Abstract
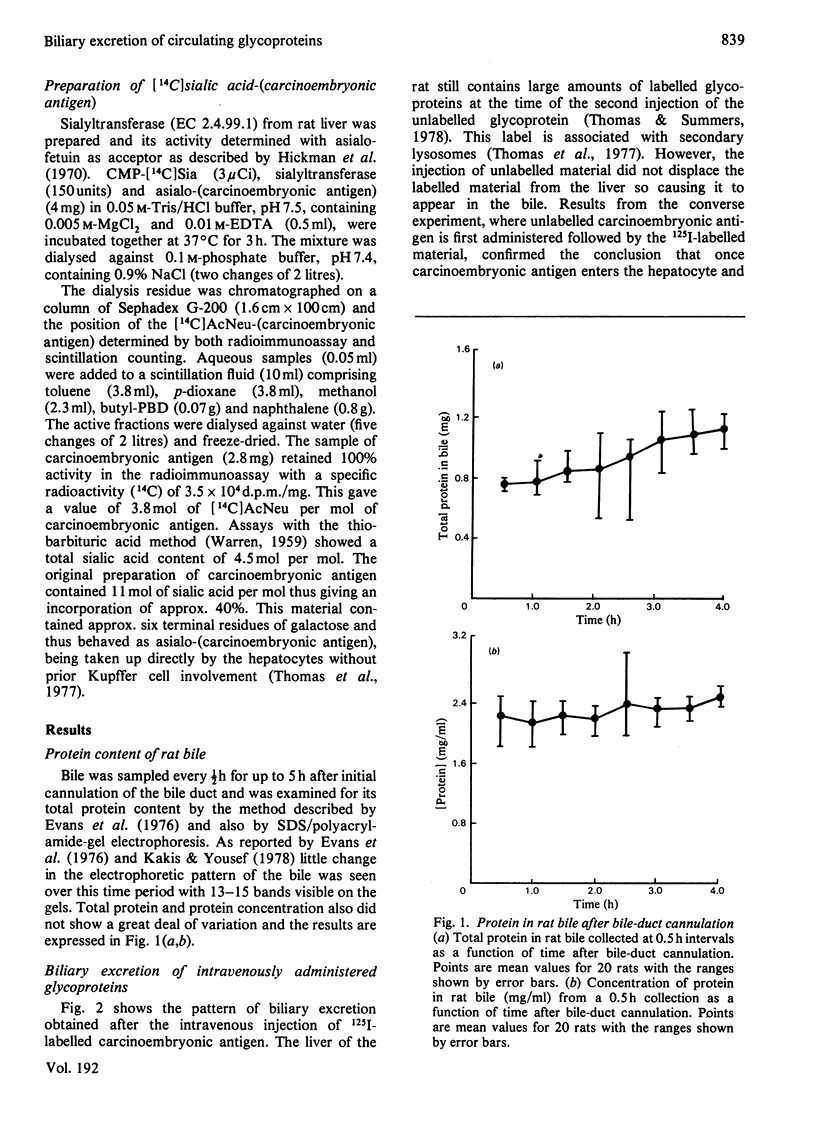
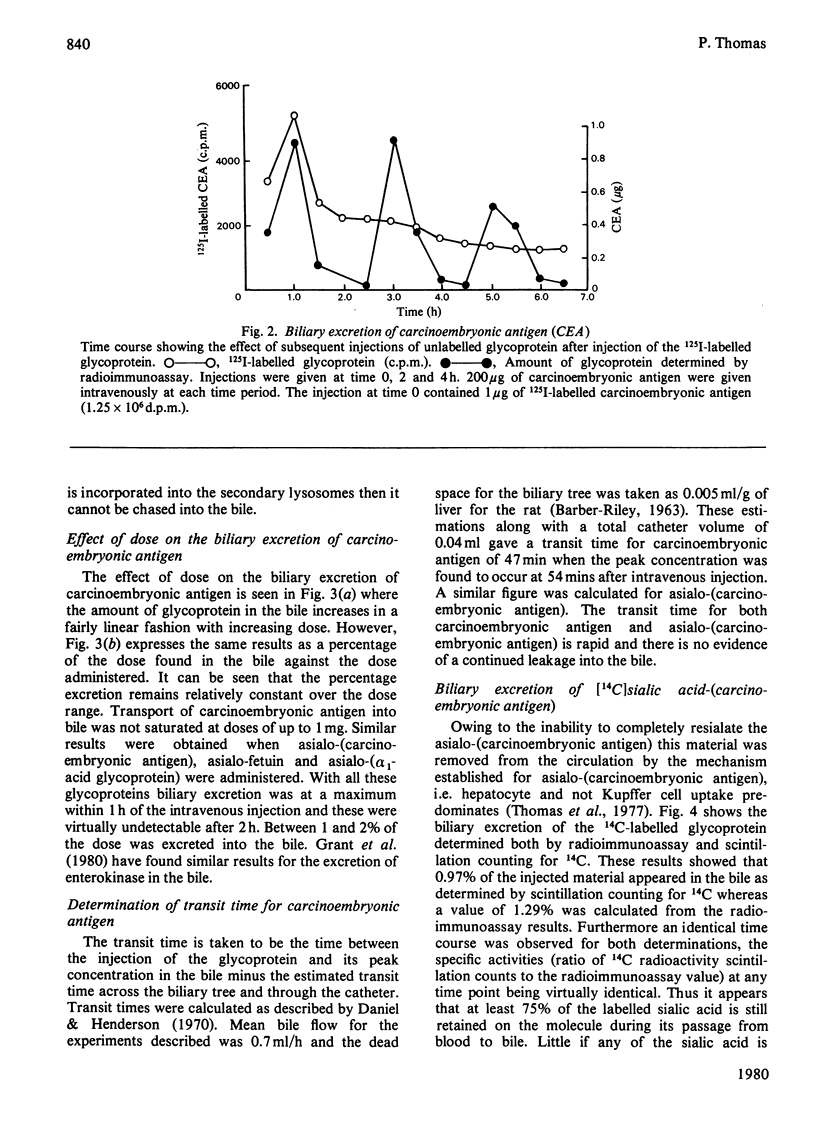
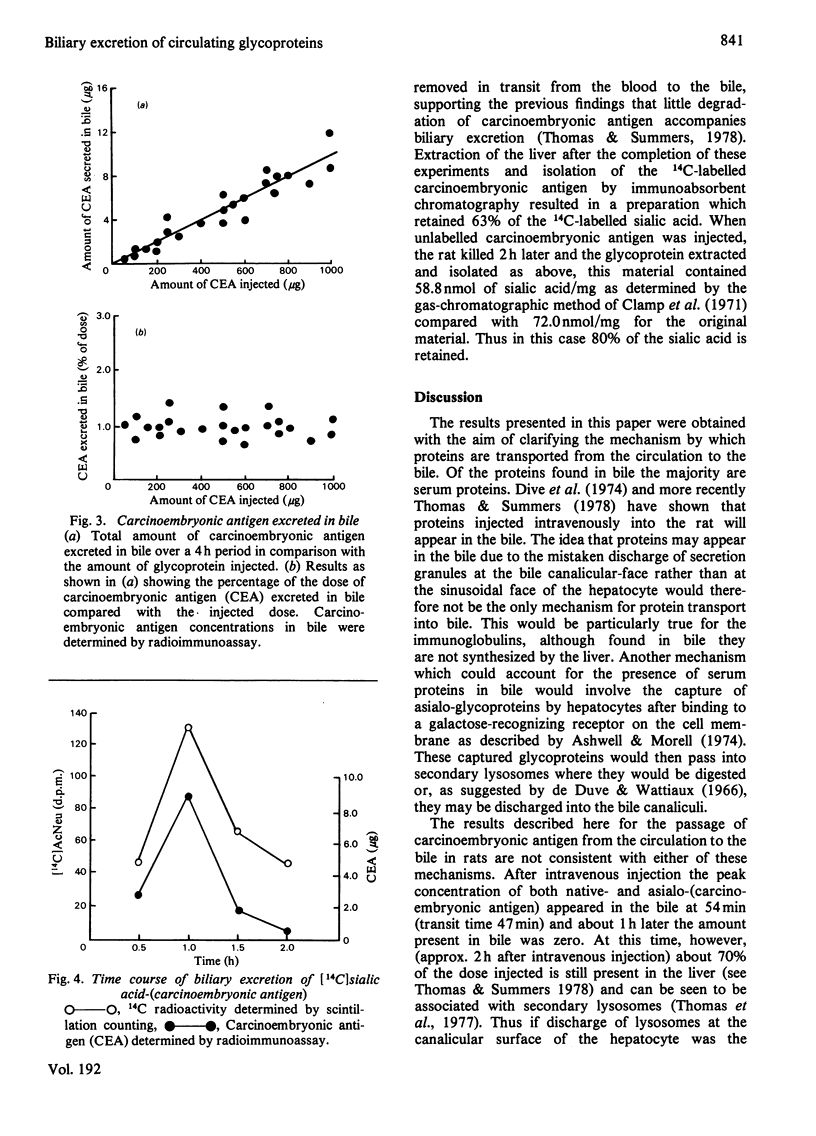
The transport of carcinoembryonic antigen from the circulation of the bile has been studied in the rat. Biliary excretion was directly proportional to the intravenously administered dose. Approximately 1-1.5% of the injected is excreted in the bile. Asialo-(carcinoembryonic antigen), asialo-fetuin and asialo-(alpha 1-acid glycoprotein) behaved similarly. The transit time through the liver for both native- and asialo-(carcinoembryonic antigen) was calculated as 47 min. Sialic acid content was not affected during biliary excretion. Glycoproteins that entered the liver cell lysosomes were not excreted in ther bile. A mechanism by which circulating proteins may by-pass the hepatocytes before being excreted in bile is suggested. Alternative mechanisms of protein entry into bile are discussed.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashwell G., Morell A. G. The role of surface carbohydrates in the hepatic recognition and transport of circulating glycoproteins. Adv Enzymol Relat Areas Mol Biol. 1974;41(0):99–128. doi: 10.1002/9780470122860.ch3. [DOI] [PubMed] [Google Scholar]
- BARBER-RILEY G. MEASUREMENT OF CAPACITY OF BILIARY TREE IN RATS. Am J Physiol. 1963 Dec;205:1122–1126. doi: 10.1152/ajplegacy.1963.205.6.1122. [DOI] [PubMed] [Google Scholar]
- Birbeck M. S., Cartwright P., Hall J. G., Orlans E., Peppard J. The transport by hepatocytes of immunoglobulin A from blood to bile visualized by autoradiography and electron microscopy. Immunology. 1979 Jun;37(2):477–484. [PMC free article] [PubMed] [Google Scholar]
- Clamp J. R., Bhatti T., Chambers R. E. The determination of carbohydrate in biological materials by gas-liquid chromatography. Methods Biochem Anal. 1971;19:229–344. doi: 10.1002/9780470110386.ch3. [DOI] [PubMed] [Google Scholar]
