Abstract
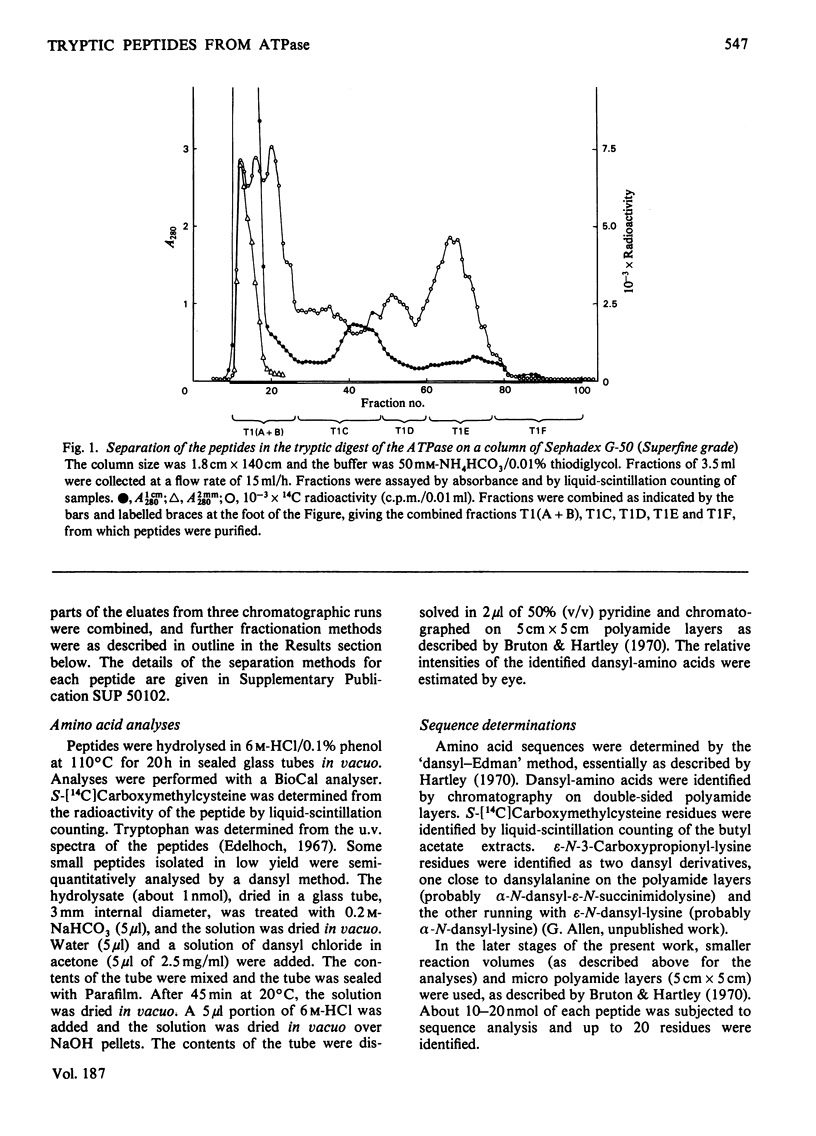
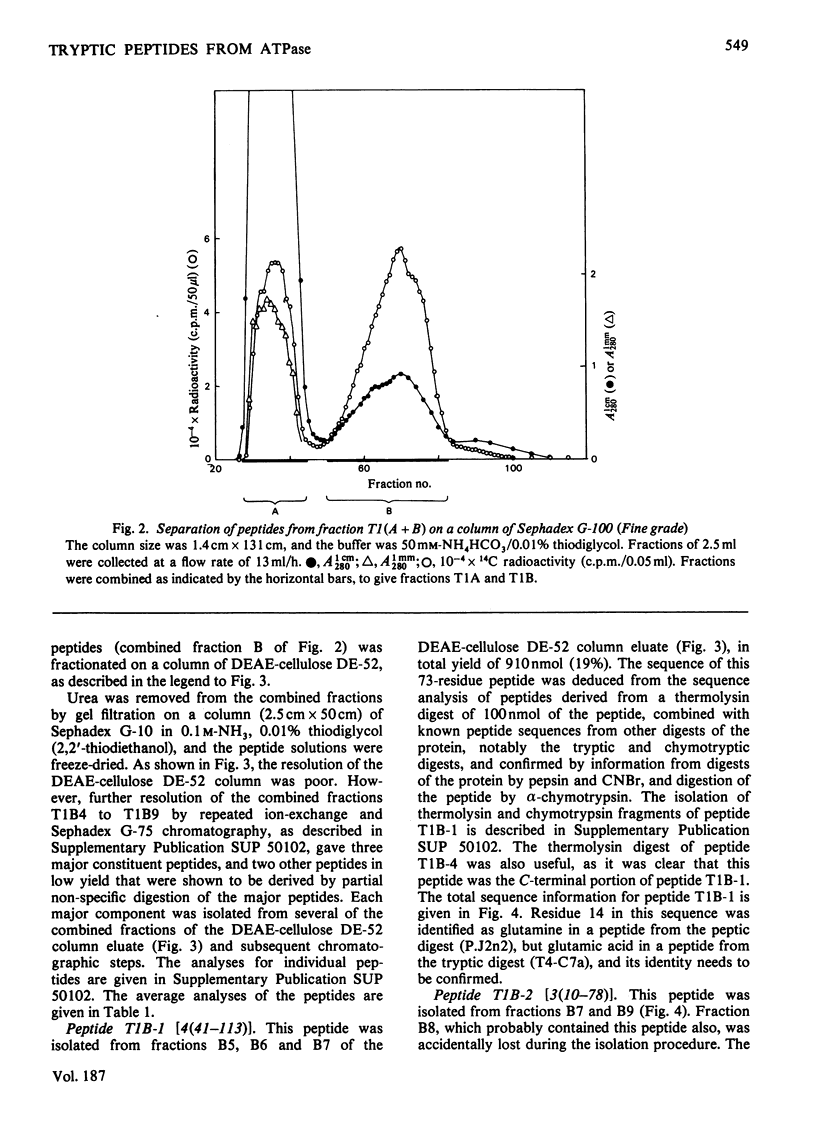
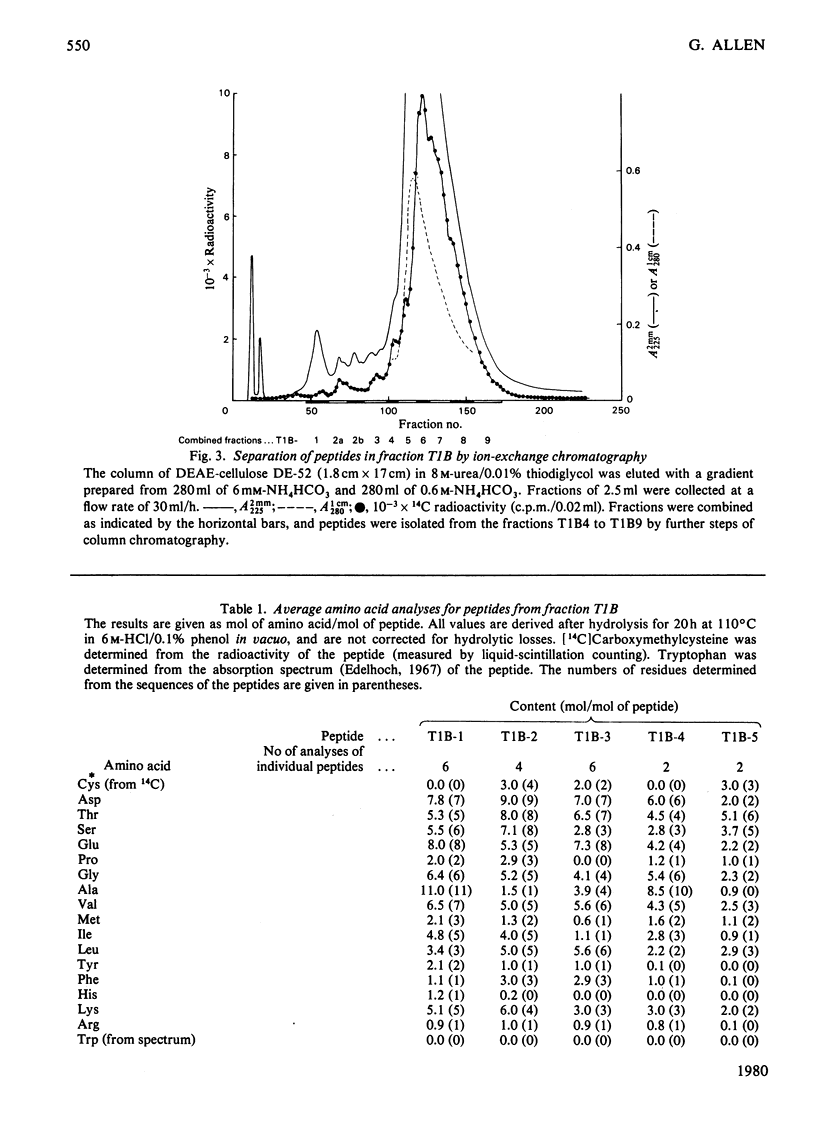
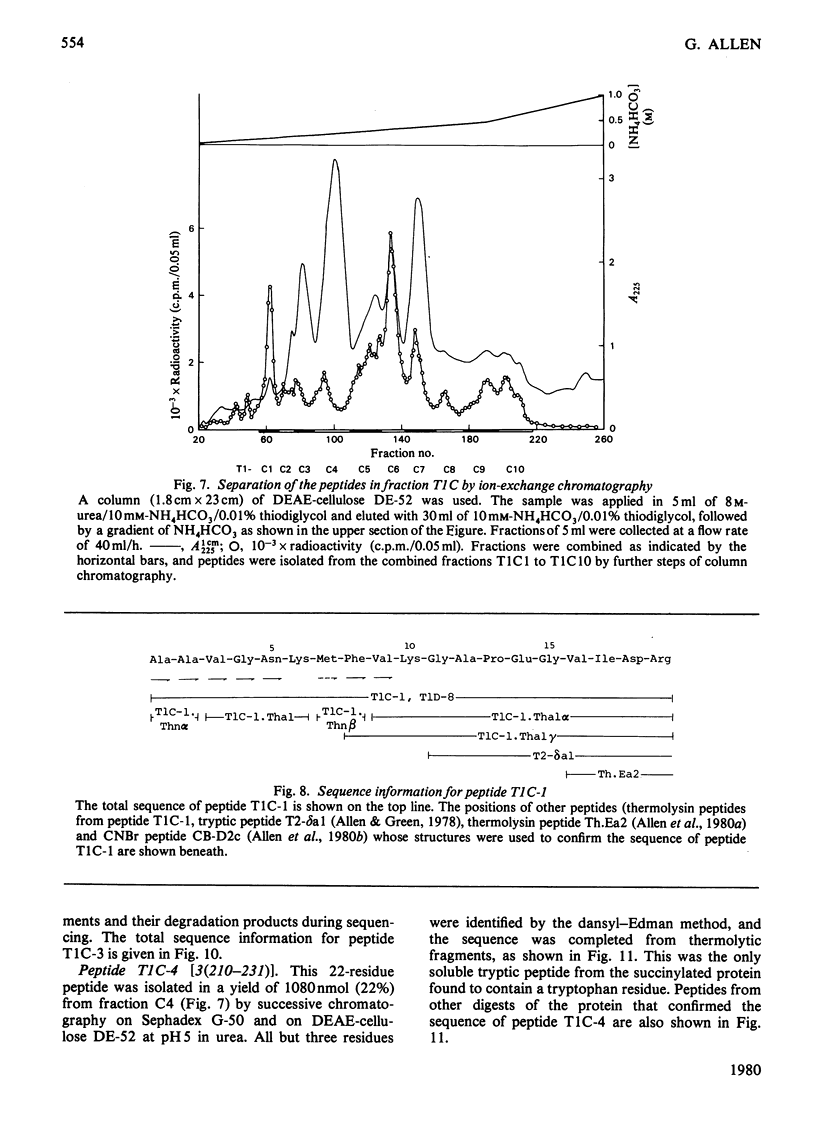
The isolation and the determination of the amino-acid sequences of the soluble tryptic peptides, derived by cleavage at arginine residues, of the succinylated (3-carboxypropionylated) S-carboxymethylated adenosine triphosphatase protein of rabbit skeletal sarcoplasmic reticulum are described. Treatment of the protein with succinic anhydride gave a derivative that was readily digested with trypsin, yielding two distinct sets of peptides. One set comprises large, relatively hydrophobic, peptides that are highly aggregated (or insoluble) in aqueous solution and that have been identified, by several criteria, with the portion of the protein embedded in the lipid bilayer in the sarcoplasmic reticulum. The second set, which is described here, comprises peptides that have properties typical of those derived from soluble globular proteins and that constitute that part of the protein external to the lipid bilayer. The sequences of these soluble tryptic peptides contain 586 unique residues. Details of the isolation of the peptides and the determination of the sequences are contained in Supplementary Publication SUP 50102 (88 pages) which has been deposited with the British Library Lending Division, Boston Spa, Wetherby, West Yorkshire LS23 7BQ, U.K., from whom copies can be obtained on the terms indicated in Biochem. J. (1978) 169, 5.
Full text
PDF











Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen G., Bottomley R. C., Trinnaman B. J. Primary structure of the calcium ion-transporting adenosine triphosphatase from rabbit skeletal sarcoplasmic reticulum. Some peptic, thermolytic, tryptic and staphylococcal-proteinase peptides. Biochem J. 1980 Jun 1;187(3):577–589. doi: 10.1042/bj1870577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen G., Green N. M. A 31-residue tryptic peptide from the active site of the [Ca++]-transporting adenosine triphosphatase of rabbit sarcoplasmic reticulum. FEBS Lett. 1976 Mar 15;63(1):188–192. doi: 10.1016/0014-5793(76)80223-2. [DOI] [PubMed] [Google Scholar]
- Allen G., Green N. M. Primary structures of cysteine-containing peptides from the calcium ion-transporting adenosine triphosphatase of rabbit sarcoplasmic reticulum. Biochem J. 1978 Aug 1;173(2):393–402. doi: 10.1042/bj1730393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen G. Primary structure of the calcium ion-transporting adenosine triphosphatase of rabbit skeletal sarcoplasmic reticulum. Soluble peptides from the alpha-chymotryptic digest of the carboxymethylated protein. Biochem J. 1980 Jun 1;187(3):565–575. doi: 10.1042/bj1870565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen G., Trinnaman B. J., Green N. M. The primary structure of the calcium ion-transporting adenosine triphosphatase protein of rabbit skeletal sarcoplasmic reticulum. Peptides derived from digestion with cyanogen bromide, and the sequences of three long extramembranous segments. Biochem J. 1980 Jun 1;187(3):591–616. doi: 10.1042/bj1870591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridgen J., Walker I. D. Photoreceptor protein from the purple membrane of Halobacterium halobium. Molecular weight and retinal binding site. Biochemistry. 1976 Feb 24;15(4):792–798. doi: 10.1021/bi00649a010. [DOI] [PubMed] [Google Scholar]
