Abstract
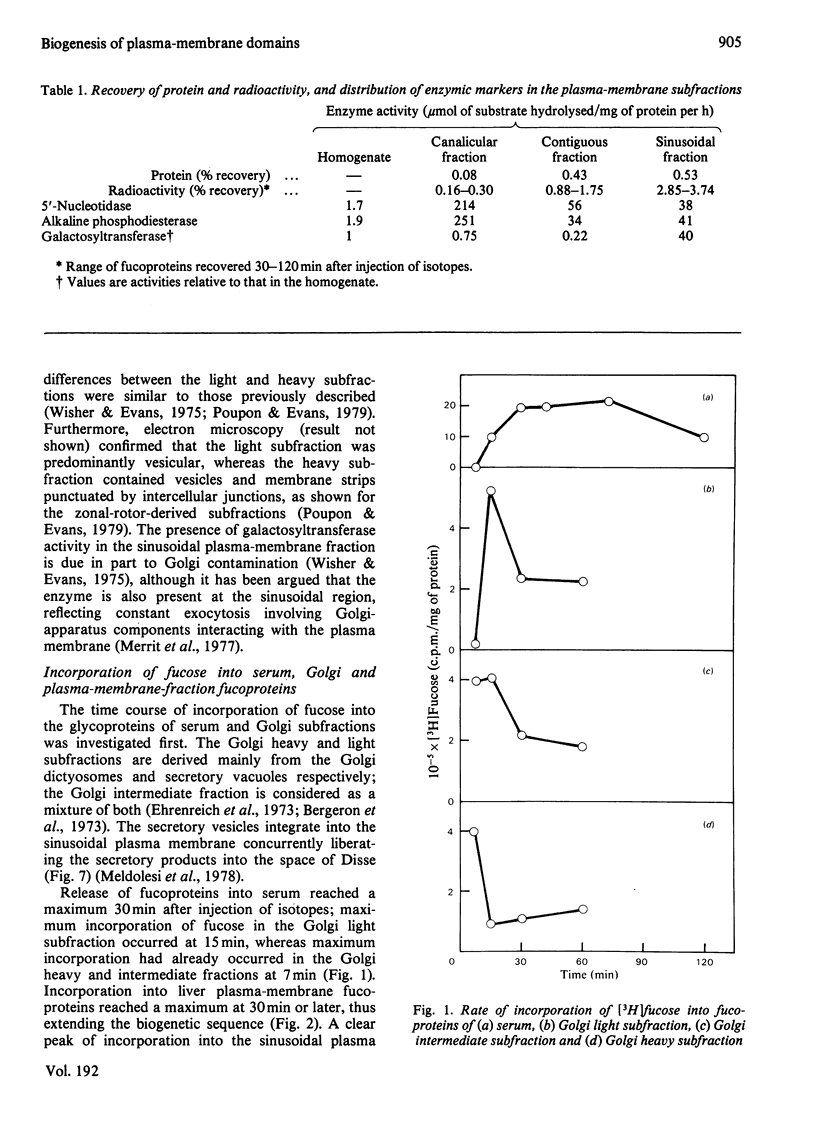
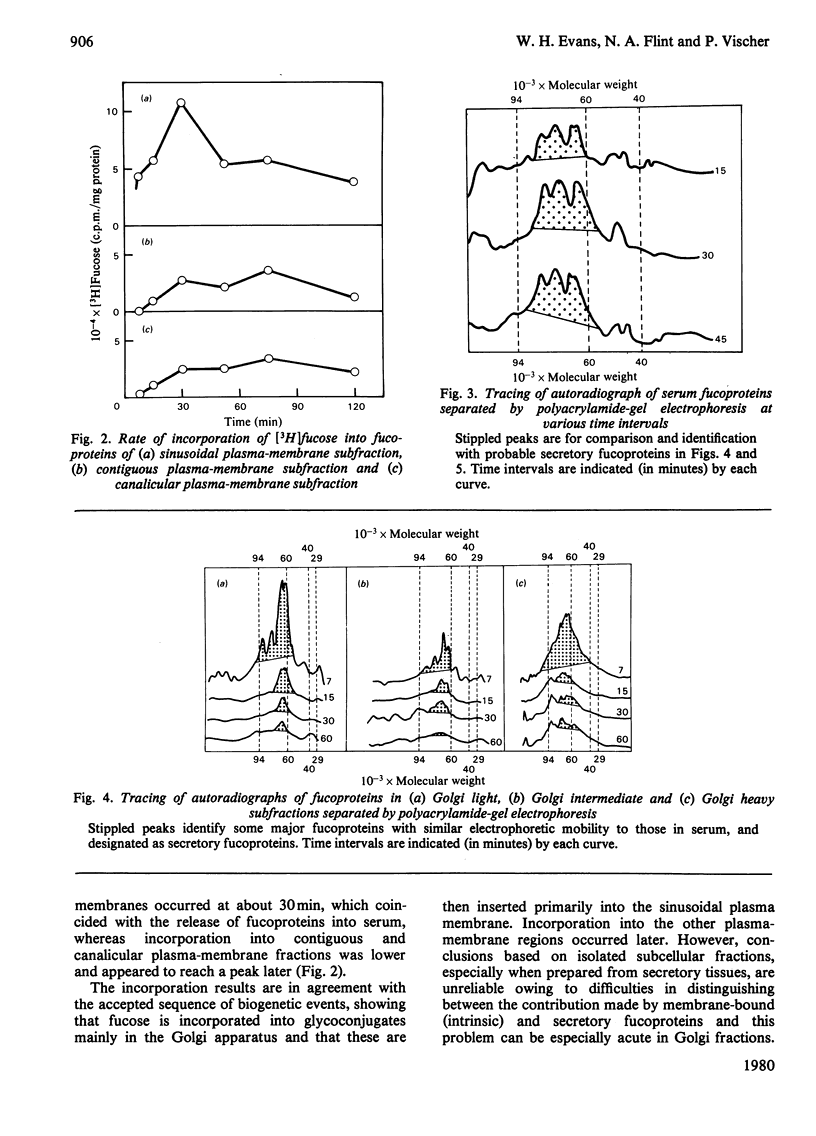
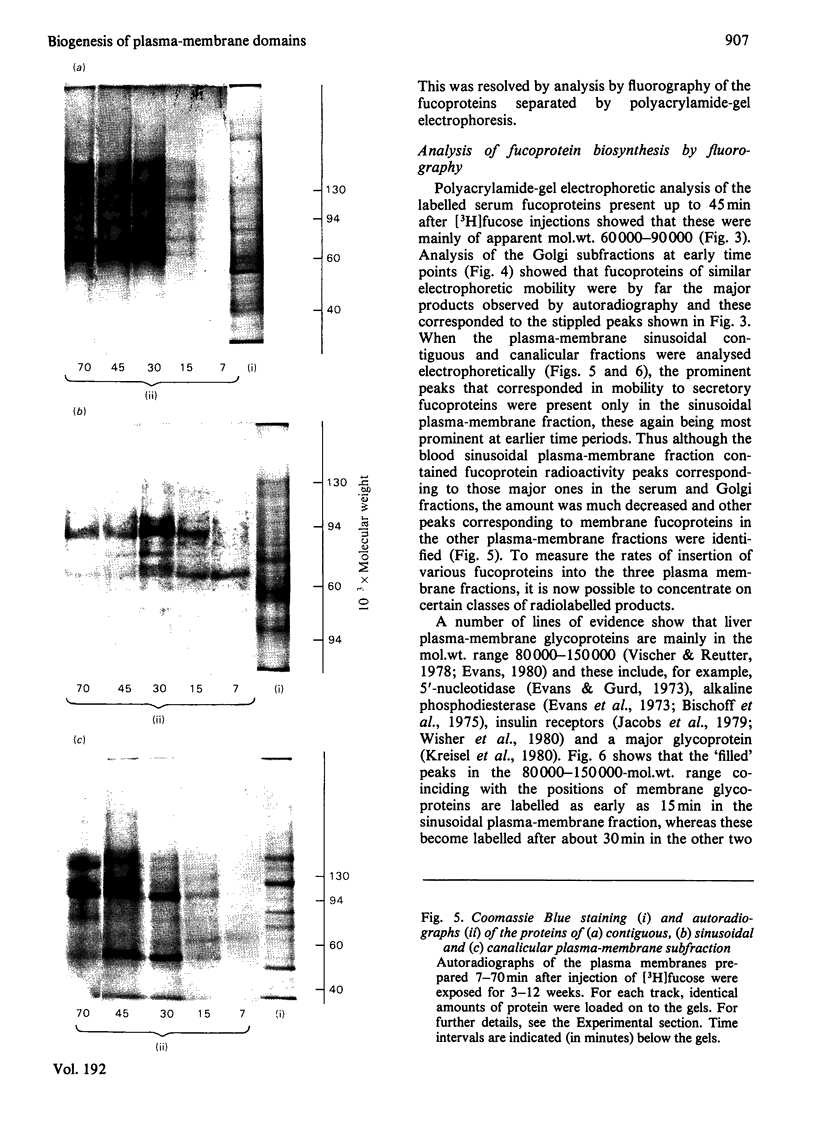
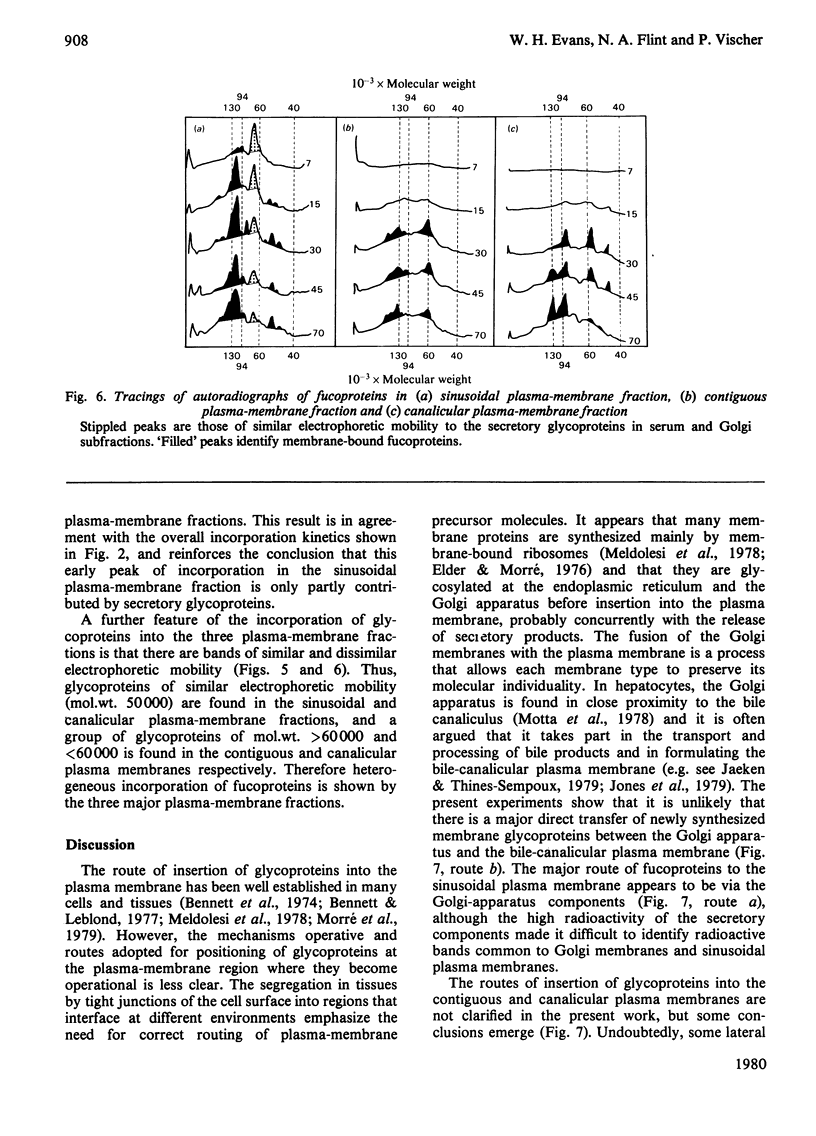
1. Rats were injected intracaudally with [3H]fucose and its rate of incorporation into the fucoproteins of serum, Golgi and plasma-membrane subfractions was followed for up tp 2h. 2. Incorporation into the Golgi dictyosome and secretory-vesicular fractions reached a maximum at 15 min or less, but most of the radioactivity was associated with classes of secretory glycoproteins. Incorporation into sinusoidal plasma-membrane fractions reached a maximum at 30 min, coinciding with the maximum release of fucoproteins into the serum. Contiguous and canalicular plasma-membrane fractions were labelled slightly later and at a lower rate and specific radioactivity. 3. Fluorography of fucoproteins separated by polyacrylamide-gel electrophoresis helped to distinguish between the major secretory and membrane-bound glycoproteins. The results show that a major biogenetic sequence is probably from Golgi dictyosomes to Golgi secretory elements to a sinusoidal plasma membrane. 4. The kinetics of incorporation make it unlikely that there is rapid and direct insertion of glycoproteins into the bile-canalicular plasma membrane. A route involving direct transfer of glycoproteins via a membrane-mediated intracellular path from the blood sinusoidal to the bile-canalicular plasma membranes is proposed.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bennett G., Leblond C. P. Biosynthesis of the glycoproteins present in plasma membrane, lysosomes and secretory materials, as visualized by radioautography. Histochem J. 1977 Jul;9(4):393–417. doi: 10.1007/BF01002973. [DOI] [PubMed] [Google Scholar]
