Abstract
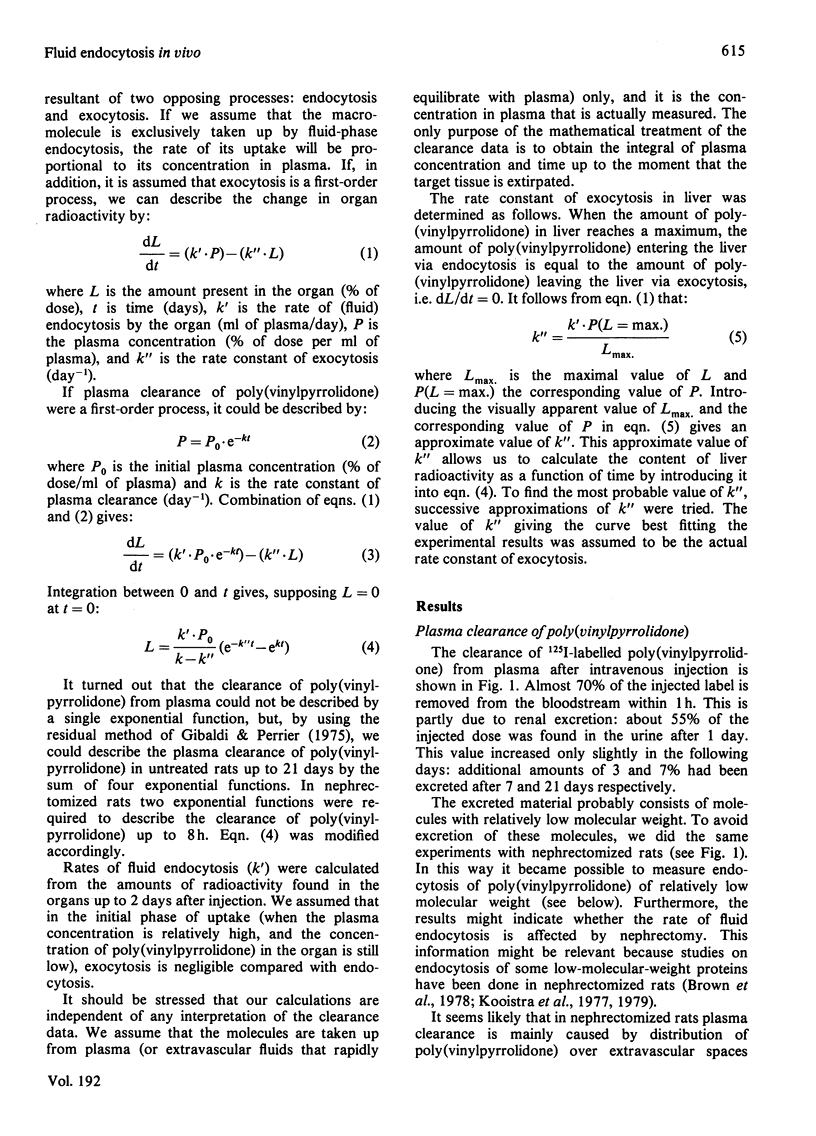
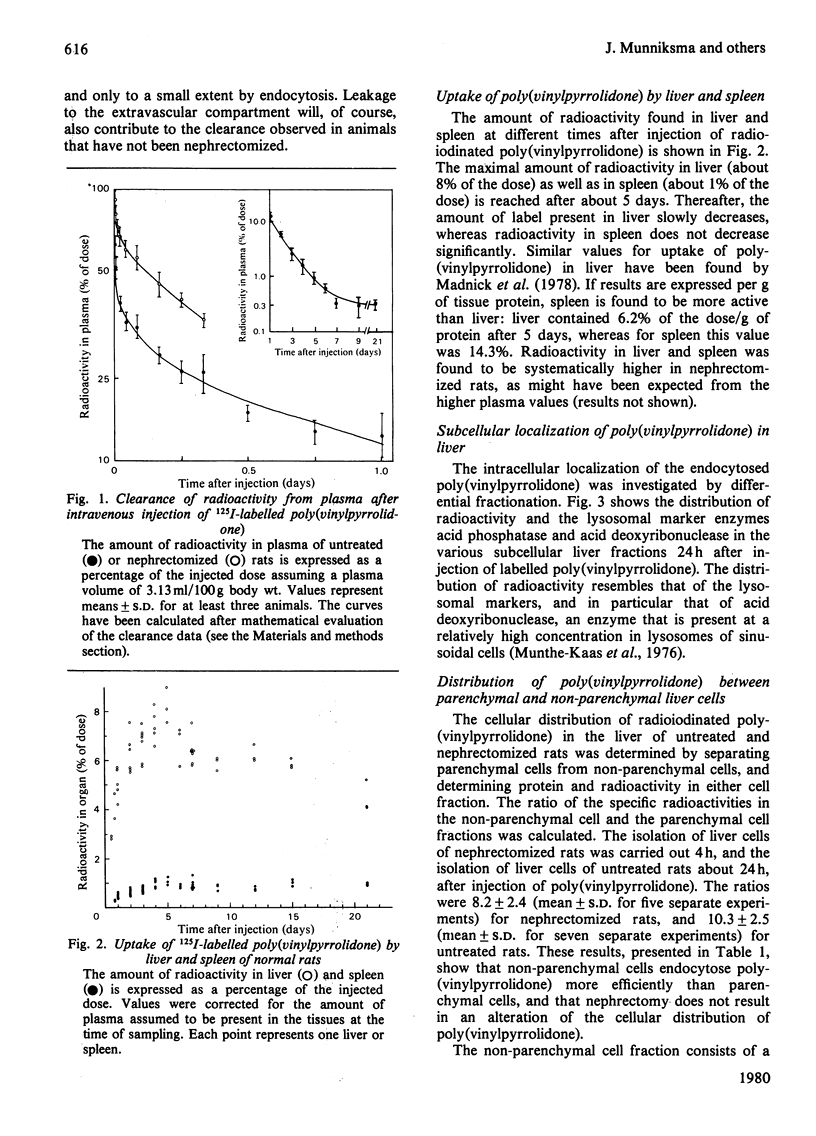
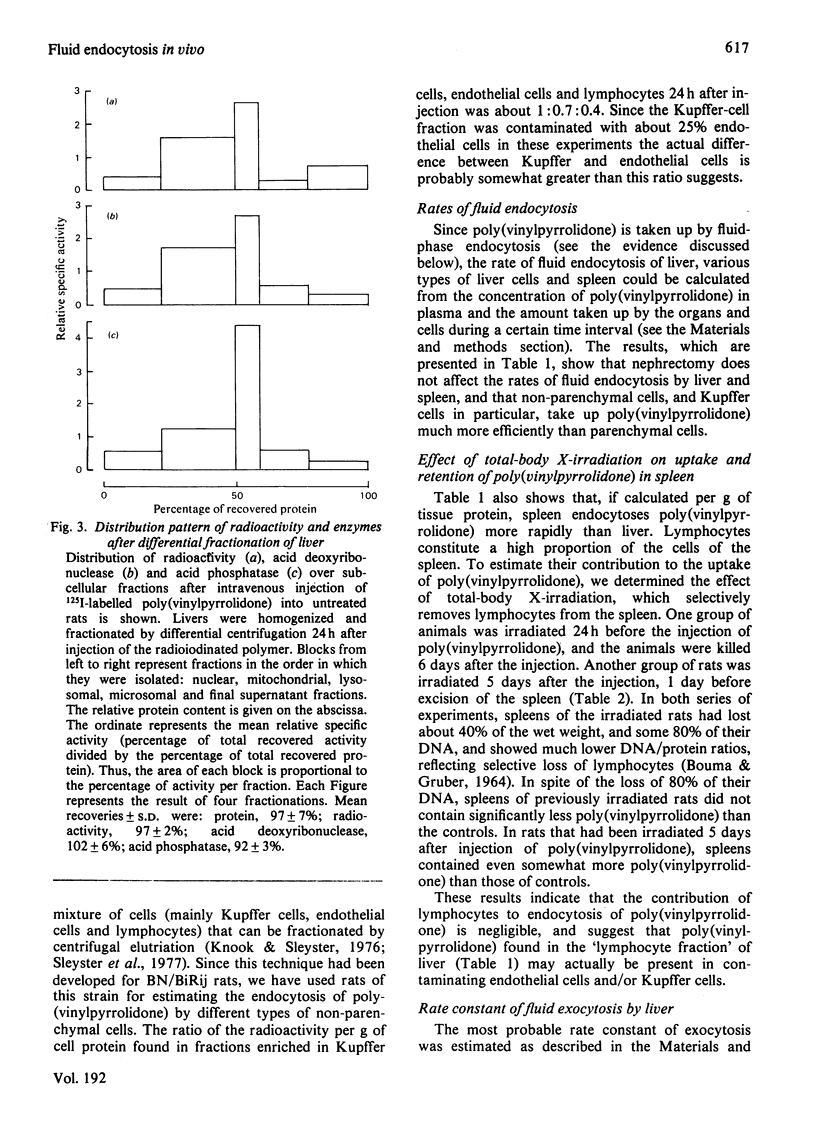
1. Rates of fluid endocytosis of rat liver, spleen, hepatocytes and sinusoidal liver cells have been determined, by using 125I-labelled poly(vinylpyrrolidone) as marker. Poly(vinylpyrrolidone) was injected intravenously into rats, and plasma clearance and uptake by liver and spleen were estimated. From these data, rates of fluid endocytosis of 1.2 and 1.8 ml of plasma/g of protein per day were calculated for liver and spleen respectively. Essentially the same results were found in nephrectomized rats. 2. Hepatocytes and sinusoidal cells were separately isolated by the collagenase/Pronase method, and sinusoidal cells were further fractionated by centrifugal elutriation. Hepatocytes, sinusoidal cells, Kupffer cells and endothelial cells showed rates of fluid endocytosis of 0.96, 9.0, 19 and 13 ml of plasma/g of cell protein per day respectively. Total-body X-irradiation did not influence uptake of poly(vinylpyrrolidone) by spleen, indicating that spleen lymphocytes are not significantly involved in fluid endocytosis. 3. For liver a rate constant of exocytosis of 5% per day was found, whereas for spleen no significant loss of accumulated label could be demonstrated during a 21-day period. 4. Distribution of label over a great number of organs and tissues was measured 9 days after the injection. Liver, skin, bone and muscle together contained about 70% of the label present in the carcass; only spleen and lymph nodes contained more label per g fresh weight of tissue than liver.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BOUMA J. M., GRUBER M. THE DISTRIBUTION OF CATHEPSINS B AND C IN RAT TISSUES. Biochim Biophys Acta. 1964 Sep 18;89:545–547. doi: 10.1016/0926-6569(64)90082-3. [DOI] [PubMed] [Google Scholar]