- Daniel P. M., Henderson J. R. Some factors affecting the passage of insulin into bile in the rabbit, with some observations on glucagon. Proc R Soc Lond B Biol Sci. 1970 Apr 7;175(1039):167–181. doi: 10.1098/rspb.1970.0017. [DOI] [PubMed] [Google Scholar]
- David G. S., Reisfeld R. A. Protein iodination with solid state lactoperoxidase. Biochemistry. 1974 Feb 26;13(5):1014–1021. doi: 10.1021/bi00702a028. [DOI] [PubMed] [Google Scholar]
- De Duve C., Wattiaux R. Functions of lysosomes. Annu Rev Physiol. 1966;28:435–492. doi: 10.1146/annurev.ph.28.030166.002251. [DOI] [PubMed] [Google Scholar]
- Dive C., Heremans J. F. Nature and origin of the proteins of bile. I. A comparative analysis of serum and bile proteins in man. Eur J Clin Invest. 1974 Aug;4(4):235–239. doi: 10.1111/j.1365-2362.1974.tb00398.x. [DOI] [PubMed] [Google Scholar]
- Dive C., Nadalini R. A., Vaerman J. P., Heremans J. F. Origin and nature of the proteins of bile. II. A comparative analysis of serum, hepatic lymph and bile proteins in the dog. Eur J Clin Invest. 1974 Aug;4(4):241–246. doi: 10.1111/j.1365-2362.1974.tb00399.x. [DOI] [PubMed] [Google Scholar]
- Evans W. H., Kremmmer T., Culvenor J. G. Role of membranes in bile formation. Comparison of the composition of bile and a liver bile-canalicular plasma-membrane subfraction. Biochem J. 1976 Mar 15;154(3):589–595. doi: 10.1042/bj1540589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher M. M., Nagy B., Bazin H., Underdown B. J. Biliary transport of IgA: role of secretory component. Proc Natl Acad Sci U S A. 1979 Apr;76(4):2008–2012. doi: 10.1073/pnas.76.4.