Abstract
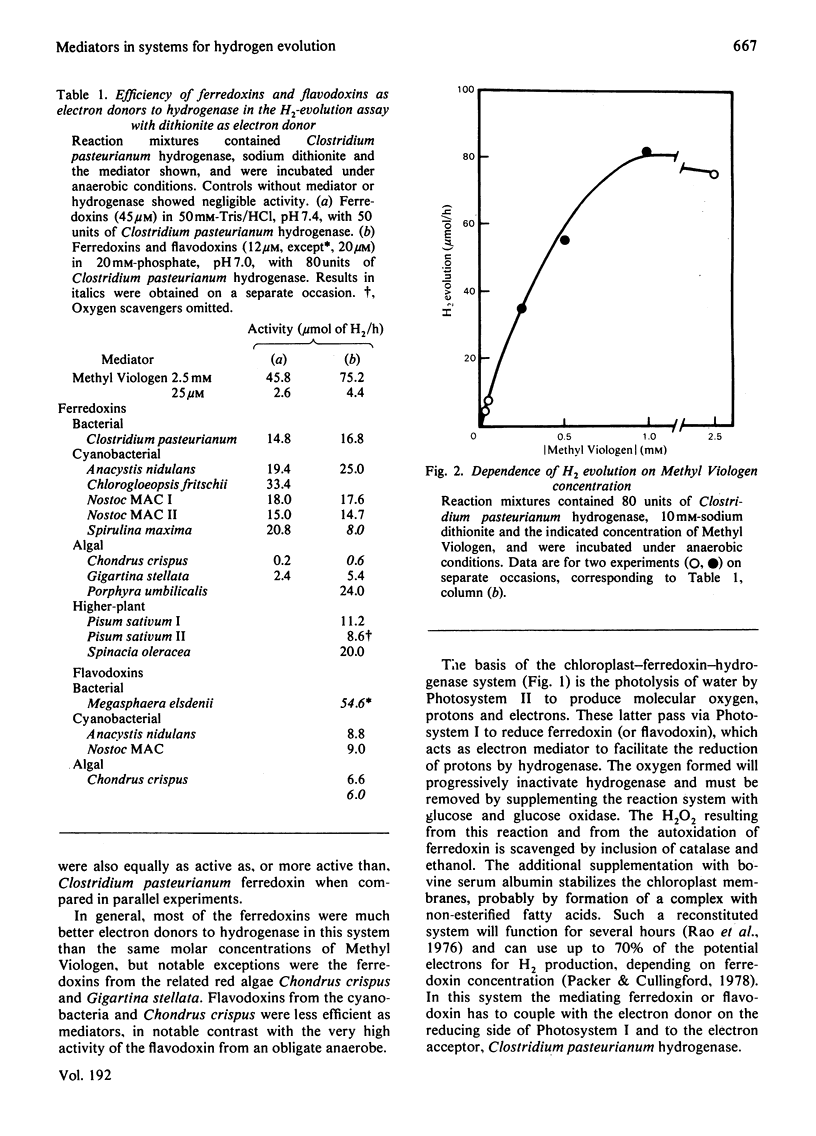
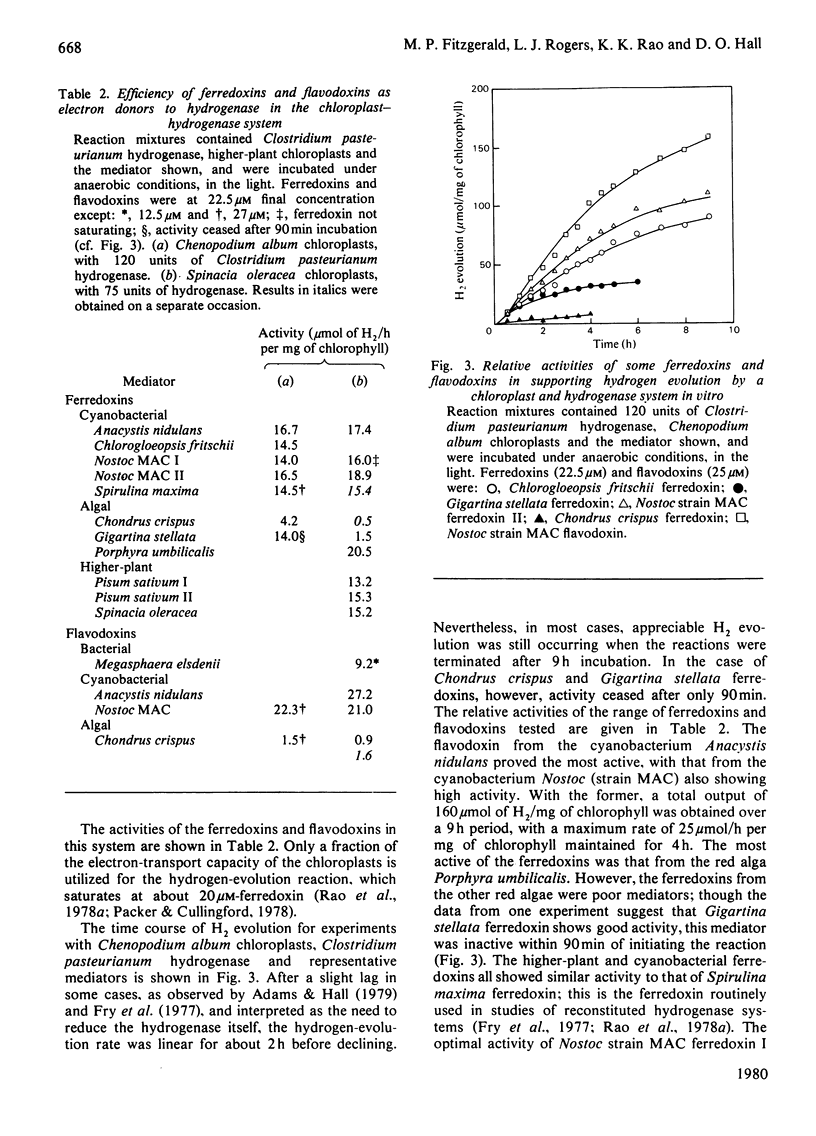
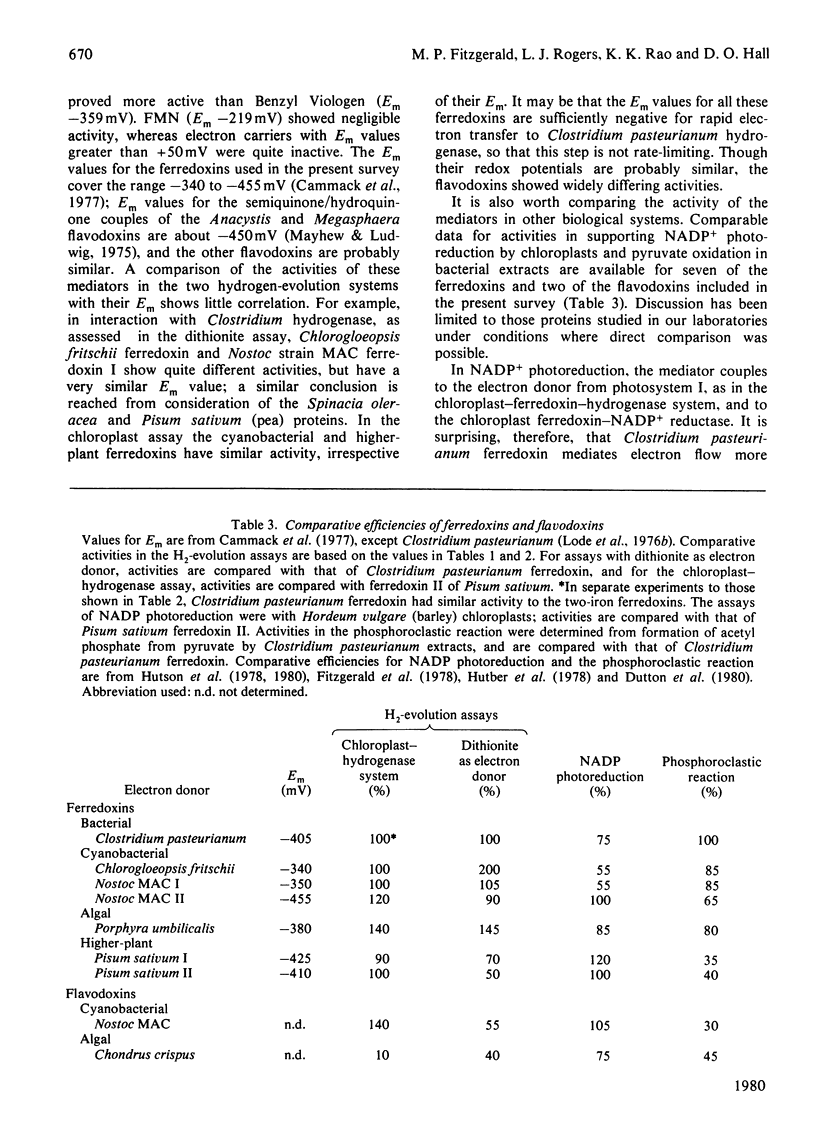
1. The efficiencies of ferredoxins and flavodoxins from a range of sources as mediators in systems for hydrogen evolution were assessed. 2. In supporting electron transfer from dithionite to hydrogenase of the bacterium Clostridium pasteurianum, highest activity was shown by the ferredoxin from the cyanobacterium Chlorogloeopsis fritschii and flavodoxin from the bacterium Megasphaera elsdenii. The latter was some twenty times as active as comparable concentrations of Methyl Viologen. Ferredoxins from the cyanobacterium Anacystis nidulans and the red alga Porphyra umbilicalis also showed high activity. 3. In mediating electron transfer from chloroplast membranes to Clostridium pasteurianum hydrogenase the flavodoxin from Anacystis nidulans proved the most active with Nostoc strain MAC flavodoxin and Porphyra umbilicalis ferredoxin also being appreciably more active than other cyanobacterial and higher plant ferredoxins. 4. In both hydrogenase systems the ferredoxin and flavodoxin from the red alga Chondrus crispus and the ferredoxin from another red alga Gigartina stellata showed very low activity. 5. There appeared to be no apparent correlation of efficiency in supporting hydrogenase activity with midpoint redox potential (Em) of the mediators, though some correlation of Em with the efficiency of the mediators in supporting NADP+ photoreduction by chloroplasts, or pyruvate oxidation by a Clostridium pasteurianum system, was evident. 