Abstract
Purpose/Objectives: Cancer treatment survivors often report impaired functioning and increased falls. Not all survivors experience the same symptom burden, suggesting individual susceptibilities. APOE genotype is a potential genetic risk factor for cancer treatment related side effects. Lifestyle factors such as physical activity can mitigate the effect of APOE genotype on measures of clinical interest in individuals without a history of cancer. We tested the hypothesis that APOE genotype influences cancer treatment related side effects and symptoms as well as response to exercise intervention.
Materials and Methods: Data from a subsample of a study of fall prevention exercise in post-treatment female cancer survivors aged 50–75 years old (https://clinicaltrials.gov NCT01635413) were used to conduct a secondary data analysis. ApoE genotype was determined by serum sampling. Physical functioning, frequency of falls, and symptom burden were assessed using survey instruments.
Results: Data from 126 female cancer survivors a median of 49 months out from cancer diagnosis were analyzed. ApoE4 carriers trended toward a higher fall rate at baseline (p = 0.059), but after exercise intervention had a fall rate lower than E4 non-carriers both immediately after structured intervention (p = 0.013) and after 6 months of follow up (p = 0.002). E2 carriers did not show improved measures of depressive symptoms and self-report disability after exercise intervention. E3 homozygotes showed increased self report physical activity after the 6 month exercise intervention, but E4 and E2 carriers did not.
Conclusions: APOE genotype may modulate cancer treatment related side effects and symptoms and response to exercise intervention.
Keywords: apoE, breast cancer, exercise intervention, fall rate, functional status
INTRODUCTION
The number of cancer survivors in the United States continues to increase due to a growing, aging population and from improved cancer detection and treatment. As of January 2019, there were an estimated 17 million cancer survivors living in the United States [1]. Within 10 years, the number of cancer survivors in the US is projected to increase by 31% [1], meaning more people will be living longer with the side effects of cancer and cancer treatment. Many of these cancer survivors will be elderly and may experience reduced quality of life from a preventable or treatable toxicity related to cancer or cancer treatment. Although these long-term effects are often attributed to cytotoxic chemotherapy, the underlying mechanisms are multifactorial and may also include contributions of the malignancy itself, surgery, radiotherapy, hormonal therapy, immunotherapy, and targeted therapy [2–4]. It is important to understand how individual genetic susceptibility factors influence symptom burden and efficacy of mitigation strategies in cancer survivors to best tailor rehabilitation programs.
Cancer survivors often experience impaired functioning even years after cancer treatment [5–7]. Frequently reported symptoms include behavioral and cognitive changes such as difficulty concentrating, memory impairment, fatigue, and increased anxiety [8, 9]. These symptoms are important not only to quality of life, but also have been associated with increased risk of falls, decreased activity levels, and increased disability [10–13]. Functional impairments and increased risk of fall have also been associated with chemotherapy-induced peripheral neuropathy [14]. Importantly, there may be subsets of survivors who are particularly vulnerable to late functional impairments as a result of cancer treatment [5, 15].
Assessing genetic factors of neurological vulnerability may increase the understanding of behavioral and cognitive impairments among cancer survivors. One such genetic risk factor is apolipoprotein E (apoE) isoform [16]. ApoE is involved in cholesterol and lipid homeostasis and synaptic functions [17]. More recently, the role of apoE in immunomodulation, especially in the central nervous system (CNS), has also been recognized [18]. There are three major isoforms of apoE present in humans: E2, E3, E4. In the general population, E3 is encoded by far the most commonly possessed ε3 allele (79.8%), followed by E4 that is encoded by the ε4 allele (14.9%) and E2 encoded by the least common ε2 allele (5.3%).[19]. Possession of one or two ε4 alleles is associated with increased risk of developing cardiovascular disease, Alzheimer’s disease (AD), and cognitive impairments following various environmental challenges [20–23]. Even among healthy middle-aged populations, compared to ε3, possession of an ε4 allele is associated with accelerated cognitive decline [24]. Compared to E3, E2 is associated with a relative protective effect in risk to develop AD, but is associated with increased propensity toward developing more severe symptoms in survivors with post-traumatic stress disorder (PTSD) [19]. Less is known regarding the role of apoE isoform in influencing cancer survivorship. Several studies have identified an association between the ε4 allele and increased vulnerability to cognitive dysfunction after cancer treatment in survivors with breast cancer, lymphoma, and testicular cancer receiving chemotherapy [16, 25–27]. as well as in survivors with brain tumors [28, 29].
Prior studies have identified the impact of lifestyle-related factors on mediating the relationship between apoE isoform and long-term cancer-related toxicity. Remarkably, smoking has been identified as a protective factor against cancer-related cognitive impairment among apoE4 carriers [25, 29]. However, despite recognition of exercise as a salient protective factor against functional decline in apoE4 carriers in the setting of other medical comorbidities, this relationship has not yet been explored in the context of cancer [30, 31]. Epidemiologic evidence suggests that exercise may not only curb side effects during active cancer treatment, but may also lower the risk of cancer recurrence and improve quality of life in cancer survivors [32–34]. Exercise has cardio-metabolic benefits [35] and may also attenuate the increased risk of cardiovascular disease following cancer treatment, which is now a competing cause of morbidity and mortality for female cancer survivors [36–38].
In the current analyses, the modulating effect of apoE genotype on functional status and symptom burden in response to exercise intervention was investigated in a subsample of trial participants. Data were analyzed from the GET FIT (“Group Exercise Training for Functional Improvement after Treatment;” NCT01635413) study, a single-blind, parallel group, prospective controlled trial involving randomized underactive female cancer survivors previously treated with chemotherapy assigned to one of three study arms: (1) tai chi training, (2) lower body strength training, or (3) an exercise placebo (stretching and relaxation classes) [39]. More specifically, we testes the hypothesis that apoE isoform modulates cancer- and cancer treatment-related side effects and symptoms in response to exercise intervention.
RESULTS
Participant characteristics
APOE genotyping was performed on 133 participants in the GET FIT trial. Seven genotyped individuals were excluded for having the E2/E4 genotype, due to conflicting literature on relative advantages of the E2 and E4. APOE allele frequency data are shown in the Supplementary Table 1. Seventeen participants carried an ε2 allele while 29 carried an ε4 allele. To determine whether there was an enrichment of any specific genotype in our participant pool, APOE allele frequencies were plotted against reference frequencies in the general population using chi-square goodness of fit. The study population demonstrated similar allele frequencies to a reference population [19] for E2 (6.7% vs. 5.3%) and E4 (11.5% versus 14.9%) [Chi-Square = 3.06, p = 0.217].