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREENWOOD F. C., HUNTER W. M., GLOVER J. S. THE PREPARATION OF I-131-LABELLED HUMAN GROWTH HORMONE OF HIGH SPECIFIC RADIOACTIVITY. Biochem J. 1963 Oct;89:114–123. doi: 10.1042/bj0890114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickman J., Ashwell G., Morell A. G., van den Hamer C. J., Scheinberg I. H. Physical and chemical studies on ceruloplasmin. 8. Preparation of N-acetylneuraminic acid-1-14C-labeled ceruloplasmin. J Biol Chem. 1970 Feb 25;245(4):759–766. [PubMed] [Google Scholar]
- Hinton R. H., Mullock B. M. Bile proteins in the serum of jaundiced rats. Clin Chim Acta. 1977 Jul 1;78(1):159–162. doi: 10.1016/0009-8981(77)90349-7. [DOI] [PubMed] [Google Scholar]
- Holdsworth G., Coleman R. Enzyme profiles of mammalian bile. Biochim Biophys Acta. 1975 Apr 21;389(1):47–50. doi: 10.1016/0005-2736(75)90384-3. [DOI] [PubMed] [Google Scholar]
- Jackson G. D., Lemaître-Coelho I., Vaerman J. P., Bazin H., Beckers A. Rapid disappearance from serum of intravenously injected rat myeloma IgA and its secretion into bile. Eur J Immunol. 1978 Feb;8(2):123–126. doi: 10.1002/eji.1830080210. [DOI] [PubMed] [Google Scholar]
- Kakis G., Yousef I. M. Protein composition of rat bile. Can J Biochem. 1978 May;56(5):287–290. doi: 10.1139/o78-044. [DOI] [PubMed] [Google Scholar]
- Krupey J., Wilson T., Freedman S. O., Gold P. The preparation of purified carcinoembryonic antigen of the human digestive system from large quantities of tumor tissue. Immunochemistry. 1972 Jun;9(6):617–622. doi: 10.1016/0019-2791(72)90247-9. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laurence D. J., Stevens U., Bettelheim R., Darcy D., Leese C., Turberville C., Alexander P., Johns E. W., Neville A. M. Role of plasma carcinoembryonic antigen in diagnosis of gastrointestinal, mammary, and bronchial carcinoma. Br Med J. 1972 Sep 9;3(5827):605–609. doi: 10.1136/bmj.3.5827.