- Bruton C. J., Hartley B. S. Chemical studies on methionyl-tRNA synthetase from Escherichia coli. J Mol Biol. 1970 Sep 14;52(2):165–178. doi: 10.1016/0022-2836(70)90023-9. [DOI] [PubMed] [Google Scholar]
- Edelhoch H. Spectroscopic determination of tryptophan and tyrosine in proteins. Biochemistry. 1967 Jul;6(7):1948–1954. doi: 10.1021/bi00859a010. [DOI] [PubMed] [Google Scholar]
- Fowler A. V., Zabin I. The amino acid sequence of beta-galactosidase of Escherichia coli. Proc Natl Acad Sci U S A. 1977 Apr;74(4):1507–1510. doi: 10.1073/pnas.74.4.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HEILMANN J., BARROLLIER J., WATZKE E. Beitrag zur Aminosäurebestimmung auf Papierchromatogrammen. Hoppe Seylers Z Physiol Chem. 1957;309(4-6):219–220. [PubMed] [Google Scholar]
- HIRS C. H., MOORE S., STEIN W. H. Peptides obtained by tryptic hydrolysis of performic acid-oxidized ribonuclease. J Biol Chem. 1956 Apr;219(2):623–642. [PubMed] [Google Scholar]
- Hardwicke M. D. The binding of lipid to the lipid-free adenosine triphosphatase protein of sarcoplasmic reticulum. Eur J Biochem. 1976 Mar 1;62(3):431–438. doi: 10.1111/j.1432-1033.1976.tb10176.x. [DOI] [PubMed] [Google Scholar]
- Hardwicke P. M., Green N. M. The effect of delipidation on the adenosine triphosphatase of sarcoplasmic reticulum. Electron microscopy and physical properties. Eur J Biochem. 1974 Feb 15;42(1):183–193. doi: 10.1111/j.1432-1033.1974.tb03328.x. [DOI] [PubMed] [Google Scholar]
