Abstract
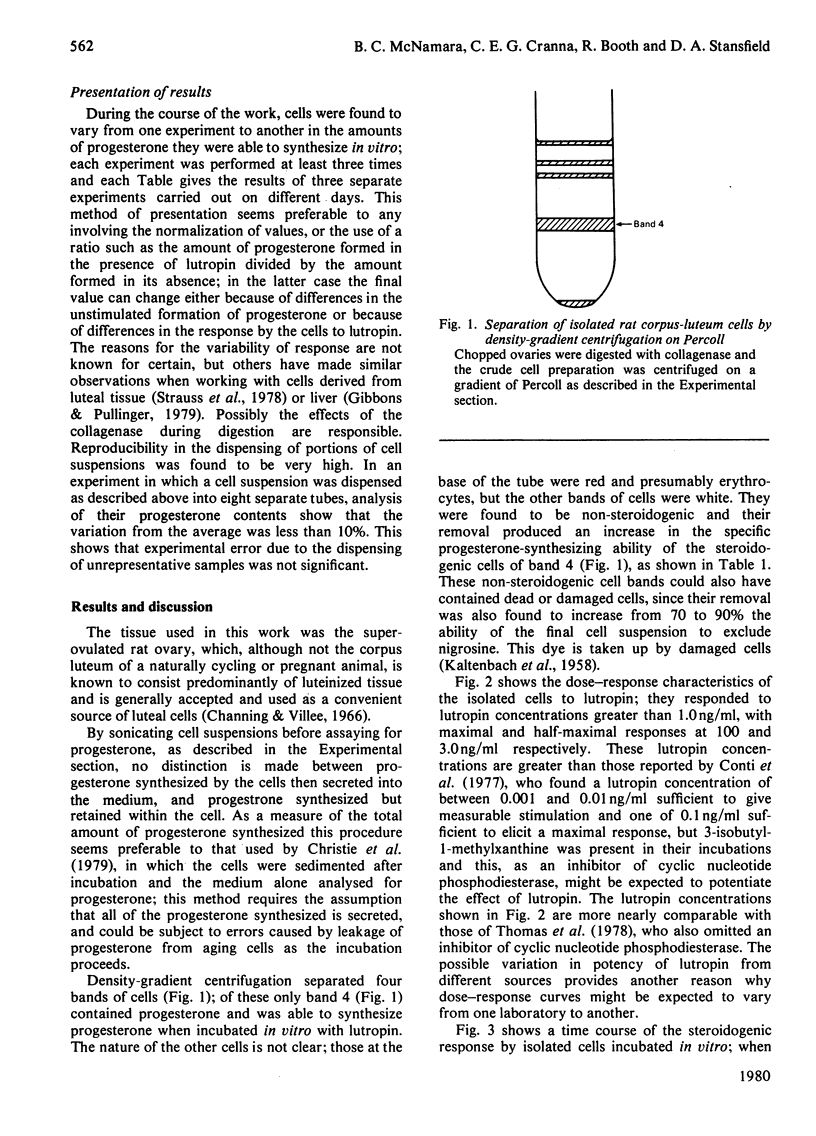
Isolated luteal cells, prepared from superovulated rat ovaries by digestion with collagenase, were subjected to density-gradient centrifugation on Percoll to give a more highly purified preparation of luteal cells than has been reported previously. The cells formed progesterone when incubated in vitro; lutropin stimulated this steroidogenesis. Progesterone formation was linear for at least 2 h; a minimal lutropin concentration of 1.0 ng/ml was needed for stimulation and concentrations of 3.0 and 100 ng/ml gave half-maximal and maximal responses respectively. The cells were unresponsive towards hormones other than lutropin. Exposure to lutropin raised the cellular cyclic AMP concentration, and dibutyryl cyclic AMP, but not dibutyryl cyclic GMP, was as effective in stimulating steroidogenesis as was lutropin. Aminoglutethimide, an inhibitor of cholesterol side-chain cleavage, completely blocked progesterone formation by the cells, showing cholesterol side-chain cleavage to be an obligatory step in steroidogenesis by these cells. Neither the activity of 3-hydroxy-3-methylglutaryl-CoA reductase nor the incorporation of radioactively labelled acetate or mevalonate into cholesterol by cells incubated in vitro were detectable unless the rats had been treated previously with 4-aminopyrazolo[3,4-d]pyrimidine. In cells from rats so treated, compactin was found to block almost completely the incorporation of radioactively labelled acetate, but not of mevalonate, into cholesterol, indicating that this inhibitor acts in corpus luteum in the same way as it does in other tissues. In cells from rats not treated with 4-aminopyrazolo[3,4-d]pyrimidine compactin had no effect on progesterone formation in vitro, showing cholesterol biosynthesis to be unnecessary for the rapid steroidogenic response by luteal cells to lutropin.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersen J. M., Dietschy J. M. Cholesterogenesis: derepression in extrahepatic tissues with 4-aminopyrazolo (3,4-d) pyrimidine. Science. 1976 Sep 3;193(4256):903–905. doi: 10.1126/science.948751. [DOI] [PubMed] [Google Scholar]
- Andersen J. M., Dietschy J. M. Regulation of sterol synthesis in 15 tissues of rat. II. Role of rat and human high and low density plasma lipoproteins and of rat chylomicron remnants. J Biol Chem. 1977 Jun 10;252(11):3652–3659. [PubMed] [Google Scholar]
- Armstrong D. T., Lee T. P., Miller L. S. Stimulation of progesterone biosynthesis in bovine corpora lutea by luteinizing hormone in the presence of an inhibitor of cholesterol synthesis. Biol Reprod. 1970 Feb;2(1):29–36. doi: 10.1095/biolreprod2.1.29. [DOI] [PubMed] [Google Scholar]
- Arthur J. R., Blair H. A., Boyd G. S., Mason J. I., Suckling K. E. Oxidation of cholesterol and cholesterol analogues by mitochondrial preparations of steroid-hormone-producing tissue. Biochem J. 1976 Jul 15;158(1):47–51. doi: 10.1042/bj1580047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BAILLIE L. A. Determination of liquid scientillation counting efficiency by pulse height shift. Int J Appl Radiat Isot. 1960 May;8:1–7. doi: 10.1016/0020-708x(60)90153-8. [DOI] [PubMed] [Google Scholar]