Participant characteristics are shown in Table 1. The median age at enrollment in the trial was 65 years (interquartile range [IQR] 61.4 to 68.7 years) with a median time since cancer diagnosis of 49 months (IQR 18.3 to 79.8 months). The cohort was largely non-Hispanic (97.5%, 121/124; 2 declined to answer), Caucasian (89.7%, 113/126), and highly-educated (49.2% with undergraduate or postgraduate degree, 62/126). Most participants were married (57.9%, 73/126) and many were retired (49.2%, 62/126) at the time of study enrollment. E4 carriers were younger (mean age at enrollment 61.1 vs. 64.2; p = 0.012) and had been diagnosed with cancer at an earlier age (mean age at diagnosis 55.6 vs. 59.0; p = 0.015) than non-E4 carriers. There were no differences in participant characteristics between E2 carriers and non-carriers.
Table 1. Demographic data of study participants (n = 126).
| Genotype | ||||
|---|---|---|---|---|
| E2− | E2+ | E4− | E4+ | |
| Age at enrollment Median (IQR) | 64.0 (60–68) | 66.0 (62.3–69.8) | 66.0 (62.3–69.8) | 62.0 (56.5–67.5) |
| Age at cancer diagnosis Median (IQR) | 58.0 (52.4–63.6) | 60.6 (58.9–62.4) | 59.1 (54.3–63.9) | 55.8 (49.8–61.9) |
| Ethnicity | ||||
| Hispanic | 3% (3) | 0% (0) | 3% (3) | 0% (0) |
| Non-Hispanic | 96% (105) | 94% (16) | 96% (93) | 97% (28) |
| Decline to answer | 1% (1) | 6% (1) | 1% (1) | 3% (1) |
| Race | ||||
| Caucasian/White | 89% (97) | 94% (16) | 90% (87) | 90% (26) |
| African-American/Black | 1% (1) | 6% (1) | 1% (1) | 3% (1) |
| Native Hawaiian/Pacific Islander | 1% (1) | 0% (0) | 0% (0) | 3% (1) |
| American Indian/Alaskan Native | 2% (2) | 0% (0) | 1% (1) | 3% (1) |
| Asian | 2% (2) | 0% (0) | 2% (2) | 0% (0) |
| More than 1 race | 6% (6) | 0% (0) | 6% (6) | 0% (0) |
| Highest degree attained | ||||
| High school diploma | 24% (26) | 35% (6) | 23% (22) | 34% (10) |
| Associate/technical | 26% (28) | 24% (4) | 30% (29) | 10% (3) |
| Undergraduate degree | 28% (30) | 24% (4) | 25% (24) | 34% (10) |
| Postgraduate degree | 23% (25) | 18% (3) | 23% (22) | 21% (6) |
| Marital status | ||||
| Married/Partnered | 60% (65) | 47% (8) | 56% (54) | 66% (19) |
| Divorced/Separated | 19% (21) | 29% (5) | 21% (20) | 21% (6) |
| Widowed | 12% (13) | 24% (4) | 16% (16) | 3% (1) |
| Single | 9% (10) | 0% (0) | 7% (7) | 10% (3) |
| Employment | ||||
| Retired | 47% (51) | 65% (11) | 51% (49) | 45% (13) |
| Full time | 21% (23) | 18% (3) | 22% (21) | 17% (5) |
| Part time | 20% (22) | 12% (2) | 18% (17) | 24% (7) |
| Homemaker | 5% (5) | 6% (1) | 5% (5) | 3% (1) |
| Unemployed | 7% (8) | 0% (0) | 5% (5) | 10% (3) |
| Total | 100% (109) | 100% (17) | 100% (97) | 100% (29) |
Disease and treatment characteristics of the subsample are listed in Table 2. Breast cancer was the most common cancer diagnosis (69%, 87/126). Most participants had early stage cancer (61.9%, 78/126 with stage I or II disease) and received multimodal therapy including radiation (65.1%, 82/126) and/or surgery (88.1%, 111/126) in addition to chemotherapy (100%, 126/126).
Table 2. Participant cancer and cancer treatment history (n = 126).
| Genotype | ||||
|---|---|---|---|---|
| E2− | E2+ | E4− | E4+ | |
| Cancer type | n (%) | |||
| Breast | 77 (71) | 10 (59) | 67 (69) | 20 (69) |
| Cervical | 3 (3) | 0 (0) | 1 (1) | 2 (7) |
| Colon | 6 (6) | 2 (12) | 6 (6) | 2 (7) |
| Lung | 3 (3) | 2 (12) | 5 (5) | 0 (0) |
| Lymphoma | 5 (5) | 1 (6) | 4 (4) | 2 (7) |
| Ovarian | 6 (6) | 1 (6) | 6 (6) | 1 (3) |
| Uterine | 5 (5) | 1 (6) | 4 (4) | 2 (7) |
| Other | 4 (4) | 0 (0) | 4 (4) | 0 (0) |
| Cancer stage | ||||
| I | 29 (27) | 6 (35) | 24 (25) | 11 (38) |
| II | 39 (36) | 4 (24) | 34 (35) | 9 (31) |
| III | 30 (28) | 5 (29) | 30 (31) | 5 (17) |
| IV or metastatic | 0 (0%) | 0 (0) | 0 (0%) | 0 (0) |
| No stage/don’t remember | 11 (10) | 2 (12) | 9 (9) | 4 (14) |
| Cancer treatment received | 108 (99) | 16 (94) | 96 (99) | 28 (97) |
| Chemotherapy | 109 (100) | 17 (100) | 97 (100) | 29 (100) |
| Surgery | 99 (91) | 13 (76) | 85 (88) | 27 (93) |
| Radiation | 74 (68) | 9 (53) | 63 (65) | 20 (69) |
| Hormone therapy | 40 (37) | 3 (18) | 33 (34) | 10 (34) |
| Diagnosed with any other type of cancer? | 21 (19) | 3 (18) | 21 (22) | 3 (10) |
| Total | 109 (100) | 17 (100) | 97 (100) | 29 (100) |
Fall data
Thirty five participants recalled a fall in the 6 months prior to study enrollment. Of these 35 participants, 11 did not experience another fall, 10 fell again during the 6 month exercise intervention time period, 8 fell again during the 6 month post-intervention follow up time period, and 6 fell again during both the intervention and post-intervention time points. Of the 86 participants who reported no recent falls at the time of enrollment, 50 went on to report no future fall events. Of the remaining 36 participants who had not fallen recently prior to enrollment, 20 fell during the intervention time period only, 12 fell during the post-intervention time period, and 4 fell during both time periods.