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemaître-Coelho I., Jackson G. D., Vaerman J. P. Rat bile as a convenient source of secretory IgA and free secretory component. Eur J Immunol. 1977 Aug;7(8):588–590. doi: 10.1002/eji.1830070818. [DOI] [PubMed] [Google Scholar]
- Lurie B. B., Loewenstein M. S., Zamcheck N. Elevated carcinoembryonic antigen levels and biliary tract obstruction. JAMA. 1975 Jul 28;233(4):326–330. [PubMed] [Google Scholar]
- Mullock B. M., Hinton R. H., Dobrota M., Peppard J., Orlans E. Endocytic vesicles in liver carry polymeric IgA from serum to bile. Biochim Biophys Acta. 1979 Oct 18;587(3):381–391. doi: 10.1016/0304-4165(79)90442-2. [DOI] [PubMed] [Google Scholar]
- Mullock B. M., Hinton R. H. Mechanisms by which newly made glycoproteins are transferred from hepatocytes into bile. FEBS Lett. 1979 Oct 1;106(1):121–124. doi: 10.1016/0014-5793(79)80707-3. [DOI] [PubMed] [Google Scholar]
- Mullock B. M., Hinton R. H. The relation of bile proteins to serum and liver plasma membrane [proceedings]. Biochem Soc Trans. 1978;6(1):274–276. doi: 10.1042/bst0060274. [DOI] [PubMed] [Google Scholar]
- Mullock B. M., Issa F. S., Hinton R. H. Bile 5'-nucleotidase in the serum of jaundiced rats. Clin Chim Acta. 1977 Aug 15;79(1):129–140. doi: 10.1016/0009-8981(77)90470-3. [DOI] [PubMed] [Google Scholar]
- Orlans E., Peppard J., Reynolds J., Hall J. Rapid active transport of immunoglobulin A from blood to bile. J Exp Med. 1978 Feb 1;147(2):588–592. doi: 10.1084/jem.147.2.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternlieb I. Special article: functional implications of human portal and bile ductular ultrastructure. Gastroenterology. 1972 Aug;63(2):321–327. [PubMed] [Google Scholar]