- Balasubramaniam S., Goldstein J. L., Faust J. R., Brown M. S. Evidence for regulation of 3-hydroxy-3-methylglutaryl coenzyme A reductase activity and cholesterol synthesis in nonhepatic tissues of rat. Proc Natl Acad Sci U S A. 1976 Aug;73(8):2564–2568. doi: 10.1073/pnas.73.8.2564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balasubramaniam S., Goldstein J. L., Faust J. R., Brunschede G. Y., Brown M. S. Lipoprotein-mediated regulation of 3-hydroxy-3-methylglutaryl coenzyme A reductase activity and cholesteryl ester metabolism in the adrenal gland of the rat. J Biol Chem. 1977 Mar 10;252(5):1771–1779. [PubMed] [Google Scholar]
- Brown M. S., Faust J. R., Goldstein J. L., Kaneko I., Endo A. Induction of 3-hydroxy-3-methylglutaryl coenzyme A reductase activity in human fibroblasts incubated with compactin (ML-236B), a competitive inhibitor of the reductase. J Biol Chem. 1978 Feb 25;253(4):1121–1128. [PubMed] [Google Scholar]
- Catt K. J., Harwood J. P., Aguilera G., Dufau M. L. Hormonal regulation of peptide receptors and target cell responses. Nature. 1979 Jul 12;280(5718):109–116. doi: 10.1038/280109a0. [DOI] [PubMed] [Google Scholar]
- Channing C. P., Villee C. A. Stimulation of cholesterol metabolism in the luteinized rat ovary by luteinizing hormone. Biochim Biophys Acta. 1966 Sep 26;127(1):1–17. doi: 10.1016/0304-4165(66)90469-7. [DOI] [PubMed] [Google Scholar]
- Christie M. H., Strauss J. F., 3rd, Flickinger G. L. Effect of reduced blood cholesterol on sterol and steroid metabolism by rat luteal tissue. Endocrinology. 1979 Jul;105(1):92–98. doi: 10.1210/endo-105-1-92. [DOI] [PubMed] [Google Scholar]
- Conti M., Harwood J. P., Dufau M. L., Catt K. J. Effect of gonadotropin-induced receptor regulation on biological responses of isolated rat luteal cells. J Biol Chem. 1977 Dec 25;252(24):8869–8874. [PubMed] [Google Scholar]
- Coutts J. R., Stansfield D. A. Cholesteryl esterase and cholesteryl ester pools in corpus luteum. J Lipid Res. 1968 Sep;9(5):647–651. [PubMed] [Google Scholar]
- DeVilla G. O., Jr, Roberts K., Wiest W. G., Mikhail G., Flickinger G. A specific radioimmunoassay of plasma progesterone. J Clin Endocrinol Metab. 1972 Sep;35(3):458–460. doi: 10.1210/jcem-35-3-458. [DOI] [PubMed] [Google Scholar]
- Endo A., Kuroda M., Tanzawa K. Competitive inhibition of 3-hydroxy-3-methylglutaryl coenzyme A reductase by ML-236A and ML-236B fungal metabolites, having hypocholesterolemic activity. FEBS Lett. 1976 Dec 31;72(2):323–326. doi: 10.1016/0014-5793(76)80996-9. [DOI] [PubMed] [Google Scholar]
- Gibbons G. F., Pullinger C. R. Utilization of endogenous and exogenous sources of substrate for cholesterol biosynthesis by isolated hepatocytes. Biochem J. 1979 Jan 1;177(1):255–263. doi: 10.1042/bj1770255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein J. L., Brown M. S. The low-density lipoprotein pathway and its relation to atherosclerosis. Annu Rev Biochem. 1977;46:897–930. doi: 10.1146/annurev.bi.46.070177.004341. [DOI] [PubMed] [Google Scholar]
- Goldstein J. L., Helgeson J. A., Brown M. S. Inhibition of cholesterol synthesis with compactin renders growth of cultured cells dependent on the low density lipoprotein receptor. J Biol Chem. 1979 Jun 25;254(12):5403–5409. [PubMed] [Google Scholar]