There were no differences in fall rates seen between exercise groups. Therefore, these groups were collapsed for analysis of fall rate. In order to compare between the different genotype groups, a standardized fall rate per 1000 participant-days was calculated and is shown in Table 3 broken down by E2 and E4 carrier groups. In the 6 months prior to enrollment, or any intervention and based on participant recall, there was a trend toward a higher fall rate among E4 carriers than non-E4 carriers (2.52 per 1000 participant-days versus 5.56, p = 0.059). By the final month of the study (Month 12), there was a decrease in fall rate per 1000 participant-days among all participants (p = 0.043). However, this effect was significantly greater in E4 carriers than non-E4 carriers at both measurements during the 9-month (p = 0.013) and 12-month (p = 0.0002) post-intervention time points.
Table 3. Exercise intervention significantly decreases fall rate in E4 carriers.
| Fall rate per 1,000 participant-days1 | |||||||
|---|---|---|---|---|---|---|---|
| Genotype | Month | N | Mean fall rate | Genotype | Month | N | Mean fall rate |
| E2− | B | 109 | 3.47 | E4− | B | 97 | 2.52 |
| 1 | 109 | 4.89 | 1 | 97 | 5.50 | ||
| 3 | 106 | 1.89 | 3 | 94 | 2.13 | ||
| 6 | 105 | 2.54 | 6 | 93 | 2.87 | ||
| 9 | 105 | 1.59 | 9 | 93 | 2.15 | ||
| 12 | 105 | 2.54 | 12 | 93 | 3.94 | ||
| E2+ | B | 17 | 1.63 | E4+ | B | 29 | 5.56 |
| 1 | 17 | 7.84 | 1 | 29 | 4.60 | ||
| 3 | 16 | 4.17 | 3 | 28 | 2.38 | ||
| 6 | 16 | 6.25 | 6 | 28 | 3.57 | ||
| 9 | 16 | 2.08 | 9 | 28 | 0* | ||
| 12 | 16 | 6.25 | 12 | 28 | 0*** | ||
1Fall rate was calculated as (# participant-falls/# participant-days) × 1000. The fall rate of E4 carriers per 1000 participant-days decreased from 5.56 falls per 1000 participant days to 0 from baseline to month 12 (p = 0.043). There was a trend toward higher fall rate among E4 carriers versus E4 non-carriers at baseline (2.52 per 1000 participant-days versus 5.56, p = 0.059). However, after exercise intervention, E4 carriers demonstrated a significantly lower fall rate when compared with E4 non-carriers at both the 9 (2.15 versus 0, * p = 0.013) and 12 month (3.94 versus 0, *** p = 0.002) time periods. Abbreviation: B: baseline.
Cancer treatment related side effects and symptoms
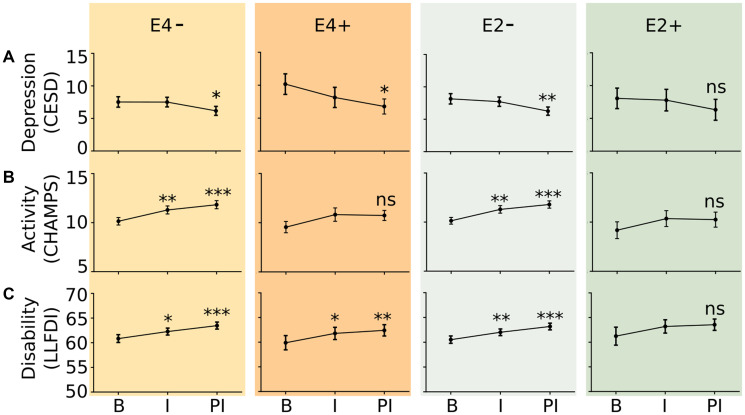
There were no differences in changes in self-reported depressive symptoms, physical activity, and physical functioning by exercise intervention arm. Therefore, these groups were collapsed for further analysis. Assessments were then compared by E4 and E2 carrier status (Figure 1). E4 carriers had a reduction in depressive symptoms over the entire 12 month study time period, as measured by the CESD (p = 0.003) but E2 carriers did not (p = 0.244). Non-E2 and non-E4 carriers demonstrated improved activity levels after the 6 month exercise intervention as measured by the CHAMPS questionnaire, while those who possessed at least one ε2 or ε4 allele did not demonstrate significant change. Except for E2 carriers, all genotype groups demonstrated significant improvement in the disability component LLFDI questionnaire, meaning that they could perform more activities independently than before.
Figure 1.
Effect of exercise intervention on survey indices of depressive symptoms (A), activity (B), and physical disability (C) during the baseline (B), intervention (I), and post-intervention (PI) timepoints. Depressive symptoms were measured by the Center for Epidemiological Studies Depression Scale. Activity was measured using the CHAMPS Activity Questionnaire for Older Adults; Physical disability was measured using the Late Life Functionality and Disability Instrument Limitation Questionnaire. E4−: n = 87 study participants; E4+: n = 27 study participants; E2−: n = 99 study participants; E2+: n = 16 study participants. * p < 0.05, ** p < 0.05, *** p < 0.005 versus B.
Neuropathy data
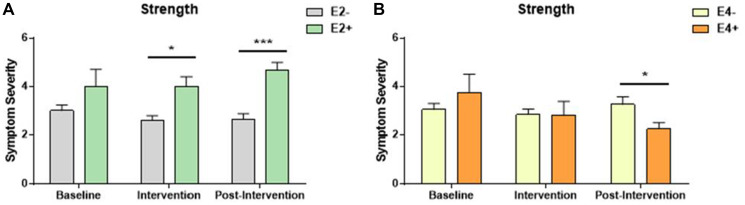
Among participants who reported neuropathy symptoms, neuropathy symptom severity was similar at baseline among all intervention and genotype groups (Figure 2A and 2B, Table 4). However, among participants who underwent the strength training intervention, those who lacked E2 had significantly lower neuropathy severity than heterozygous or homozygous E2 carriers, both during the exercise intervention and during the post-intervention time periods (Figure 2A). In the stretching control group, E2 carriers reported lower neuropathy severity in the post-intervention time period compared to non-E2 carriers (3.56 versus 2.00, p < 0.001; Table 4). E4 carriers in the strength training group had significantly lower neuropathy severity scores during the post intervention time point than non-E4 carriers (Figure 2B).
Figure 2. Symptom severity of individuals who reported neuropathy symptom incidence.
