Abstract
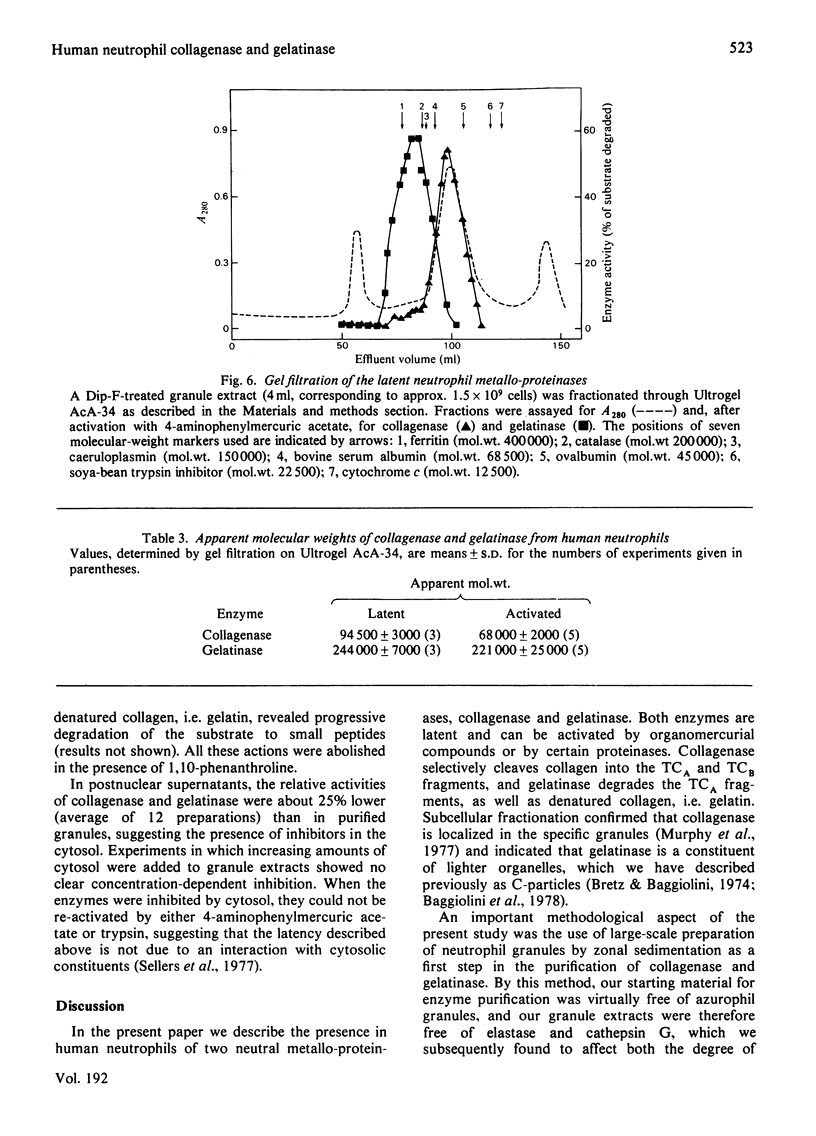
Two metallo-proteinases of human neutrophil leucocytes, collagenase and gelatinase, were studied. Collagenase specifically cleaved native collagen into the TCA and TCB fragments, whereas gelatinase degraded denatured collagen, i.e. gelatin, and the TCA fragments produced by collagenase. On subcellular fractionation by zonal sedimentation, collagenase was found to be localized in the specific granules, separate from gelatinase, which was recovered in smaller subcellular organelles known as C-particles. Neither enzyme was present in the azurophil granules, which contain the two major serine proteinases of neutrophils, elastase and cathepsin G. Collagenase and gelatinase were separated by gel filtration from extracts of partially purified granules. Both enzymes were found to occur in latent forms and were activated either by trypsin or by 4-aminophenylmercuric acetate. Gelatinase was also activated by cathepsin G, which, however, destroyed collagenase. Both enzymes were destroyed by neutrophil elastase. Activation resulted in a decrease by 25 000 in the apparent mol. wt. of both latent metallo-proteinases.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews P. Estimation of the molecular weights of proteins by Sephadex gel-filtration. Biochem J. 1964 May;91(2):222–233. doi: 10.1042/bj0910222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baugh R. J., Travis J. Human leukocyte granule elastase: rapid isolation and characterization. Biochemistry. 1976 Feb 24;15(4):836–841. doi: 10.1021/bi00649a017. [DOI] [PubMed] [Google Scholar]
- Bretz U., Baggiolini M. Biochemical and morphological characterization of azurophil and specific granules of human neutrophilic polymorphonuclear leukocytes. J Cell Biol. 1974 Oct;63(1):251–269. doi: 10.1083/jcb.63.1.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cawston T. E., Barrett A. J. A rapid and reproducible assay for collagenase using [1-14C]acetylated collagen. Anal Biochem. 1979 Nov 1;99(2):340–345. doi: 10.1016/s0003-2697(79)80017-2. [DOI] [PubMed] [Google Scholar]
- Dewald B., Rindler-Ludwig R., Bretz U., Baggiolini M. Subcellular localization and heterogeneity of neutral proteases in neutrophilic polymorphonuclear leukocytes. J Exp Med. 1975 Apr 1;141(4):709–723. doi: 10.1084/jem.141.4.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingle J. T., Blow A. M., Barrett A. J., Martin P. E. Proteoglycan-degrading enzymes. A radiochemical assay method and the detection of a new enzyme cathepsin F. Biochem J. 1977 Dec 1;167(3):775–785. doi: 10.1042/bj1670775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eeckhout Y., Vaes G. Further studies on the activation of procollagenase, the latent precursor of bone collagenase. Effects of lysosomal cathepsin B, plasmin and kallikrein, and spontaneous activation. Biochem J. 1977 Jul 15;166(1):21–31. doi: 10.1042/bj1660021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Gallin J. I., Wright D. G., Schiffmann E. Role of secretory events in modulating human neutrophil chemotaxis. J Clin Invest. 1978 Dec;62(6):1364–1374. doi: 10.1172/JCI109257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granelli-Piperno A., Vassalli J. D., Reich E. Secretion of plasminogen activator by human polymorphonuclear leukocytes. Modulation by glucocorticoids and other effectors. J Exp Med. 1977 Dec 1;146(6):1693–1706. doi: 10.1084/jem.146.6.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris E. D., Jr, Krane S. M. An endopeptidase from rheumatoid synovial tissue culture. Biochim Biophys Acta. 1972 Feb 28;258(2):566–576. doi: 10.1016/0005-2744(72)90249-5. [DOI] [PubMed] [Google Scholar]