- Gregory K. W., Smith C. Z., Booth R. Diurnal variations in rat liver 3-hydroxy-3-methylglutaryl-Coenzyme A reductase activity in relation to feeding. Biochem J. 1972 Dec;130(4):1163–1165. doi: 10.1042/bj1301163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HELLIG H. R., SAVARD K. BIOSYNTHESIS OF PROGESTERONE, STEROLS, AND SQUALENE FROM ACETATE-1-14-C AND MEVALONATE-2-14-C BY THE BOVINE CORPUS LUTEUM IN VITRO. J Biol Chem. 1965 May;240:1957–1961. [PubMed] [Google Scholar]
- Hardie D. G., Stansfield D. A. Endogenous phosphorylation of microsomal proteins in bovine corpus luteum. Tenfold activation by adenosine 3':5'-cyclic monophosphate. Biochem J. 1977 Apr 15;164(1):213–221. doi: 10.1042/bj1640213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KALTENBACH J. P., KALTENBACH M. H., LYONS W. B. Nigrosin as a dye for differentiating live and dead ascites cells. Exp Cell Res. 1958 Aug;15(1):112–117. doi: 10.1016/0014-4827(58)90067-3. [DOI] [PubMed] [Google Scholar]
- Kowal J. Adrenal cells in tissue culture. IV. Use of an inhibitor of steroid synthesis for the study of ACTH action. Endocrinology. 1969 Aug;85(2):270–279. doi: 10.1210/endo-85-2-270. [DOI] [PubMed] [Google Scholar]
- Ling W. Y., Marsh J. M. Reevaluation of the role of cyclic adenosine 3',5'-monophosphate and protein kinase in the stimulation of steroidogenesis by luteinizing hormone in bovine corpus luteum slices. Endocrinology. 1977 Jun;100(6):1571–1578. doi: 10.1210/endo-100-6-1571. [DOI] [PubMed] [Google Scholar]
- Marsh J. M. The role of cyclic AMP in gonadal function. Adv Cyclic Nucleotide Res. 1975;6:137–199. [PubMed] [Google Scholar]
- Morgan C. A., Cooke I. D. A comparison of the competitive protein-binding assay and radioimmunoassay for plasma progesterone during the normal menstrual cycle. J Endocrinol. 1972 Sep;54(3):445–456. doi: 10.1677/joe.0.0540445. [DOI] [PubMed] [Google Scholar]
- Sala G. B., Dufau M. L., Catt K. J. Gonadotropin action in isolated ovarian luteal cells. The intermediate role of adenosine 3':5'-monophosphate in hormonal stimulation of progesterone synthesis. J Biol Chem. 1979 Mar 25;254(6):2077–2083. [PubMed] [Google Scholar]
- Shima S., Urata Y., Pincus G. Progestins biosynthesis in rats in vivo following infusion of cholesterol-7-alpha-3H and acetate-1-14C into luteinized ovaries. Proc Soc Exp Biol Med. 1968 Jul;128(3):673–676. doi: 10.3181/00379727-128-33096. [DOI] [PubMed] [Google Scholar]
- Strauss J. F., 3rd, Kirsch T., Flickinger G. L. Effects of lysosomotropic agents on progestin secretion by rat ovarian cells. J Steroid Biochem. 1978 Aug;9(8):731–738. doi: 10.1016/0022-4731(78)90192-9. [DOI] [PubMed] [Google Scholar]
- Thomas J. P., Dorflinger L. J., Behrman H. R. Mechanism of the rapid antigonadotropic action of prostaglandins in cultured luteal cells. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1344–1348. doi: 10.1073/pnas.75.3.1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb B. A., Stansfield D. A. Proceedings: Rat luteal adenosine 3':5'-cyclic monophosphate-dependent protein kinase. Biochem Soc Trans. 1974;2(3):446–448. doi: 10.1042/bst0020446. [DOI] [PubMed] [Google Scholar]
- Young J. L., Stansfield D. A. Use of a competitive protein-binding assay for adenosine 3':5'-phosphate for the study of bovine corpus luteum adenylate cyclase. J Endocrinol. 1977 Apr;73(1):123–134. doi: 10.1677/joe.0.0730123. [DOI] [PubMed] [Google Scholar]