Participants ranked severity between 2 and 5, with 5 being the most severe. (A) E2 carriers in the strength intervention experienced significantly more neuropathy symptoms than E2 non-carriers both during and after the intervention time point. * p = 0.012, *** p = 0.003, respectively. (B) During the post-intervention timepoint, the opposite pattern was noted with E4 carriers experiencing less neuropathy symptoms in the strength intervention group. * p = 0.014).
Table 4. Neuropathy symptom burden noted by APOE genotype in the flexibility and Tai chi training group before, during, and after intervention.
| Exercise Group | Genotype | Baseline | Intervention | Post-intervention | |||
|---|---|---|---|---|---|---|---|
| Severity | N | Severity | N | Severity | N | ||
| Flexibility | E2− | 3.53 ± 0.30 | 17 | 3.53 ± 0.30 | 17 | 3.56 ± 0.27 | 18 |
| E2+ | 2 | 1 | 3.00 | 2 | 2.00 | 3 | |
| p | 0.245 | 0.095 | <0.001 | ||||
| E4− | 3.43 ± 0.34 | 14 | 3.53 ± 0.29 | 15 | 3.38 ± 0.32 | 16 | |
| E4+ | 3.50 ± 0.65 | 4 | 3.25 ± 0.75 | 4 | 3.20 ± 0.49 | 5 | |
| p | 0.926 | 0.743 | 0.772 | ||||
| Tai chi training | E2− | 3.18 ± 0.246 | 17 | 3.28 ± 0.30 | 18 | 3.06 ± 0.21 | 18 |
| E2+ | 4.50 ± 0.500 | 2 | 4.00 ± 1.0 | 2 | 2.50 ± 0.50 | 2 | |
| p | 0.177 | 0.599 | 0.452 | ||||
| E4− | 3.46 ± 0.33 | 13 | 3.33 ± 0.32 | 15 | 3.07 ± 0.25 | 14 | |
| E4+ | 3.00 ± 0.26 | 6 | 3.40 ± 0.60 | 5 | 2.83 ± 0.31 | 6 | |
| p | 0.289 | 0.925 | 0.556 | ||||
DISCUSSION
This is the first examination of the relationship among exercise, apoE genotype, and side effects and symptoms of cancer in a subset of older female cancer survivors participating in a large clinical exercise trial. The study findings suggest that APOE genotype may be associated with presence and severity of cancer treatment-related side effects and symptoms and also influence the response to exercise-based interventions in cancer survivors.
Cancer survivors carrying at least one ε4 allele fell less after undergoing exercise intervention both in comparison to their baseline and to non-E4 carriers. The same pattern was not seen when comparing E2 and non-E2 carriers. Cancer survivors who carry an ε4 allele seem to experience greater benefit from a strength training program in terms of neuropathy symptom burden than those who do not carry an ε4 allele. However, the opposite trend is shown in E2 carriers: participants carrying an ε2 allele have significantly higher neuropathy symptom burden when compared to non-E2 carriers after a strength training intervention. This ε4 effect was not seen after tai chi training or flexibility control intervention.
APOE genotype may modulate long-term cancer-related toxicity through a number of pathways. Cancer treatment may cause CNS injury through vascular damage, depletion of glial progenitor cells, oxidative stress, neuroinflammation, demyelination, and disruption of hippocampal neurogenesis [40, 41]. The pleiotropic effects of apoE involving the hypothalamic-pituitary-adrenal axis [19, 42], and the immune system [43] may modulate these effects [43]. Previously, E3 has been described as functioning in an antitumor capacity through suppression of angiogenesis and cell invasion [44], while E2 has been associated with decreased risk of gastric cancer [44–46]. A recent study found E4 was associated with significantly prolonged survival in survivors with melanoma, while E2 was associated with shorter survival [43].
Our study does not show long-term differences after cancer treatment in symptoms of depression, functional status, or neuropathy symptoms burden based on E2 or E4 status. This finding aligns with several prior studies [47–52]. The dissociation between the effects of E4 status on a reduction in falls but not on depressive and neuropathy phenotypes suggests that this reduction in falls might be related to improved vestibular or motor function in E4 carriers following exercise. Consistent with this beneficial effect of exercise in E4 carriers, in patients with mild Alzheimer’s disease, E4 carriers benefitted more from physical exercise than non-E4 carriers with regard to improvement in physical and cognitive measures [53].
In female breast cancer survivors, those with a history of falls at baseline performed worse when integration of vestibular input was critical for maintaining balance, but balance problems at baseline did not predict falls over six month [54]. Similarly, in prostate cancer survivors a history of falls but not balance at baseline predicted falls over twelve month [55]. In contrast, in a study of female and male cancer survivors, impaired balance predicted falls over twenty four months [56]. In the context of those studies, it is remarkable that in the current study the beneficial effect of exercise on falls in E4 carriers is seen while there was a trend toward a higher fall rate in E4 carriers than E4 non-carriers at baseline.
The current study has the following limitations. The first limitation is the sample size of the subsample and the relatively low numbers of E2 or E4 carriers. It is possible that our study was not sufficiently powered to detect relatively subtle differences between E2 or E4 carriers versus non-carriers, but sufficiently large to reveal this difference once exercise intervention was introduced. We could not explore dose-effect relationships, as our study population did not contain any survivors with the E4/E4 or E2/E2 genotypes. Another limitation is that only women were included in the fall prevention exercise in post-treatment cancer survivors study. Future studies of the relationship of APOE genotype with long-term toxicity burden and functional outcomes in male cancer survivors enrolled in a clinical exercise trial are warranted.
MATERIALS AND METHODS
Study design
After approval by the Oregon Health & Science University (OHSU) institutional review board, 444 female participants were enrolled and provided informed consent between September 2012 and October 2016. After baseline testing women were randomly assigned to one of three exercise groups: strength training, tai chi training, or the stretching control group. An ancillary study was conducted to compare changes in inflammatory markers in response to each of the exercise interventions. Participation in the ancillary study included an additional blood draw and was completely voluntary. For 133 of these ancillary study participants serum samples, were available for APOE genotyping and were considered for the current analysis.
Study population
Participant recruitment for the GET FIT trial has been previously described [39]. Inclusion criteria included the following: female sex, diagnosed with stage I-IIIc cancer other than cancers of the brain or spinal cord, completion of chemotherapy >3 months prior to enrollment, no ongoing adjuvant therapy other than hormone therapy for breast cancer, aged 50–75 on the date of enrollment, underactive at baseline (<60 minutes of moderate intensity exercise per week at the time of enrollment), cognitive ability sufficient to answer survey questions and to participate in exercise classes, and free of any medical condition that contraindicates participation in moderate intensity exercise. Exclusion criteria included male sex.