- Bartholeyns J., Baudhuin P. Inhibition of tumor cell proliferation by dimerized ribonuclease. Proc Natl Acad Sci U S A. 1976 Feb;73(2):573–576. doi: 10.1073/pnas.73.2.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blouin A., Bolender R. P., Weibel E. R. Distribution of organelles and membranes between hepatocytes and nonhepatocytes in the rat liver parenchyma. A stereological study. J Cell Biol. 1977 Feb;72(2):441–455. doi: 10.1083/jcb.72.2.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boman D., Berg T. The influence of Cortisone on the hepatic uptake of Triton WR-1339 in adrenalectomized rats. Hoppe Seylers Z Physiol Chem. 1975 Mar;356(3):301–308. doi: 10.1515/bchm2.1975.356.1.301. [DOI] [PubMed] [Google Scholar]
- Bouma J. M., Gruber M. Intracellular distribution of cathepsin B and cathepsin C in rat liver. Biochim Biophys Acta. 1966 Feb 14;113(2):350–358. doi: 10.1016/s0926-6593(66)80074-7. [DOI] [PubMed] [Google Scholar]
- Brown T. L., Henderson L. A., Thorpe S. R., Baynes J. W. The effect of alpha-mannose-terminal oligosaccharides on the survival of glycoproteins in the circulation. Rapid uptake and catabolism of bovine pancreatic ribonuclease B by nonparenchymal cells of rat liver. Arch Biochem Biophys. 1978 Jun;188(2):418–428. doi: 10.1016/s0003-9861(78)80026-5. [DOI] [PubMed] [Google Scholar]
- Edelson P. J., Cohn Z. A. Effects of concanavalin A on mouse peritoneal macrophages. I. Stimulation of endocytic activity and inhibition of phago-lysosome formation. J Exp Med. 1974 Nov 1;140(5):1364–1386. doi: 10.1084/jem.140.5.1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GIANETTO R., DE DUVE C. Tissue fractionation studies. 4. Comparative study of the binding of acid phosphatase, beta-glucuronidase and cathepsin by rat-liver particles. Biochem J. 1955 Mar;59(3):433–438. doi: 10.1042/bj0590433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRUBER M. The influence of carbohydrate on the origin of symptoms and the onset of death in thiamine-deficient pigeons. Biochim Biophys Acta. 1953 Jan;10(1):136–142. doi: 10.1016/0006-3002(53)90222-4. [DOI] [PubMed] [Google Scholar]