- Bennett G., Leblond C. P., Haddad A. Migration of glycoprotein from the Golgi apparatus to the surface of various cell types as shown by radioautography after labelled fucose injection into rats. J Cell Biol. 1974 Jan;60(1):258–284. doi: 10.1083/jcb.60.1.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergeron J. J., Ehrenreich J. H., Siekevitz P., Palade G. E. Golgi fractions prepared from rat liver homogenates. II. Biochemical characterization. J Cell Biol. 1973 Oct;59(1):73–88. doi: 10.1083/jcb.59.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergeron J. J. Golgi fractions from livers of control and ethanol-intoxicated rats. Enzymic and morphologic properties following rapid isolation. Biochim Biophys Acta. 1979 Aug 23;555(3):493–503. doi: 10.1016/0005-2736(79)90402-4. [DOI] [PubMed] [Google Scholar]
- Bischoff E., Tran-Thi T. A., Decker K. F. Nucleotide pyrophosphatase of rat liver. A comparative study on the enzymes solubilized and purified from plasma membrane and endoplasmic reticulum. Eur J Biochem. 1975 Feb 21;51(2):353–361. doi: 10.1111/j.1432-1033.1975.tb03935.x. [DOI] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Büchsel R., Berger D., Reutter W. Routes of fucoproteins in plasma membrane domains. FEBS Lett. 1980 Apr 21;113(1):95–98. doi: 10.1016/0014-5793(80)80503-5. [DOI] [PubMed] [Google Scholar]
- Carey F., Evans W. H. Identification of blood-sinusoidal plasma-membrane fractions from rat liver homogenates by radioiodinated-ligand binding. Biochem Soc Trans. 1977;5(1):103–104. doi: 10.1042/bst0050103. [DOI] [PubMed] [Google Scholar]
- Castle J. D., Jamieson J. D., Palade G. E. Secretion granules of the rabbit parotid gland. Isolation, subfractionation, and characterization of the membrane and content subfractions. J Cell Biol. 1975 Jan;64(1):182–210. doi: 10.1083/jcb.64.1.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle D., Hou E., Warren R. Transfer of the hepatocyte receptor for serum asialo-glycoproteins to the plasma membrane of a fibroblast. Acquisition of the hepatocyte receptor functions by mouse L-cells. J Biol Chem. 1979 Aug 10;254(15):6853–6856. [PubMed] [Google Scholar]