- Hartley B. S. Strategy and tactics in protein chemistry. Biochem J. 1970 Oct;119(5):805–822. doi: 10.1042/bj1190805f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- INGRAM V. M. A specific chemical difference between the globins of normal human and sickle-cell anaemia haemoglobin. Nature. 1956 Oct 13;178(4537):792–794. doi: 10.1038/178792a0. [DOI] [PubMed] [Google Scholar]
- KOSTKA V., CARPENTER F. H. INHIBITION OF CHYMOTRYPSIN ACTIVITY IN CRYSTALLINE TRYPSIN PREPARATIONS. J Biol Chem. 1964 Jun;239:1799–1803. [PubMed] [Google Scholar]
- Keefer L. M., Bradshaw R. A. Structural studies on Halobacterium halobium bacteriorhodopsin. Fed Proc. 1977 May;36(6):1799–1804. [PubMed] [Google Scholar]
- Louis C., Shooter E. M. The proteins of rabbit skeletal muscle sarcoplasmic reticulum. Arch Biochem Biophys. 1972 Dec;153(2):641–655. doi: 10.1016/0003-9861(72)90383-9. [DOI] [PubMed] [Google Scholar]
- MacLennan D. H. Isolation of proteins of the sarcoplasmic reticulum. Methods Enzymol. 1974;32:291–302. doi: 10.1016/0076-6879(74)32030-7. [DOI] [PubMed] [Google Scholar]
- MacLennan D. H. Purification and properties of an adenosine triphosphatase from sarcoplasmic reticulum. J Biol Chem. 1970 Sep 10;245(17):4508–4518. [PubMed] [Google Scholar]
- MacLennan D. H., Seeman P., Iles G. H., Yip C. C. Membrane formation by the adenosine triphosphatase of sarcoplasmic reticulum. J Biol Chem. 1971 Apr 25;246(8):2702–2710. [PubMed] [Google Scholar]
- Martonosi A., Halpin R. A. Sarcoplasmic reticulum. X. The protein composition of sarcoplasmic reticulum membranes. Arch Biochem Biophys. 1971 May;144(1):66–77. doi: 10.1016/0003-9861(71)90455-3. [DOI] [PubMed] [Google Scholar]
- Meissner G., Conner G. E., Fleischer S. Isolation of sarcoplasmic reticulum by zonal centrifugation and purification of Ca 2+ -pump and Ca 2+ -binding proteins. Biochim Biophys Acta. 1973 Mar 16;298(2):246–269. doi: 10.1016/0005-2736(73)90355-6. [DOI] [PubMed] [Google Scholar]