6. Activity of the mediators in the hydrogenase systems therefore primarily reflects differences in tertiary structure conferring differing affinities for the other components of the systems.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams M. W., Hall D. O. Properties of the solubilized membrane-bound hydrogenase from the photosynthetic bacterium Rhodospirillum rubrum. Arch Biochem Biophys. 1979 Jul;195(2):288–299. doi: 10.1016/0003-9861(79)90355-2. [DOI] [PubMed] [Google Scholar]
- Adams M. W., Hall D. O. Solubilization and partial purification of the membrane-bound hydrogenase of Escherichia coli [proceedings]. Biochem Soc Trans. 1978;6(6):1339–1341. doi: 10.1042/bst0061339. [DOI] [PubMed] [Google Scholar]
- Adams M. W., Rao K. K., Hall D. O., Christou G., Garner C. D. Biological activity of synthetic molybdenum-iron-sulphur, iron-sulphur and iron-selenium analogues of ferredoxin-type centres. Biochim Biophys Acta. 1980 Jan 4;589(1):1–9. doi: 10.1016/0005-2728(80)90127-9. [DOI] [PubMed] [Google Scholar]
- Andrew P. W., Rogers L. J., Boulter D., Haslett B. G. Ferredoxin from a red alga, Porphyra umbilicalis. Eur J Biochem. 1976 Oct 1;69(1):243–248. doi: 10.1111/j.1432-1033.1976.tb10879.x. [DOI] [PubMed] [Google Scholar]