- Ehrenreich J. H., Bergeron J. J., Siekevitz P., Palade G. E. Golgi fractions prepared from rat liver homogenates. I. Isolation procedure and morphological characterization. J Cell Biol. 1973 Oct;59(1):45–72. doi: 10.1083/jcb.59.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elder J. H., Morré D. J. Synthesis in vitro of intrinsic membrane proteins by free, membrane-bound, and Golgi apparatus-associated polyribosomes from rat liver. J Biol Chem. 1976 Aug 25;251(16):5054–5068. [PubMed] [Google Scholar]
- Evans W. H. A biochemical dissection of the functional polarity of the plasma membrane of the hepatocyte. Biochim Biophys Acta. 1980 May 27;604(1):27–64. doi: 10.1016/0005-2736(80)90584-2. [DOI] [PubMed] [Google Scholar]
- Evans W. H. Fractionation of liver plasma membranes prepared by zonal centrifugation. Biochem J. 1970 Mar;116(5):833–842. doi: 10.1042/bj1160833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans W. H., Gurd J. W. Properties of a 5'-nucleotidase purified from mouse liver plasma membranes. Biochem J. 1973 May;133(1):189–199. doi: 10.1042/bj1330189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans W. H., Hood D. O., Gurd J. W. Purification and properties of a mouse liver plasma-membrane glycoprotein hydrolysing nucleotide pyrophosphate and phosphodiester bonds. Biochem J. 1973 Dec;135(4):819–826. doi: 10.1042/bj1350819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAMPTON J. C. An electron microscope study of the hepatic uptake and excretion of submicroscopic particles injected into the blood stream and into the bile duct. Acta Anat (Basel) 1958;32(3):262–291. doi: 10.1159/000141328. [DOI] [PubMed] [Google Scholar]