- Henning R., Kaulen H. D., Stoffel W. Biochemical analysis of the pinocytotic process. 3. Subcellular distribution and metabolic effects of ( 3 H)Triton WR-1339. Hoppe Seylers Z Physiol Chem. 1971 Oct;352(10):1347–1358. doi: 10.1515/bchm2.1971.352.2.1347. [DOI] [PubMed] [Google Scholar]
- Kaplan J. Cell contact induces an increase in pinocytotic rate in cultured epithelial cells. Nature. 1976 Oct 14;263(5578):596–597. doi: 10.1038/263596a0. [DOI] [PubMed] [Google Scholar]
- Keeler R. The effect of bilateral nephrectomy on the production and distribution of muramidase (lysozyme) in the rat. Can J Physiol Pharmacol. 1970 Feb;48(2):131–138. doi: 10.1139/y70-021. [DOI] [PubMed] [Google Scholar]
- Knook D. L., Sleyster E. C. Separation of Kupffer and endothelial cells of the rat liver by centrifugal elutriation. Exp Cell Res. 1976 May;99(2):444–449. doi: 10.1016/0014-4827(76)90605-4. [DOI] [PubMed] [Google Scholar]
- Kooistra T., Duursma A. M., Bouma J. M., Gruber M. Effect of size and charge on endocytosis of lysozyme derivatives by sinusoidal rat liver cells in vivo. Biochim Biophys Acta. 1980 Sep 1;631(3):439–450. doi: 10.1016/0304-4165(80)90020-3. [DOI] [PubMed] [Google Scholar]
- Kooistra T., Duursma A. M., Bouma J. M., Gruber M. Endocytosis and breakdown of ribonuclease oligomers by sinusoidal rat liver cells in vivo. I. Effect of size. Biochim Biophys Acta. 1979 Oct 4;587(2):282–298. doi: 10.1016/0304-4165(79)90361-1. [DOI] [PubMed] [Google Scholar]
- Kooistra T., Duursma A., Bouma J. M., Gruber M. Endocytosis and breakdown of proteins by sinusoidal liver cells. Acta Biol Med Ger. 1977;36(11-12):1763–1776. [PubMed] [Google Scholar]