- Bell G. R., Lee J. P., Peck H. D., Jr, Gall J. L. Reactivity of Desulfovibrio gigas hydrogenase toward artificial and natural electron donors or acceptors. Biochimie. 1978;60(3):315–320. doi: 10.1016/s0300-9084(78)80828-1. [DOI] [PubMed] [Google Scholar]
- Benemann J. R., Berenson J. A., Kaplan N. O., Kamen M. D. Hydrogen evolution by a chloroplast-ferredoxin-hydrogenase system. Proc Natl Acad Sci U S A. 1973 Aug;70(8):2317–2320. doi: 10.1073/pnas.70.8.2317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bothe H., Distler E., Eisbrenner G. Hydrogen metabolism in blue-green algae. Biochimie. 1978;60(3):277–289. doi: 10.1016/s0300-9084(78)80824-4. [DOI] [PubMed] [Google Scholar]
- Cammack R., Rao K. K., Bargeron C. P., Hutson K. G., Andrew P. W., Rogers L. J. Midpoint redox potentials of plant and algal ferredoxins. Biochem J. 1977 Nov 15;168(2):205–209. doi: 10.1042/bj1680205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRENKEL A. W., RIEGER C. Photoreduction in algae. Nature. 1951 Jun 23;167(4260):1030–1030. doi: 10.1038/1671030a0. [DOI] [PubMed] [Google Scholar]
- Fitzgerald M. P., Husain A., Rogers L. J. A constitutive flavodoxin from a eukaryotic alga. Biochem Biophys Res Commun. 1978 Mar 30;81(2):630–635. doi: 10.1016/0006-291x(78)91582-6. [DOI] [PubMed] [Google Scholar]
- Hutson K. G., Rogers L. J., Haslett B. G., Boulter D., Cammack R. Comparative studies on two ferredoxins from the cyanobacterium Nostoc strain MAC. Biochem J. 1978 Jun 15;172(3):465–477. doi: 10.1042/bj1720465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llama M. J., Serra J. L., Rao K. K., Hall D. O. Characterization of hydrogenases from blue--green algae [proceedings]. Biochem Soc Trans. 