Participant assessments
At the time of enrollment, participants self-reported their demographics and medical history using an in-house questionnaire. Participants were also asked to complete survey questionnaires including the Functional Comorbidity Index (FCI; a self-administered 18-item scale of comorbidities effect on physical functioning) [57], Late Life Function & Disability Instrument (LLFDI; an assessment of functional limitations and performance of socially defined life tasks) [58], the Community Healthy Activities Model Program for Seniors Activity Questionnaire for Older Adults (CHAMPS; an assessment of weekly frequency and duration of lifestyle physical activities) [59], and Center for Epidemiological Studies Depression Scale (CES-D; a screening test for depression and depressive disorder) [60]. Participants were also asked about current symptom burden including neuropathy. These assessments were repeated at the 3 month (mid-intervention), 6 month (post-intervention), and 12 month (6 month follow up to supervised training) data collection visits. Falls during the study period were assessed prospectively by monthly reports [39]. A fall was defined as unintentionally coming to rest on the ground, not as a result of extenuating circumstances. Baseline fall rate was assessed through a 6-month recall.
Exercise interventions
The exercise protocols used in the GET FIT study have been previously published in detail [39]. The strength training program was based on training programs that improved neuromuscular function and reduced fall risk factors in our prior studies in women without cancer [61]. The tai chi training protocol consisted primarily of 8 purposeful movement forms, developed on the basis of the original simplified 24-form Yang-style tai chi training and also shown to prevent falls in non-cancer populations [62, 63]. In the exercise placebo stretching group, participants performed a series of seated or lying whole body flexibility and progressive neuromuscular relaxation exercises of the same frequency, duration, and length as the other groups, but intended to have little effect on fall risk factors [64]. Participants in each study group attended supervised one hour classes two days per week for six months, and were followed for an additional six months after the supervised intervention period finished.
Genotyping
Serum samples collected from the study participants were used for APOE genotyping, as previously described [65]. APOE genotypes were determined by Dr. Clive Woffendin at the Oregon Clinical Translational Research Institute (OCTRI) of OHSU using the Oragene self-collection methodology (DNA Genotek Inc., Ottawa, ON, Canada) as previously described [66]. Following polymerase chain reaction (PCR) amplification and restriction digestion with HhaI, DNA fragments were resolved on an 8% polyacrylamide nondenaturing gel, stained with ethidium bromide and visualized by ultraviolet illumination. Sizes of HhaI fragments were estimated by comparison with DNA size markers and the APOE genotype determined according to the unique pattern for each isoform. Known control samples of each APOE genotype were run alongside the unknown samples in each genotyping procedure.
Statistical analysis
Participants were divided into carriers versus non-carriers for E2 (E2+ versus E2−) and E4 (E4+ versus E4−). Individuals with the E3/E3 genotype were included as non-carriers in both analyses. Continuous data between groups were compared with a two-sample t test. Fisher’s exact test was used to compare categorical variables. Repeated measures ANOVA were used to evaluate continuous variables over the study time period. Percentages were rounded to the nearest percentage point. Means ± SEM are reported. All tests were two-sided, and p values of <0.05 were considered significant. SPSS Statistical Software v25 (Chicago, IL, USA) was used for statistical analysis and Graphpad Prism software (San Diego, CA, USA) for the generation of the figures.
CONCLUSIONS
Long-term cancer survivorship is increasingly common. Cancer survivors often lose functional ability and experience behavioral and cognitive dysfunction. The aim of the present investigation was to examine the relationship of E2 and E4 with long-term toxicity burden and functional outcomes in a sample of female cancer survivors treated with chemotherapy enrolled in a clinical exercise trial. Our data suggest that APOE genotype determines who may benefit the most from exercise interventions in long-term measures of mood, functional status, and toxicity burden. E4 carriers appear to benefit significantly from a strength training intervention. Increased efforts are warranted to assess the role of apoE isoforms in cancer survivors and the mechanisms underlying these apoE isoform-dependent effects.
SUPPLEMENTARY MATERIALS
Footnotes
Author contributions
GJM, JR, and KWS designed the study. GC, BSD, KD recruited the study participants and acquired all the data besides APOE genotyping. GJM, SH, BY, and CR performed the analyses. GJM wrote a first draft of the manuscript. All authors reviewed and provided feedback on the manuscript.
CONFLICTS OF INTEREST
Authors have no conflicts of interest to declare.
Ethical statement and consent
After approval by the Oregon Health & Science University (OHSU) institutional review board, 444 female participants were enrolled and provided informed consent between September 2012 and October 2016. After baseline testing women were randomly assigned to one of three exercise groups: strength training, tai chi training, or the stretching control group.
FUNDING
G.J.M. was supported by an HHMI Medical Research Fellowship, the Collins Medical Trust, RSNA Research Medical Student Grants (RMS1703, RMS1416), an N.L. Tartar Research Fellowship, the Oregon Clinical and Translational Research Institute (OCTRI) grant number UL1 RR024140 from the National Center for Research Resources (NCRR), the William Moss Kenneth Stevens Academic Development Fund of the Department of Radiation Medicine, and the development account of J.R. B.Y. was supported by the John S. Rogers Science Research Program as well as the James F. and Marion L. Miller Foundation. J.R. is partially supported by NASA NSCOR NNX15AK13G, NIH R21 CA223461, and NIH RF1 AG059088.