- Kopitar M., Lebez D. Intracellular distribution of neutral proteinases and inhibitors in pig leucocytes. Isolation of two inhibitors of neutral proteinases. Eur J Biochem. 1975 Aug 15;56(2):571–581. doi: 10.1111/j.1432-1033.1975.tb02264.x. [DOI] [PubMed] [Google Scholar]
- Kruze D., Wojtecka E. Activation of leucocyte collagenase proenzyme by rheumatoid synovial fluid. Biochim Biophys Acta. 1972 Dec 28;285(2):436–446. doi: 10.1016/0005-2795(72)90330-3. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K., Favre M. Maturation of the head of bacteriophage T4. I. DNA packaging events. J Mol Biol. 1973 Nov 15;80(4):575–599. doi: 10.1016/0022-2836(73)90198-8. [DOI] [PubMed] [Google Scholar]
- Lazarus G. S., Daniels J. R., Lian J., Burleigh M. C. Role of granulocyte collagenase in collagen degradation. Am J Pathol. 1972 Sep;68(3):565–578. [PMC free article] [PubMed] [Google Scholar]
- Murphy G., Reynolds J. J., Bretz U., Baggiolini M. Collagenase is a component of the specific granules of human neutrophil leucocytes. Biochem J. 1977 Jan 15;162(1):195–197. doi: 10.1042/bj1620195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oronsky A. L., Perper R. J., Schroder H. C. Phagocytic release and activation of human leukocyte procollagenase. Nature. 1973 Dec 14;246(5433):417–419. doi: 10.1038/246417a0. [DOI] [PubMed] [Google Scholar]
- Reynolds J. J., Murphy G., Sellers A., Cartwright E. A new factor that may control collagen resorption. Lancet. 1977 Aug 13;2(8033):333–335. doi: 10.1016/s0140-6736(77)91490-8. [DOI] [PubMed] [Google Scholar]
- Sellers A., Cartwright E., Murphy G., Reynolds J. J. Evidence that latent collagenases are enzyme-inhibitor complexes. Biochem J. 1977 May 1;163(2):303–307. doi: 10.1042/bj1630303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellers A., Murphy G., Meikle M. C., Reynolds J. J. Rabbit bone collagenase inhibitor blocks the activity of other neutral metalloproteinases. Biochem Biophys Res Commun. 1979 Mar 30;87(2):581–587. doi: 10.1016/0006-291x(79)91834-5. [DOI] [PubMed] [Google Scholar]
- Sopata I., Dancewicz A. M. Presence of a gelatin-specific proteinase and its latent form in human leucocytes. Biochim Biophys Acta. 1974 Dec 29;370(2):510–523. doi: 10.1016/0005-2744(74)90112-0. [DOI] [PubMed] [Google Scholar]
- Starkey P. M., Barrett A. J., Burleigh M. C. The degradation of articular collagen by neutrophil proteinases. Biochim Biophys Acta. 1977 Aug 11;483(2):386–397. doi: 10.1016/0005-2744(77)90066-3. [DOI] [PubMed] [Google Scholar]
- Werb Z., Mainardi C. L., Vater C. A., Harris E. D., Jr Endogenous activiation of latent collagenase by rheumatoid synovial cells. Evidence for a role of plasminogen activator. N Engl J Med. 1977 May 5;296(18):1017–1023. doi: 10.1056/NEJM197705052961801. [DOI] [PubMed] [Google Scholar]
- Wize J., Abgarowicz T., Wojtecka-Lukasik E., Ksiezny S., Dancewicz A. M. Activation of human leucocyte procollagenase by rheumatoid synovial tissue culture medium. Ann Rheum Dis. 1975 Dec;34(6):520–523. doi: 10.1136/ard.34.6.520. [DOI] [PMC free article] [PubMed] [Google Scholar]



