proc. Natl. Acad. Sci. 107, 20228–20233 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gattuso J. P., Williamson P., Duarte C. M., Magnan A. K., The potential for ocean-based climate action: Negative emissions technologies and beyond. Front. Clim. 2, doi.org/10.3389/fclim.2020.575716 (2021). [Google Scholar]
- 8.Eisaman M. D., Geilert S., Renforth P., Bastianini L., Campbell J., Dale A. W., Foteinis S., Grasse P., Hawrot O., Löscher C. R., Rau G. H., Rønning J., “ Assessing the technical aspects of ocean-alkalinity-enhancement approaches” in Guide to Best Practices in Ocean Alkalinity Enhancement Research (Copernicus Publications, 2023) pp. 1–29. [Google Scholar]
- 9.Caserini S., Cappello G., Righi D., Raos G., Campo F., De Marco S., Renforth P., Varliero S., Grosso M., Buffered accelerated weathering of limestone for storing CO2: Chemical background. Int. J. Greenhouse Gas Control 112, 103517 (2021). [Google Scholar]
- 10.Caserini S., Pagano D., Campo F., Abbà A., De Marco S., Righi D., Renforth P., Grosso M., Potential of maritime transport for ocean liming and atmospheric CO2 removal. Front. Clim. 3, 575900 (2021). [Google Scholar]
- 11.FAO. In Brief to The State of World Fisheries and Aquaculture. Towards Blue Tranformation (FAO, Rome, 2022). [Google Scholar]
- 12.Steinberg D. K., Landry M. R., Zooplankton and the ocean carbon cycle. Ann. Rev. Mar. Sci. 9, 413–444 (2017). [DOI] [PubMed] [Google Scholar]
- 13.Wang M., Jeffs A. G., Nutritional composition of potential zooplankton prey of spiny lobster larvae: A review. Rev. Aquac. 6, 270–299 (2014). [Google Scholar]
- 14.Kiørboe T., Zooplankton body composition. Limnol. Oceanogr. 58, 1843–1850 (2013). [Google Scholar]
- 15.Jaspers C., Hopcroft R. R., Kiørboe T., Lombard F., López-Urrutia A., Everett J. D., Richardson A. J., Gelatinous larvacean zooplankton can enhance trophic transfer and carbon sequestration. Trends Ecol. Evol. 38, 980–993 (2023). [DOI] [PubMed] [Google Scholar]
- 16.Dessier A., Dupuy C., Kerric A., Mornet F., Authier M., Bustamante P., Spitz J., Variability of energy density among mesozooplankton community: New insights in functional diversity to forage fish. Prog. Oceanogr. 166, 121–128 (2018). [Google Scholar]
- 17.DeLorenzo Costa A., Durbin E. G., Mayo C. A., Variability in the nutritional value of the major copepods in Cape Cod Bay (Massachusetts, USA) with implications for right whales. Marine Ecol. 27, 109–123 (2006). [Google Scholar]
- 18.Dupont S., Thorndyke M. C., Impact of CO2-driven ocean acidification on invertebrates early life-history-What we know, what we need to know and what we can do. Biogeosciences 6, 3109–3131 (2009). [Google Scholar]
- 19.M. A. Teodósio, A. B. Barbosa, Ocean acidification impacts on zooplankton in Zooplankton ecology (CRC Press, 2020) pp. 64–84. [Google Scholar]
- 20.Thor P., Dupont S., Transgenerational effects alleviate severe fecundity loss during ocean acidification in a ubiquitous planktonic copepod. Glob. Chang. Biol. 21, 2261–2271 (2015). [DOI] [PubMed] [Google Scholar]
- 21.Gattuso J.-P., Magnan A., Billé R., Cheung W. W. L., Howes E. L., Joos F., Allemand D., Bopp L., Cooley S. R., Eakin C. M., Hoegh-Guldberg O., Kelly R. P., Pörtner H.-O., Rogers A. D., Baxter J. M., Laffoley D., Osborn D., Rankovic A., Rochette J., Sumaila U. R., Treyer S., Turley C., Contrasting futures for ocean and society from different anthropogenic CO2 emissions scenarios. Science 349, aac4722 (2015). [DOI] [PubMed] [Google Scholar]