- Jacobs S., Hazum E., Shechter Y., Cuatrecasas P. Insulin receptor: covalent labeling and identification of subunits. Proc Natl Acad Sci U S A. 1979 Oct;76(10):4918–4921. doi: 10.1073/pnas.76.10.4918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaeken L., Thines-Sempoux D. A three-dimensional study of organelle interrelationships in regenerating rat liver. 3. Organelles related to bile. Cell Biol Int Rep. 1979 Aug;3(5):453–462. doi: 10.1016/0309-1651(79)90007-9. [DOI] [PubMed] [Google Scholar]
- Jones A. L., Schmucker D. L., Mooney J. S., Ockner R. K., Adler R. D. Alterations in hepatic pericanalicular cytoplasm during enhanced bile secretory activity. Lab Invest. 1979 Apr;40(4):512–517. [PubMed] [Google Scholar]
- Kreisel W., Volk B. A., Büchsel R., Reutter W. Different half-lives of the carbohydrate and protein moieties of a 110,000-dalton glycoprotein isolated from plasma membranes of rat liver. Proc Natl Acad Sci U S A. 1980 Apr;77(4):1828–1831. doi: 10.1073/pnas.77.4.1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Merritt W. D., Morre D. J., Franke W. W., Keenan T. W. Glycosyltransferases with endogenous acceptor activity in plasma membranes isolated from rat liver. Biochim Biophys Acta. 1977 May 26;497(3):820–824. doi: 10.1016/0304-4165(77)90305-1. [DOI] [PubMed] [Google Scholar]
- Morré D. J., Kartenbeck J., Franke W. W. Membrane flow and intercoversions among endomembranes. Biochim Biophys Acta. 1979 Apr 23;559(1):71–52. doi: 10.1016/0304-4157(79)90008-x. [DOI] [PubMed] [Google Scholar]
- Mullock B. M., Hinton R. H., Dobrota M., Peppard J., Orlans E. Endocytic vesicles in liver carry polymeric IgA from serum to bile. Biochim Biophys Acta. 1979 Oct 18;587(3):381–391. doi: 10.1016/0304-4165(79)90442-2. [DOI] [PubMed] [Google Scholar]
- Neville D. M., Jr Isolation of an organ specific protein antigen from cell-surface membrane of rat liver. Biochim Biophys Acta. 1968 Apr 9;154(3):540–552. doi: 10.1016/0005-2795(68)90014-7. [DOI] [PubMed] [Google Scholar]