- Thomas P., Birbeck M. S., Cartwright P. A radioautographic study of the hepatic uptake of circulating carcinoembryonic antigen by the mouse. Biochem Soc Trans. 1977;5(1):312–313. doi: 10.1042/bst0050312. [DOI] [PubMed] [Google Scholar]
- Thomas P., Edwards R. G., Westwood J. H. The hepatic uptake of the non-specific cross-reacting antigen, a glycoprotein related to carcinoembryonic antigen [proceedings]. Biochem Soc Trans. 1979 Aug;7(4):699–701. doi: 10.1042/bst0070699. [DOI] [PubMed] [Google Scholar]
- Thomas P., Hems D. A. The hepatic clearance of circulating native and asialo carcinoembronic antigen by the rat. Biochem Biophys Res Commun. 1975 Dec 1;67(3):1205–1209. doi: 10.1016/0006-291x(75)90801-3. [DOI] [PubMed] [Google Scholar]
- Thomas P., Jones M. The effects of triton WR1339 and asialo-fetuin on the hepatic uptake of circulating native and asialo-carcinoembryonic antigen. Biochem J. 1978 Sep 1;173(3):981–983. doi: 10.1042/bj1730981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P., Summers J. W. The biliary excretion of circulating asialoglycoproteins in the rat. Biochem Biophys Res Commun. 1978 Jan 30;80(2):335–339. doi: 10.1016/0006-291x(78)90681-2. [DOI] [PubMed] [Google Scholar]
- WARREN L. The thiobarbituric acid assay of sialic acids. J Biol Chem. 1959 Aug;234(8):1971–1975. [PubMed] [Google Scholar]
- Westwood J. H., Bessell E. M., Bukhari M. A., Thomas P., Walker J. M. Studies on the structure of the carcinoembryonic antigen. I. some deductions on the basis of chemical degradations. Immunochemistry. 1974 Dec;11(12):811–818. doi: 10.1016/0019-2791(74)90302-4. [DOI] [PubMed] [Google Scholar]
- Whitehead P. H., Sammons H. G. A simple technique for the isolation of orosomucoid from normal and pathological sera. Biochim Biophys Acta. 1966 Jul 27;124(1):209–211. doi: 10.1016/0304-4165(66)90336-9. [DOI] [PubMed] [Google Scholar]