- 22.Bouquet J.-M., Troedsson C., Novac A., Reeve M., Lechtenbörger A. K., Massart W., Skaar K. S., Aasjord A., Dupont S., Thompson E. M., Increased fitness of a key appendicularian zooplankton species under warmer, acidified seawater conditions. PLOS ONE 13, e0190625 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Troedsson C., Bouquet J.-M., Lobon C. M., Novac A., Nejstgaard J. C., Dupont S., Bosak S., Jakobsen H. H., Romanova N., Pankoke L. M., Isla A., Dutz J., Sazhin A. F., Thompson E. M., Effects of ocean acidification, temperature and nutrient regimes on the appendicularian Oikopleura dioica: A mesocosm study. Mar. Biol. 160, 2175–2187 (2013). [Google Scholar]
- 24.Gately J. A., Kim S. M., Jin B., Brzezinski M. A., Iglesias-Rodriguez M. D., Coccolithophores and diatoms resilient to ocean alkalinity enhancement: A glimpse of hope? Sci. Adv. 9, eadg6066 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ferderer A., Chase Z., Kennedy F., Schulz K. G., Bach L. T., Assessing the influence of ocean alkalinity enhancement on a coastal phytoplankton community. Biogeosciences 19, 5375–5399 (2022). [Google Scholar]
- 26.Subhas A. V., Marx L., Reynolds S., Flohr A., Mawji E. W., Brown P. J., Cael B. B., Microbial ecosystem responses to alkalinity enhancement in the North Atlantic Subtropical Gyre. Front. Clim. 4, doi.org/10.3389/fclim.2022.784997 (2022). [Google Scholar]
- 27.Taucher J., Haunost M., Boxhammer T., Bach L. T., Algueró-Muñiz M., Riebesell U., Influence of ocean acidification on plankton community structure during a winter-to-summer succession: An imaging approach indicates that copepods can benefit from elevated CO2 via indirect food web effects. PLOS ONE 12, e0169737 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sswat M., Stiasny M. H., Taucher J., Algueró-Muñiz M., Bach L. T., Jutfelt F., Riebesell U., Clemmesen C., Food web changes under ocean acidification promote herring larvae survival. Nat. Ecol. Evol. 2, 836–840 (2018). [DOI] [PubMed] [Google Scholar]
- 29.Spisla J., Taucher M., Sswat H., Wunderow P., Kohnert C., Clemmesen U., Riebesell, Ocean Acidification alters the predator-prey relationship between hydrozoa and fish larvae. Front. Mar. Sci. 9, doi.org/10.3389/fmars.2022.831488 (2022). [Google Scholar]
- 30.Goldenberg S. U., Nagelkerken I., Ferreira C. M., Ullah H., Connell S. D., Boosted food web productivity through ocean acidification collapses under warming. Glob. Chang. Biol. 23, 4177–4184 (2017). [DOI] [PubMed] [Google Scholar]
- 31.Algueró-Muñiz M., Horn H. G., Alvarez-Fernandez S., Spisla C., Aberle N., Bach L. T., Guan W., Achterberg E. P., Riebesell U., Boersma M., Analyzing the impacts of elevated-CO2 levels on the development of a subtropical zooplankton community during oligotrophic conditions and simulated upwelling. Front. Mar. Sci. 6, doi.org/10.3389/fmars.2019.00061 (2019). [Google Scholar]
- 32.Algueró-Muñiz M., Alvarez-Fernandez S., Thor P., Bach L. T., Esposito M., Horn H. G., Ecker U., Langer J. A. F., Taucher J., Malzahn A. M., Riebesell U., Boersma M., Ocean acidification effects on mesozooplankton community development: Results from a long-term mesocosm experiment. PLOS ONE 12, e0175851 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Meunier C. L., Algueró-Muñiz M., Horn H. G., Lange J. A. F., Boersma M., Direct and indirect effects of near-future pCO2 levels on zooplankton dynamics. Mar. Freshw. Res. 68, 373–380 (2016). [Google Scholar]