- LaRusso N. F., Fowler S. Coordinate secretion of acid hydrolases in rat bile. J Clin Invest. 1979 Oct;64(4):948–954. doi: 10.1172/JCI109561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippman M. E., Finch S. C. A quantitative study of muramidase distribution in normal and nitrogen mustard-treated rats. Yale J Biol Med. 1972 Oct;45(5):463–470. [PMC free article] [PubMed] [Google Scholar]
- Madnick H. M., Winkler J. R., Segal H. L. Uptake of yeast invertase by rat liver cells in vivo and in vitro. Arch Biochem Biophys. 1978 Nov;191(1):385–392. doi: 10.1016/0003-9861(78)90102-9. [DOI] [PubMed] [Google Scholar]
- Munthe-Kaas A. C., Berg T., Seljelid R. Distribution of lysosomal enzymes in different types of rat liver cells. Exp Cell Res. 1976 Apr;99(1):146–154. doi: 10.1016/0014-4827(76)90689-3. [DOI] [PubMed] [Google Scholar]
- Munthe-Kass A. C. Uptake of macromolecules by rat Kupffer cells in vitro. Exp Cell Res. 1977 Jun;107(1):55–62. doi: 10.1016/0014-4827(77)90385-8. [DOI] [PubMed] [Google Scholar]
- Ose L., Ose T., Reinertsen R., Berg T. Fluid endocytosis in isolated rat parenchymal and non-parenchymal liver cells. Exp Cell Res. 1980 Mar;126(1):109–119. doi: 10.1016/0014-4827(80)90475-9. [DOI] [PubMed] [Google Scholar]
- Pratten M. K., Williams K. E., Lloyd J. B. A quantitative study of pinocytosis and intracellular proteolysis in rat peritoneal macrophages. Biochem J. 1977 Dec 15;168(3):365–372. doi: 10.1042/bj1680365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAVIN H. A., SELIGMAN A. M., FINE J. Polyvinyl pyrrolidone as a plasma expander; studies on its excretion, distribution and metabolism. N Engl J Med. 1952 Dec 11;247(24):921–929. doi: 10.1056/NEJM195212112472403. [DOI] [PubMed] [Google Scholar]
- Reske-Nielsen E., Bojsen-Moller M., Vetner M., Hansen J. C. Polyvinylpyrrolidone-storage disease. Light microscopical, ultrastructural and chemical verification. Acta Pathol Microbiol Scand A. 1976 Sep;84(5):397–405. [PubMed] [Google Scholar]