REFERENCES
- 1. Miller KD, Nogueira L, Mariotto AB, Rowland JH, Yabroff KR, Alfano CM, Jemal A, Kramer JL, Siegel RL. Cancer treatment and survivorship statistics, 2019. CA Cancer J Clin. 2019; 69:363–85. 10.3322/caac.21565. [DOI] [PubMed] [Google Scholar]
- 2. McGinnis GJ, Friedman D, Young KH, Torres ER, Thomas CR Jr, Gough MJ, Raber J. Neuroinflammatory and cognitive consequences of combined radiation and immunotherapy in a novel preclinical model. Oncotarget. 2017; 8:9155–73. 10.18632/oncotarget.13551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Janelsins MC, Heckler CE, Peppone LJ, Kamen C, Mustian KM, Mohile SG, Magnuson A, Kleckner IR, Guido JJ, Young KL, Conlin AK, Weiselberg LR, Mitchell JW, et al. Cognitive Complaints in Survivors of Breast Cancer After Chemotherapy Compared With Age-Matched Controls: An Analysis From a Nationwide, Multicenter, Prospective Longitudinal Study. J Clin Oncol. 2017; 35:506–14. 10.1200/JCO.2016.68.5826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Phillips KM, Jim HS, Small BJ, Laronga C, Andrykowski MA, Jacobsen PB. Cognitive functioning after cancer treatment: a 3-year longitudinal comparison of breast cancer survivors treated with chemotherapy or radiation and noncancer controls. Cancer. 2012; 118:1925–32. 10.1002/cncr.26432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ahles TA, Root JC, Ryan EL. Cancer- and cancer treatment-associated cognitive change: an update on the state of the science. J Clin Oncol. 2012; 30:3675–86. 10.1200/JCO.2012.43.0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jim HS, Donovan KA, Small BJ, Andrykowski MA, Munster PN, Jacobsen PB. Cognitive functioning in breast cancer survivors: a controlled comparison. Cancer. 2009; 115:1776–83. 10.1002/cncr.24192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hardy SJ, Krull KR, Wefel JS, Janelsins M. Cognitive Changes in Cancer Survivors. Am Soc Clin Oncol Educ Book. 2018; 38:795–806. 10.1200/EDBK_201179. [DOI] [PubMed] [Google Scholar]
- 8. Bower JE, Ganz PA, Tao ML, Hu W, Belin TR, Sepah S, Cole S, Aziz N. Inflammatory biomarkers and fatigue during radiation therapy for breast and prostate cancer. Clin Cancer Res. 2009; 15:5534–40. 10.1158/1078-0432.CCR-08-2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Janelsins MC, Kohli S, Mohile SG, Usuki K, Ahles TA, Morrow GR. An update on cancer- and chemotherapy-related cognitive dysfunction: current status. Semin Oncol. 2011; 38:431–38. 10.1053/j.seminoncol.2011.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pamoukdjian F, Aparicio T, Zelek L, Boubaya M, Caillet P, François V, de Decker L, Lévy V, Sebbane G, Paillaud E. Impaired mobility, depressed mood, cognitive impairment and polypharmacy are independently associated with disability in older cancer outpatients: The prospective Physical Frailty in Elderly Cancer patients (PF-EC) cohort study. J Geriatr Oncol. 2017; 8:190–95. 10.1016/j.jgo.2017.02.003. [DOI] [PubMed] [Google Scholar]
- 11. Mirelman A, Herman T, Brozgol M, Dorfman M, Sprecher E, Schweiger A, Giladi N, Hausdorff JM. Executive function and falls in older adults: new findings from a five-year prospective study link fall risk to cognition. PLoS One. 2012; 7:e40297. 10.1371/journal.pone.0040297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Minton O, Stone PC. A comparison of cognitive function, sleep and activity levels in disease-free breast cancer patients with or without cancer-related fatigue syndrome. BMJ Support Palliat Care. 2012; 2:231–38. 10.1136/bmjspcare-2011-000172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chen Z, Maricic M, Aragaki AK, Mouton C, Arendell L, Lopez AM, Bassford T, Chlebowski RT. Fracture risk increases after diagnosis of breast or other cancers in postmenopausal women: results from the Women’s Health Initiative. Osteoporos Int. 2009; 20:527–36. 10.1007/s00198-008-0721-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tofthagen C, Overcash J, Kip K. Falls in persons with chemotherapy-induced peripheral neuropathy. Support Care Cancer. 2012; 20:583–89. 10.1007/s00520-011-1127-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wefel JS, Vardy J, Ahles T, Schagen SB. International Cognition and Cancer Task Force recommendations to harmonise studies of cognitive function in patients with cancer. Lancet Oncol. 2011; 12:703–8. 10.1016/S1470-2045(10)70294-1. [DOI] [PubMed] [Google Scholar]
- 16. Ahles TA, Saykin AJ, Noll WW, Furstenberg CT, Guerin S, Cole B, Mott LA. The relationship of APOE genotype to neuropsychological performance in long-term cancer survivors treated with standard dose chemotherapy. Psychooncology. 2003; 12:612–19. 10.1002/pon.742. [DOI] [PubMed] [Google Scholar]
- 17. Mahley RW. Apolipoprotein E: from cardiovascular disease to neurodegenerative disorders. J Mol Med (Berl). 2016; 94:739–46. 10.1007/s00109-016-1427-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Shi Y, Holtzman DM. Interplay between innate immunity and Alzheimer disease: APOE and TREM2 in the spotlight. Nat Rev Immunol. 2018; 18:759–72. 10.1038/s41577-018-0051-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Johnson LA, Zuloaga DG, Bidiman E, Marzulla T, Weber S, Wahbeh H, Raber J. ApoE2 Exaggerates PTSD-Related Behavioral, Cognitive, and Neuroendocrine Alterations. Neuropsychopharmacology. 2015; 40:2443–53. 10.1038/npp.2015.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Strittmatter WJ, Roses AD. Apolipoprotein E and Alzheimer disease. Proc Natl Acad Sci U S A. 1995; 92:4725–27. 10.1073/pnas.92.11.4725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Weisgraber KH, Mahley RW. Human apolipoprotein E: the Alzheimer’s disease connection. FASEB J. 1996; 10:1485–94. 10.1096/fasebj.10.13.8940294. [DOI] [PubMed] [Google Scholar]
- 22. Raber J, Huang Y, Ashford JW. ApoE genotype accounts for the vast majority of AD risk and AD pathology. Neurobiol Aging. 2004; 25:641–50. 10.1016/j.neurobiolaging.2003.12.023. [DOI] [PubMed] [Google Scholar]
- 23. Raber J. Androgens, apoE, and Alzheimer’s disease. Sci Aging Knowledge Environ. 2004; 2004:re2. 10.1126/sageke.2004.11.re2. [DOI] [PubMed] [Google Scholar]