- 34.Osma N., Varga C. A., Algueró-Muñíz M., Bach L. T., Gómez M., Horn H. G., Ludwig A., Packard T. T., Riebesell U., Romero-Kutzner V., Taucher J., Fernández-Urruzola I., Ocean acidification induces distinct metabolic responses in subtropical zooplankton under oligotrophic conditions and after simulated upwelling. Sci. Total Environ. 810, 152252 (2022). [DOI] [PubMed] [Google Scholar]
- 35.Riebesell U., Aberle-Malzahn N., Achterberg E. P., Algueró-Muñiz M., Alvarez-Fernandez S., Arístegui J., Bach L. T., Boersma M., Boxhammer T., Guan W., Haunost M., Horn H. G., Löscher C. R., Ludwig A., Spisla C., Sswat M., Stange P., Taucher J., Toxic algal bloom induced by ocean acidification disrupts the pelagic food web. Nat. Clim. Change 8, 1082–1086 (2018). [Google Scholar]
- 36.Rossoll D., Bermúdez R., Hauss H., Schulz K. G., Riebesell U., Sommer U., Winder M., Ocean acidification-induced food quality deterioration constrains trophic transfer. PLOS ONE 7, e34737 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bach L. T., Gill S. J., Rickaby R. E. M., Gore S., Renforth P., CO2 removal with enhanced weathering and ocean alkalinity enhancement: Potential risks and co-benefits for marine pelagic ecosystems. Front. Clim. 1, doi.org/10.3389/fclim.2019.00007 (2019). [Google Scholar]
- 38.Marín-Samper L., Arístegui J., Hernández-Hernández N., Ortiz J., Archer S. D., Ludwig A., Riebesell U., Assessing the impact of CO2-equilibrated ocean alkalinity enhancement on microbial metabolic rates in an oligotrophic system. Biogeosciences 21, 2859–2876 (2024). [Google Scholar]
- 39.Mitra A., Caron D. A., Faure E., Flynn K. J., Leles S. G., Hansen P. J., McManus G. B., Not F., Gomes H. R., Santoferrara L. F., Stoecker D. K., Tillmann U., The mixoplankton database (MDB): Diversity of photo-phago-trophic plankton in form, function, and distribution across the global ocean. J. Eukaryot. Microbiol. 70, e12972 (2023). [DOI] [PubMed] [Google Scholar]
- 40.Hagino K., Onuma R., Kawachi M., Horiguchi T., Discovery of an endosymbiotic nitrogen-fixing Cyanobacterium UCYN-A in Braarudosphaera bigelowii (Prymnesiophyceae). PLOS ONE 8, e81749 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Intergovernmental Oceanographic Commission. “Biogeochemical characteristics of the marine ecosystem” in Oceanographic and biological features in the Canary Current Large Marine Ecosystem (IOC-UNESCO, 2015) pp. 133–196. [Google Scholar]
- 42.Parra G., Yúfera M., Tolerance response to ammonia and nitrite exposure in larvae of two marine fish species (gilthead seabream Sparus aurata L. and Senegal sole Solea senegalensis Kaup). Aquacult. Res. 30, 857–863 (2002). [Google Scholar]
- 43.Brownell C. L., Water quality requirements for first-feeding in marine fish larvae. II. pH, oxygen and carbon dioxide. J. Exp. Mar. Bio. Ecol. 44, 285–298 (1980). [Google Scholar]
- 44.Meunier C. L., Boersma M., El-Sabaawi R., Halvorson H. M., Herstoff E. M., Van de Waal D. B., Vogt R. J., Litchman E., From elements to function: Toward unifying ecological stoichiometry and trait-based ecology. Front. Environ. Sci. 5, doi.org/10.3389/fenvs.2017.00018 (2017). [Google Scholar]
- 45.Eddy T. D., Bernhardt J. R., Blanchard J. L., Cheung W. W. L., Colléter M., du Pontavice H., Fulton E. A., Gascuel D., Kearney K. A., Petrik C. M., Roy T., Rykaczewski R. R., Selden R., Stock C. A., Wabnitz C. C. C., Watson R. A., Energy flow through marine ecosystems: Confronting transfer efficiency. Trends Ecol. Evol. 36, 76–86 (2021). [DOI] [PubMed] [Google Scholar]
- 46.Thomas P. K., Kunze C., Van de Waal D. B., Hillebrand H., Striebel M., Elemental and biochemical nutrient limitation of zooplankton: A meta-analysis. Ecol. Lett. 25, 2776–2792 (2022). [DOI] [PubMed] [Google Scholar]