- Orlans E., Peppard J., Reynolds J., Hall J. Rapid active transport of immunoglobulin A from blood to bile. J Exp Med. 1978 Feb 1;147(2):588–592. doi: 10.1084/jem.147.2.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascaud A., Auliac P., Pascaud M. Renouvellement du cholestérol libre endogène des sinusoïdes de la membrane plasmique hépatique. Biochimie. 1979;61(9):1065–1071. [PubMed] [Google Scholar]
- Pisam M., Ripoche P. Redistribution of surface macromolecules in dissociated epithelial cells. J Cell Biol. 1976 Dec;71(3):907–920. doi: 10.1083/jcb.71.3.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poupon R. E., Evans W. H. Biochemical evidence that Na+,K+-ATPase is located at the lateral region of the hepatocyte surface membrane. FEBS Lett. 1979 Dec 15;108(2):374–378. doi: 10.1016/0014-5793(79)80567-0. [DOI] [PubMed] [Google Scholar]
- Socken D. J., Jeejeebhoy K. N., Bazin H., Underdown B. J. Identification of secretory component as an IgA receptor on rat hepatocytes. J Exp Med. 1979 Dec 1;150(6):1538–1548. doi: 10.1084/jem.150.6.1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vischer P., Reutter W. Specific alterations of fucoprotein biosynthesis in the plasma membrane of Morris hepatoma 7777. Eur J Biochem. 1978 Mar 15;84(2):363–368. doi: 10.1111/j.1432-1033.1978.tb12176.x. [DOI] [PubMed] [Google Scholar]
- Wisher M. H., Baron M. D., Jones R. H., Sönksen P. H. Photoreactive insulin analogues used to characterise the insulin receptor. Biochem Biophys Res Commun. 1980 Jan 29;92(2):492–498. doi: 10.1016/0006-291x(80)90360-5. [DOI] [PubMed] [Google Scholar]
- Wisher M. H., Evans W. H. Functional polarity of the rat hepatocyte surface membrane. Isolation and characterization of plasma-membrane subfractions from the blood-sinusoidal, bile-Canalicular and contiguous surfaces of the hepatocyte. Biochem J. 1975 Feb;146(2):375–388. doi: 10.1042/bj1460375. [DOI] [PMC free article] [PubMed] [Google Scholar]