- 24. Caselli RJ, Locke DE, Dueck AC, Knopman DS, Woodruff BK, Hoffman-Snyder C, Rademakers R, Fleisher AS, Reiman EM. The neuropsychology of normal aging and preclinical Alzheimer’s disease. Alzheimers Dement. 2014; 10:84–92. 10.1016/j.jalz.2013.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ahles TA, Li Y, McDonald BC, Schwartz GN, Kaufman PA, Tsongalis GJ, Moore JH, Saykin AJ. Longitudinal assessment of cognitive changes associated with adjuvant treatment for breast cancer: the impact of APOE and smoking. Psychooncology. 2014; 23:1382–90. 10.1002/pon.3545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Koleck TA, Bender CM, Sereika SM, Ahrendt G, Jankowitz RC, McGuire KP, Ryan CM, Conley YP. Apolipoprotein E genotype and cognitive function in postmenopausal women with early-stage breast cancer. Oncol Nurs Forum. 2014; 41:E313–25. 10.1188/14.ONF.E313-E325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Amidi A, Agerbæk M, Wu LM, Pedersen AD, Mehlsen M, Clausen CR, Demontis D, Børglum AD, Harbøll A, Zachariae R. Changes in cognitive functions and cerebral grey matter and their associations with inflammatory markers, endocrine markers, and APOE genotypes in testicular cancer patients undergoing treatment. Brain Imaging Behav. 2017; 11:769–83. 10.1007/s11682-016-9552-3. [DOI] [PubMed] [Google Scholar]
- 28. Correa DD, DeAngelis LM, Shi W, Thaler HT, Lin M, Abrey LE. Cognitive functions in low-grade gliomas: disease and treatment effects. J Neurooncol. 2007; 81:175–84. 10.1007/s11060-006-9212-3. [DOI] [PubMed] [Google Scholar]
- 29. Correa DD, Satagopan J, Baser RE, Cheung K, Richards E, Lin M, Karimi S, Lyo J, DeAngelis LM, Orlow I. APOE polymorphisms and cognitive functions in patients with brain tumors. Neurology. 2014; 83:320–27. 10.1212/WNL.0000000000000617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Shih IF, Paul K, Haan M, Yu Y, Ritz B. Physical activity modifies the influence of apolipoprotein E ε4 allele and type 2 diabetes on dementia and cognitive impairment among older Mexican Americans. Alzheimers Dement. 2018; 14:1–9. 10.1016/j.jalz.2017.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Obisesan TO, Umar N, Paluvoi N, Gillum RF. Association of leisure-time physical activity with cognition by apolipoprotein-E genotype in persons aged 60 years and over: the National Health and Nutrition Examination Survey (NHANES-III). Clin Interv Aging. 2012; 7:35–43. 10.2147/CIA.S26794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ballard-Barbash R, Friedenreich CM, Courneya KS, Siddiqi SM, McTiernan A, Alfano CM. Physical activity, biomarkers, and disease outcomes in cancer survivors: a systematic review. J Natl Cancer Inst. 2012; 104:815–40. 10.1093/jnci/djs207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Campbell KL, Winters-Stone KM, Wiskemann J, May AM, Schwartz AL, Courneya KS, Zucker DS, Matthews CE, Ligibel JA, Gerber LH, Morris GS, Patel AV, Hue TF, et al. Exercise Guidelines for Cancer Survivors: Consensus Statement from International Multidisciplinary Roundtable. Med Sci Sports Exerc. 2019; 51:2375–90. 10.1249/MSS.0000000000002116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Patel AV, Friedenreich CM, Moore SC, Hayes SC, Silver JK, Campbell KL, Winters-Stone K, Gerber LH, George SM, Fulton JE, Denlinger C, Morris GS, Hue T, et al. American College of Sports Medicine Roundtable Report on Physical Activity, Sedentary Behavior, and Cancer Prevention and Control. Med Sci Sports Exerc. 2019; 51:2391–402. 10.1249/MSS.0000000000002117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Cornelissen VA, Fagard RH, Coeckelberghs E, Vanhees L. Impact of resistance training on blood pressure and other cardiovascular risk factors: a meta-analysis of randomized, controlled trials. Hypertension. 2011; 58:950–58. 10.1161/HYPERTENSIONAHA.111.177071. [DOI] [PubMed] [Google Scholar]
- 36. Bardia A, Arieas ET, Zhang Z, Defilippis A, Tarpinian K, Jeter S, Nguyen A, Henry NL, Flockhart DA, Hayes DF, Hayden J, Storniolo AM, Armstrong DK, et al. Comparison of breast cancer recurrence risk and cardiovascular disease incidence risk among postmenopausal women with breast cancer. Breast Cancer Res Treat. 2012; 131:907–14. 10.1007/s10549-011-1843-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Patnaik JL, Byers T, Diguiseppi C, Denberg TD, Dabelea D. The influence of comorbidities on overall survival among older women diagnosed with breast cancer. J Natl Cancer Inst. 2011; 103:1101–11. 10.1093/jnci/djr188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hooning MJ, Botma A, Aleman BM, Baaijens MH, Bartelink H, Klijn JG, Taylor CW, van Leeuwen FE. Long-term risk of cardiovascular disease in 10-year survivors of breast cancer. J Natl Cancer Inst. 2007; 99:365–75. 10.1093/jnci/djk064. [DOI] [PubMed] [Google Scholar]
- 39. Winters-Stone KM, Li F, Horak F, Luoh SW, Bennett JA, Nail L, Dieckmann N. Comparison of tai chi vs. strength training for fall prevention among female cancer survivors: study protocol for the GET FIT trial. BMC Cancer. 2012; 12:577. 10.1186/1471-2407-12-577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Dietrich J, Monje M, Wefel J, Meyers C. Clinical patterns and biological correlates of cognitive dysfunction associated with cancer therapy. Oncologist. 2008; 13:1285–95. 10.1634/theoncologist.2008-0130. [DOI] [PubMed] [Google Scholar]
- 41. McGinnis GJ, Raber J. CNS side effects of immune checkpoint inhibitors: preclinical models, genetics and multimodality therapy. Immunotherapy. 2017; 9:929–41. 10.2217/imt-2017-0056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Raber J, Akana SF, Bhatnagar S, Dallman MF, Wong D, Mucke L. Hypothalamic-pituitary-adrenal dysfunction in Apoe(-/-) mice: possible role in behavioral and metabolic alterations. J Neurosci. 2000; 20:2064–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ostendorf BN, Bilanovic J, Adaku N, Tafreshian KN, Tavora B, Vaughan RD, Tavazoie SF. Common germline variants of the human APOE gene modulate melanoma progression and survival. Nat Med. 2020; 26:1048–53. 10.1038/s41591-020-0879-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Pencheva N, Tran H, Buss C, Huh D, Drobnjak M, Busam K, Tavazoie SF. Convergent multi-miRNA targeting of ApoE drives LRP1/LRP8-dependent melanoma metastasis and angiogenesis. Cell. 2012; 151:1068–82. 