- 47.Jones R. H., Flynn K. J., Anderson T. R., Effect of food quality on carbon and nitrogen growth efficiency in the copepod Acartia tonsa. Mar. Ecol. Prog. Ser. 235, 147–156 (2002). [Google Scholar]
- 48.Kiørboe T., Phytoplankton growth rate and nitrogen content: Implications for feeding and fecundity in a herbivorous copepod. Mar. Ecol. Prog. Ser. 55, 229–234 (1989). [Google Scholar]
- 49.Ramajo L., Pérez-León E., Hendriks I. E., Marbà N., Krause-Jensen D., Sejr M. K., Blicher M. E., Lagos N. A., Olsen Y. S., Duarte C. M., Food supply confers calcifiers resistance to ocean acidification. Sci. Rep. 6, 19347 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cripps G., Widdicombre S., Spicer J. I., Findlay H. S., Biological impacts of enhanced alkalinity in Carcinus maenas. Mar. Pollut. Bull. 71, 190–198 (2013). [DOI] [PubMed] [Google Scholar]
- 51.Truchot J. P., Water carbonate alkalinity as a determinant of hemolymph acid-base balance in the shore crab, Carcinus maenas: A study at two different ambient pCO2 and pO2 levels. J. Comp. Physiol. B 154, 601–606 (1984). [Google Scholar]
- 52.Johnson K. M., Hofmann G. E., Transcriptomic response of the Antarctic pteropod Limacina helicina antarctica to ocean acidification. BMC Genomics 18, 812 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lischka S., Büdenbender J., Boxhammer T., Riebesell U., Impact of ocean acidification and elevated temperatures on early juveniles of the polar shelled pteropod Limacina helicina: Mortality, shell degradation, and shell growth. Biogeosciences 8, 919–932 (2011). [Google Scholar]
- 54.Comeau S., Jeffree R., Teyssie J. L., Gattuso J. P., Response of the arctic pteropod Limacina helicina to projected future environmental conditions. PLOS ONE 5, 11362 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Anderson M. J., A chemical cue induces settlement of Sydney rock oysters, Saccostrea commercialis, in the laboratory and in the field. Biol. Bull. 190, 350–358 (1996). [DOI] [PubMed] [Google Scholar]
- 56.Schneider K., Erez J., The effect of carbonate chemistry on calcification and photosynthesis in the hermatypic coral Acropora eurystoma. Limnol. Oceanogr. 51, 1284–1293 (2006). [Google Scholar]
- 57.Saiz E., Griffell K., Isari S., Calbet A., Ecophysiological response of marine copepods to dietary elemental imbalances. Mar. Environ. Res. 186, 105940 (2023). [DOI] [PubMed] [Google Scholar]
- 58.Meunier C. L., Boersma M., Wiltshire K. H., Malzahn A. M., Zooplankton eat what they need: Copepod selective feeding and potential consequences for marine systems. Oikos 125, 50–58 (2016). [Google Scholar]
- 59.Schulz K. G., Bellerby R. G. J., Brussaard C. P. D., Büdenbender J., Czerny J., Engel A., Fischer M., Koch-Klavsen S., Krug S. A., Lischka S., Ludwig A., Meyerhöfer M., Nondal G., Silyakova A., Stuhr A., Riebesell U., Temporal biomass dynamics of an Arctic plankton bloom in response to increasing levels of atmospheric carbon dioxide. Biogeosciences 10, 161–180 (2013). [Google Scholar]
- 60.Hagens M., Middelburg J. J., Attributing seasonal pH variability in surface ocean waters to governing factors. Geophys. Res. Lett. 43, 12528–12537 (2016). [Google Scholar]
- 61.Dam H. G., deMayo J. A., Park G., Norton L., He X., Finiguerra M. B., Baumann H., Brennan R. S., Pespeni M. H., Rapid, but limited, zooplankton adaptation to simultaneous warming and acidification. Nat. Clim. Chang. 11, 780–786 (2021). [Google Scholar]