- Roberts A. V., Williams K. E., Lloyd J. B. The pinocytosis of 125I-labelled poly(vinylpyrrolidone), [14C]sucrose and colloidal [198Au]gold by rat yolk sac cultured in vitro. Biochem J. 1977 Nov 15;168(2):239–244. doi: 10.1042/bj1680239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider Y. J., Tulkens P., de Duve C., Trouet A. Fate of plasma membrane during endocytosis. II. Evidence for recycling (shuttle) of plasma membrane constituents. J Cell Biol. 1979 Aug;82(2):466–474. doi: 10.1083/jcb.82.2.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverstein S. C., Steinman R. M., Cohn Z. A. Endocytosis. Annu Rev Biochem. 1977;46:669–722. doi: 10.1146/annurev.bi.46.070177.003321. [DOI] [PubMed] [Google Scholar]
- Sinke J., Bouma J. M., Kooistra T., Gruber M. Endocytosis and breakdown of 125I-labelled lactate dehydrogenase isoenzyme M4 by rat liver and spleen in vivo. Biochem J. 1979 Apr 15;180(1):1–9. doi: 10.1042/bj1800001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinman R. M., Cohn Z. A. The interaction of soluble horseradish peroxidase with mouse peritoneal macrophages in vitro. J Cell Biol. 1972 Oct;55(1):186–204. doi: 10.1083/jcb.55.1.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolleshaug H., Berg T., Nilsson M., Norum K. R. Uptake and degradation of 125I-labelled asialo-fetuin by isolated rat hepatocytes. Biochim Biophys Acta. 1977 Aug 25;499(1):73–84. doi: 10.1016/0304-4165(77)90230-6. [DOI] [PubMed] [Google Scholar]
- Tothill P. The retention by the body of 131-I-polyvinylpyrrolidone and its effect on radiation dose. J Nucl Med. 1965 Aug;6(8):582–587. [PubMed] [Google Scholar]
- Williams K. E., Kidston E. M., Beck F., Lloyd J. B. Quantitative studies of pinocytosis. I. Kinetics of uptake of (125I)polyvinylpyrrolidone by rat yolk sac cultured in vitro. J Cell Biol. 1975 Jan;64(1):113–122. doi: 10.1083/jcb.64.1.113. [DOI] [PMC free article] [PubMed] [Google Scholar]