10.1016/j.cell.2012.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. De Feo E, Simone B, Persiani R, Cananzi F, Biondi A, Arzani D, Amore R, D’Ugo D, Ricciardi G, Boccia S. A case-control study on the effect of Apolipoprotein E genotypes on gastric cancer risk and progression. BMC Cancer. 2012; 12:494. 10.1186/1471-2407-12-494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ifere GO, Desmond R, Demark-Wahnefried W, Nagy TR. Apolipoprotein E gene polymorphism influences aggressive behavior in prostate cancer cells by deregulating cholesterol homeostasis. Int J Oncol. 2013; 43:1002–10. 10.3892/ijo.2013.2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Vardy JL, Dhillon HM, Pond GR, Rourke SB, Bekele T, Renton C, Dodd A, Zhang H, Beale P, Clarke S, Tannock IF. Cognitive Function in Patients With Colorectal Cancer Who Do and Do Not Receive Chemotherapy: A Prospective, Longitudinal, Controlled Study. J Clin Oncol. 2015; 33:4085–92. 10.1200/JCO.2015.63.0905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Vardy J, Dhillon HM, Pond GR, Rourke SB, Xu W, Dodd A, Renton C, Park A, Bekele T, Ringash J, Zhang H, Burkes R, Clarke SJ, Tannock IF. Cognitive function and fatigue after diagnosis of colorectal cancer. Ann Oncol. 2014; 25:2404–12. 10.1093/annonc/mdu448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Mandelblatt JS, Stern RA, Luta G, McGuckin M, Clapp JD, Hurria A, Jacobsen PB, Faul LA, Isaacs C, Denduluri N, Gavett B, Traina TA, Johnson P, et al. Cognitive impairment in older patients with breast cancer before systemic therapy: is there an interaction between cancer and comorbidity? J Clin Oncol. 2014; 32:1909–18. 10.1200/JCO.2013.54.2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Vardy JL, Stouten-Kemperman MM, Pond G, Booth CM, Rourke SB, Dhillon HM, Dodd A, Crawley A, Tannock IF. A mechanistic cohort study evaluating cognitive impairment in women treated for breast cancer. Brain Imaging Behav. 2019; 13:15–26. 10.1007/s11682-017-9728-5. [DOI] [PubMed] [Google Scholar]
- 51. Bender CM, Merriman JD, Sereika SM, Gentry AL, Casillo FE, Koleck TA, Rosenzweig MQ, Brufsky AM, McAuliffe P, Zhu Y, Conley YP. Trajectories of Cognitive Function and Associated Phenotypic and Genotypic Factors in Breast Cancer. Oncol Nurs Forum. 2018; 45:308–26. 10.1188/18.ONF.308-326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Cheng H, Li W, Gan C, Zhang B, Jia Q, Wang K. The COMT (rs165599) gene polymorphism contributes to chemotherapy-induced cognitive impairment in breast cancer patients. Am J Transl Res. 2016; 8:5087–97. [PMC free article] [PubMed] [Google Scholar]
- 53. Jensen CS, Simonsen AH, Siersma V, Beyer N, Frederiksen KS, Gottrup H, Hoffman K, Høgh P, Frikke-Schmidt R, Sobol NA, Waldemar G, Wermuth L, Hasselbalch SG. Patients with Alzheimer’s disease who carry the APOE ε4 allele benefit more from physical exercise. Alzheimers Dement (NY). 2019; 5:99–106. 10.1016/j.trci.2019.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Winters-Stone KM, Torgrimson B, Horak F, Eisner A, Nail L, Leo MC, Chui S, Luoh SW. Identifying factors associated with falls in postmenopausal breast cancer survivors: a multi-disciplinary approach. Arch Phys Med Rehabil. 2011; 92:646–52. 10.1016/j.apmr.2010.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Hussain S, Breunic H, Timilshina N, Alibhai S. Falls in men on androgen deprivation therapy for prostate cancer. J Geriatr Oncol. 2010; 1:32–39. 10.1016/j.jgo.2010.03.004. [DOI] [PubMed] [Google Scholar]
- 56. Chen TY, Janke MC. Predictors of falls among community-dwelling older adults with cancer: results from the health and retirement study. Support Care Cancer. 2014; 22:479–85. 10.1007/s00520-013-2000-7. [DOI] [PubMed] [Google Scholar]
- 57. Groll DL, To T, Bombardier C, Wright JG. The development of a comorbidity index with physical function as the outcome. J Clin Epidemiol. 2005; 58:595–602. 10.1016/j.jclinepi.2004.10.018. [DOI] [PubMed] [Google Scholar]
- 58. Sayers SP, Jette AM, Haley SM, Heeren TC, Guralnik JM, Fielding RA. Validation of the Late-Life Function and Disability Instrument. J Am Geriatr Soc. 2004; 52:1554–59. 10.1111/j.1532-5415.2004.52422.x. [DOI] [PubMed] [Google Scholar]
- 59. Stewart AL, Mills KM, King AC, Haskell WL, Gillis D, Ritter PL. CHAMPS physical activity questionnaire for older adults: outcomes for interventions. Med Sci Sports Exerc. 2001; 33:1126–41. 10.1097/00005768-200107000-00010. [DOI] [PubMed] [Google Scholar]
- 60. Radloff LS. The CES-D Scale: A Self-Report Depression Scale for Research in the General Population. Appl Psychol Meas. 1977; 1:385–401. https://conservancy.umn.edu/bitstream/handle/11299/98561/v01n3p385.pdf. [Google Scholar]
- 61. Winters KM, Snow CM. Detraining reverses positive effects of exercise on the musculoskeletal system in premenopausal women. J Bone Miner Res. 2000; 15:2495–503. 10.1359/jbmr.2000.15.12.2495. [DOI] [PubMed] [Google Scholar]
- 62. Li F, Harmer P, Glasgow R, Mack KA, Sleet D, Fisher KJ, Kohn MA, Millet LM, Mead J, Xu J, Lin ML, Yang T, Sutton B, Tompkins Y. Translation of an effective tai chi intervention into a community-based falls-prevention program. Am J Public Health. 2008; 98:1195–98. 10.2105/AJPH.2007.120402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Li F, Harmer P, Fisher KJ, McAuley E, Chaumeton N, Eckstrom E, Wilson NL. Tai Chi and fall reductions in older adults: a randomized controlled trial. J Gerontol A Biol Sci Med Sci. 2005; 60:187–94. 10.1093/gerona/60.2.187. [DOI] [PubMed] [Google Scholar]
- 64. Twiss JJ, Waltman NL, Berg K, Ott CD, Gross GJ, Lindsey AM. An exercise intervention for breast cancer survivors with bone loss. J Nurs Scholarsh. 2009; 41:20–27. 10.1111/j.1547-5069.2009.01247.x. [DOI] [PubMed] [Google Scholar]
- 65. Berteau-Pavy F, Park B, Raber J. Effects of sex and APOE epsilon4 on object recognition and spatial navigation in the elderly. Neuroscience. 2007; 147:6–17. 10.1016/j.neuroscience.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 66. Hixson JE, Vernier DT. Restriction isotyping of human apolipoprotein E by gene amplification and cleavage with HhaI. J Lipid Res. 1990; 31:545–48. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.