- 62.Frost P. C., Ebert D., Larson J. H., Marcus M. A., Wagner N. D., Zalewski A., Transgenerational effects of poor elemental food quality on Daphnia magna. Oecologia 162, 865–872 (2010). [DOI] [PubMed] [Google Scholar]
- 63.Riebesell U., Bellerby R. G. J., Grossart H.-P., Thingstad F., Mesocosm CO2 perturbation studies: From organism to community level. Biogeosciences 5, 1157–1164 (2008). [Google Scholar]
- 64.Hunt B. P. V., Pakhomov E. A., Hosie G. W., Siegel V., Ward P., Bernard K., Pteropods in Southern Ocean ecosystems. Prog. Oceanogr. 78, 193–221 (2008). [Google Scholar]
- 65.Poulton A. J., Adey T. R., Balch W. M., Holligan P. M., Relating coccolithophore calcification rates to phytoplankton community dynamics: Regional differences and implications for carbon export. Deep-Sea Res. II Top. Stud. Oceanogr. 54, 538–557 (2007). [Google Scholar]
- 66.Morse J. W., He S., Influences of T, S and pCO2 on the pseudo-homogeneous precipitation of CaCO3 from seawater: Implications for whiting formation. Mar. Chem. 41, 291–297 (1993). [Google Scholar]
- 67.Hartmann J., Suitner N., Lim C., Schneider J., Marín-Samper L., Arístegui J., Renforth P., Taucher J., Riebesell U., Stability of alkalinity in ocean alkalinity enhancement (OAE) approaches: Consequences for durability of CO2 storage. Biogeosciences 20, 781–802 (2023). [Google Scholar]
- 68.Dörner I., Hauss H., Aberle N., Lohbeck K., Spisla C., Riebesell U., Ismar-Rebitz S. M., Ocean acidification impacts on biomass and fatty acid composition of a post-bloom marine plankton community. Mar. Ecol. Prog. Ser. 647, 49–64 (2020). [Google Scholar]
- 69.Whiteman J. P., Smith E. A. E., Besser A. C., Newsome S. D., A guide to using compound-specific stable isotope analysis to study the fates of molecules in organisms and ecosystems. Diversity 11, 8 (2019). [Google Scholar]
- 70.Flynn K., Mitra A., Bode A., Toward a mechanistic understanding of trophic structure: Inferences from simulating stable isotope ratios. Mar. Biol. 165, 147 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Dalsgaard J., John M. S., Kattner G., Müller-Navarra D., Hagen W., Fatty acid trophic markers in the pelagic marine environment. Adv. Mar. Biol. 46, 225–340 (2003). [DOI] [PubMed] [Google Scholar]
- 72.Costalago D., Forster I., Nemcek N., Neville C., Perry R. I., Young K., Hunt B. P., Seasonal and spatial dynamics of the planktonic trophic biomarkers in the Strait of Georgia (northeast Pacific) and implications for fish. Sci. Rep. 10, 8517 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.De Kluijver A., Soetaert K., Czerny J., Schulz K. G., Boxhammer T., Riebesell U., Middelburg J. J., A 13C labelling study on carbon fluxes in Arctic plankton communities under elevated CO2 levels. Biogeosciences 10, 1425–1440 (2013). [Google Scholar]
- 74.Sommer U., Stibor H., Katechakis A., Sommer F., Hansen T., Pelagic food web configurations at different levels of nutrient richness and their implications for the ratio fish production: Primary production. Hydrobiologia 484, 11–20 (2002). [Google Scholar]
- 75.Utermohl H., Zur Vervollkommung der quantitativen phytoplankton-methodik. Mitt Int. Ver Limnol. 9, 38 (1958). [Google Scholar]
- 76.Peterson W. T., Patterns in stage duration and development among marine and freshwater calanoid and cyclopoid copepods: A review of rules, physiological constraints, and evolutionary significance. Hydrobiologia 453, 91–105 (2001). [Google Scholar]
- 77.J. Oksanen, Vegan: Community Ecology Package. https://CRAN.R-project.org/package=vegan (2022).