- Murphy A. J. Sulfhydryl group modification of sarcoplasmic reticulum membranes. Biochemistry. 1976 Oct 5;15(20):4492–4496. doi: 10.1021/bi00665a025. [DOI] [PubMed] [Google Scholar]
- Offord R. E. Electrophoretic mobilities of peptides on paper and their use in the determination of amide groups. Nature. 1966 Aug 6;211(5049):591–593. doi: 10.1038/211591a0. [DOI] [PubMed] [Google Scholar]
- Ovchinnikov Y. A., Abdulaev N. G., Feigina M. Y., Kiselev A. V., Lobanov N. A. Recent findings in the structure-functional characteristics of bacteriorhodopsin. FEBS Lett. 1977 Dec 1;84(1):1–4. doi: 10.1016/0014-5793(77)81046-6. [DOI] [PubMed] [Google Scholar]
- Ozols J., Gerard C. Primary structure of the membranous segment of cytochrome b5. Proc Natl Acad Sci U S A. 1977 Sep;74(9):3725–3729. doi: 10.1073/pnas.74.9.3725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzolo L. J., Maire M., Reynolds J. A., Tanford C. Molecular weights and hydrophobicity of the polypeptide chain of sarcoplasmic reticulum calcium(II) adenosine triphosphatase and of its primary tryptic fragments. Biochemistry. 1976 Aug 10;15(16):3433–3437. doi: 10.1021/bi00661a006. [DOI] [PubMed] [Google Scholar]
- SMITH I. Colour reactions on paper chromatograms by a dipping technique. Nature. 1953 Jan 3;171(4340):43–44. doi: 10.1038/171043a0. [DOI] [PubMed] [Google Scholar]
- Segrest J. P., Jackson R. L., Marchesi V. T., Guyer R. B., Terry W. Red cell membrane glycoprotein: amino acid sequence of an intramembranous region. Biochem Biophys Res Commun. 1972 Nov 15;49(4):964–969. doi: 10.1016/0006-291x(72)90306-3. [DOI] [PubMed] [Google Scholar]