1978;6(6):1337–1339. doi: 10.1042/bst0061337. [DOI] [PubMed] [Google Scholar]
- Lode E. T., Murray C. L., Rabinowitz J. C. Derivatives of Clostridium acidi-urici ferredoxin containing altered amino acid sequences. Semisynthetic synthesis, biological activity, and stability. J Biol Chem. 1976 Mar 25;251(6):1675–1682. [PubMed] [Google Scholar]
- Mayhew S. G. The redox potential of dithionite and SO-2 from equilibrium reactions with flavodoxins, methyl viologen and hydrogen plus hydrogenase. Eur J Biochem. 1978 Apr 17;85(2):535–547. doi: 10.1111/j.1432-1033.1978.tb12269.x. [DOI] [PubMed] [Google Scholar]
- Rao K. K., Gogotov I. N., Hall D. O. Hydrogen evolution by chloroplast-hydrogenase systems: improvements and additional observations. Biochimie. 1978;60(3):291–296. doi: 10.1016/s0300-9084(78)80825-6. [DOI] [PubMed] [Google Scholar]
- Rao K. K., Rosa L., Hall D. O. Prolonged production of hydrogen gas by a chloroplast biocatalytic system. Biochem Biophys Res Commun. 1976 Jan 12;68(1):21–28. doi: 10.1016/0006-291x(76)90004-8. [DOI] [PubMed] [Google Scholar]
- Reeves S. G., Hall D. O. The stoichiometry (ATP-2e- ratio) of non-cyclic photophosphorylation in isolated spinach chloroplasts. Biochim Biophys Acta. 1973 Jul 26;314(1):66–78. doi: 10.1016/0005-2728(73)90064-9. [DOI] [PubMed] [Google Scholar]