- 78.M. E. Q. Pilson, “Appendix F Dissociation constants and pH scales” in An Introduction to the Chemistry of the Sea (Cambridge Univ. Press, ed. 2, 2013), pp. 441–449. [Google Scholar]
- 79.R. E. Zeebe, D. Wolf-Gladrow, Equilibrium in CO2 in Seawater: Equilibrium, Kinetics, Isotopes (Elsevier Oceanography Series, ed. 3, 2005), pp. 55–59. [Google Scholar]
- 80.Bailey A., Thor P., Browman H. I., Fields D. M., Runge J., Vermont A., Bjelland R., Thompson C., Shema S., Durif C. M. F., Hop H., Early life stages of the Arctic copepod Calanus glacialis are unaffected by increased seawater pCO2. ICES J. Mar. Sci. 74, 996–1004 (2017). [Google Scholar]
- 81.Lombard F., da Rocha R. E., Bijma J., Gattuso J. P., Effect of carbonate ion concentration and irradiance on calcification in planktonic foraminifera. Biogeosciences 7, 247–255 (2010). [Google Scholar]
- 82.Oron S., Evans D., Abramovich S., Almogi-Labin A., Erez J., Differential sensitivity of a symbiont-bearing foraminifer to seawater carbonate chemistry in a decoupled DIC-pH Experiment. J. Geophys. Res. Biogeosciences 125, e2020JG005726 (2020). [Google Scholar]
- 83.Naylor M. A., Kaiser H., Jones C. L. W., The effect of dosing with sodium hydroxide (NaOH) on water pH and growth of Haliotis midae in an abalone serial-use raceway. Aquac. Int. 21, 467–479 (2013). [Google Scholar]
- 84.Vivanco-Aranda M., Gallardo-Escárate C. J., Del Río-Portilla M. A., Low-density culture of red abalone juveniles, Haliotis rufescens Swainson 1822, recirculating aquaculture system and flow-through system. Aquacult. Res. 42, 161–168 (2011). [Google Scholar]
- 85.Harris J. O., Maguire G. B., Edwards S. J., Hindrum S. M., Effect of pH on growth rate, oxygen consumption rate, and histopathology of gill and kidney tissue for juvenile greenlip abalone, Haliotis laevigata donovan and blacklip abalone, Haliotis rubra leach. J. Shellfish Res. 18, 611–619 (1999). [Google Scholar]
- 86.A. G. Dickson, C. L. Sabine, J. R. Christian, Guide to Best Practices for Ocean CO2 Measurements (North Pacific Marine Science Organization, 2007). [Google Scholar]
- 87.D. E. Pierrot, D. W. R. Wallace, E. Lewis, MS Excel program developed for CO2 system calculations (Carbon Dioxide Information Analysis Center, 2011). [Google Scholar]
- 88.Taucher J., Bach L. T., Boxhammer T., Nauendorf A.; The Gran Canaria KOSMOS Consortium, Achterberg E. P., Algueró-Muñiz M., Arístegui J., Czerny J., Esposito M., Guan W., Haunost M., Horn H. G., Ludwig A., Meyer J., Spisla C., Sswat M., Stange P., Riebesell U., Influence of ocean acidification and deep water upwelling on oligotrophic plankton communities in the subtropical North Atlantic: Insights from an in situ mesocosm study. Front. Mar. Sci. 4, doi.org/10.3389/fmars.2017.00085 (2017). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figs. S1 to S4
Tables S1 to S5