- Thorley-Lawson D. A., Green N. M. Separation and characterisation of tryptic fragments from the adenosine triphosphatase of sarcoplasmic reticulum. Eur J Biochem. 1975 Nov 1;59(1):193–200. doi: 10.1111/j.1432-1033.1975.tb02441.x. [DOI] [PubMed] [Google Scholar]
- Thorley-Lawson D. A., Green N. M. Studies on the location and orientation of proteins in the sarcoplasmic reticulum. Eur J Biochem. 1973 Dec 17;40(2):403–413. doi: 10.1111/j.1432-1033.1973.tb03209.x. [DOI] [PubMed] [Google Scholar]
- Thorley-Lawson D. A., Green N. M. The reactivity of the thiol groups of the adenosine triphosphatase of sarcoplasmic reticulum and their location on tryptic fragments of the molecule. Biochem J. 1977 Dec 1;167(3):739–748. doi: 10.1042/bj1670739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong S. W. The acetylated NH2-terminus of CaATPase from rabbit skeletal muscle sarcoplasmic reticulum: a commom NH2-terminal acetylated methionyl sequence. Biochem Biophys Res Commun. 1977 Feb 7;74(3):1242–1248. doi: 10.1016/0006-291x(77)91651-5. [DOI] [PubMed] [Google Scholar]
- WALEY S. G., WATSON J. The action of trypsin on polylysine. Biochem J. 1953 Sep;55(2):328–337. doi: 10.1042/bj0550328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K., Kuter D. J. Reversible denaturation of enzymes by sodium dodecyl sulfate. J Biol Chem. 1971 Jul 25;246(14):4504–4509. [PubMed] [Google Scholar]
- le Maire M., Jorgensen K. E., Roigaard-Petersen H., Moller J. V. Properties of deoxycholate solubilized sarcoplasmic reticulum Ca2+-ATPase. Biochemistry. 1976 Dec 28;15(26):5805–5812. doi: 10.1021/bi00671a018. [DOI] [PubMed] [Google Scholar]


