Abstract
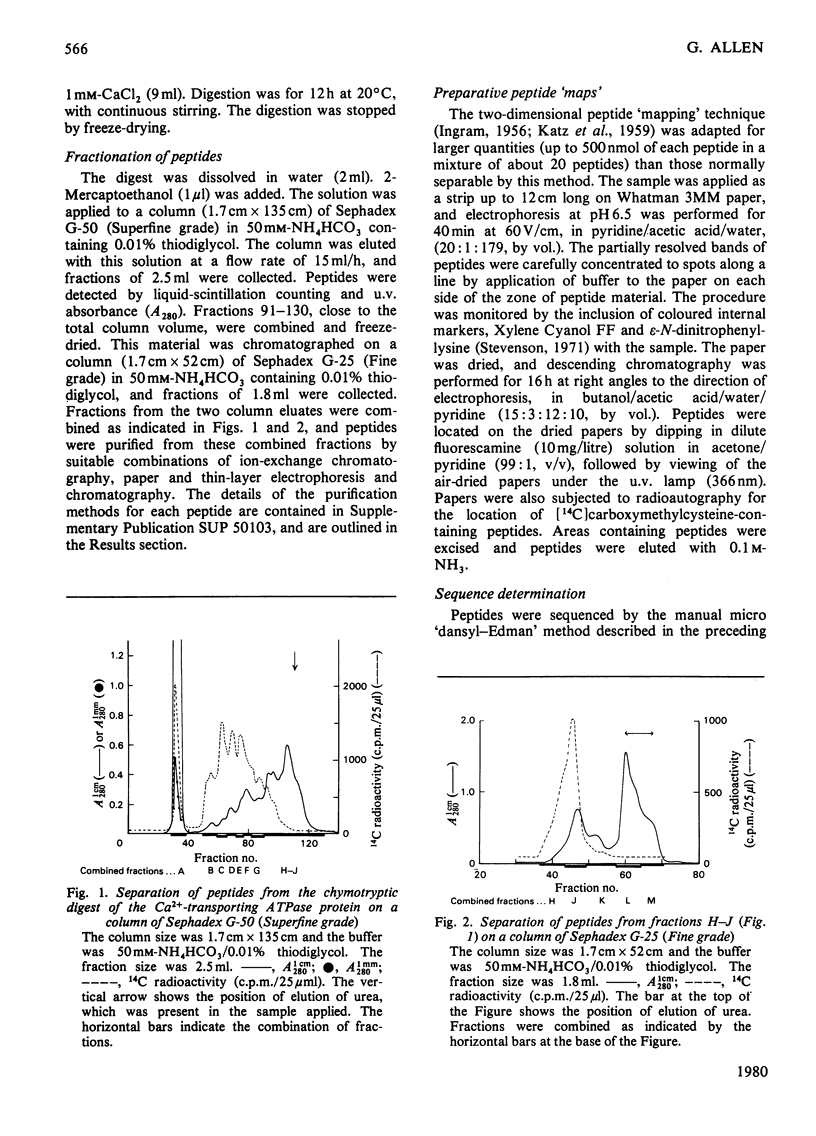
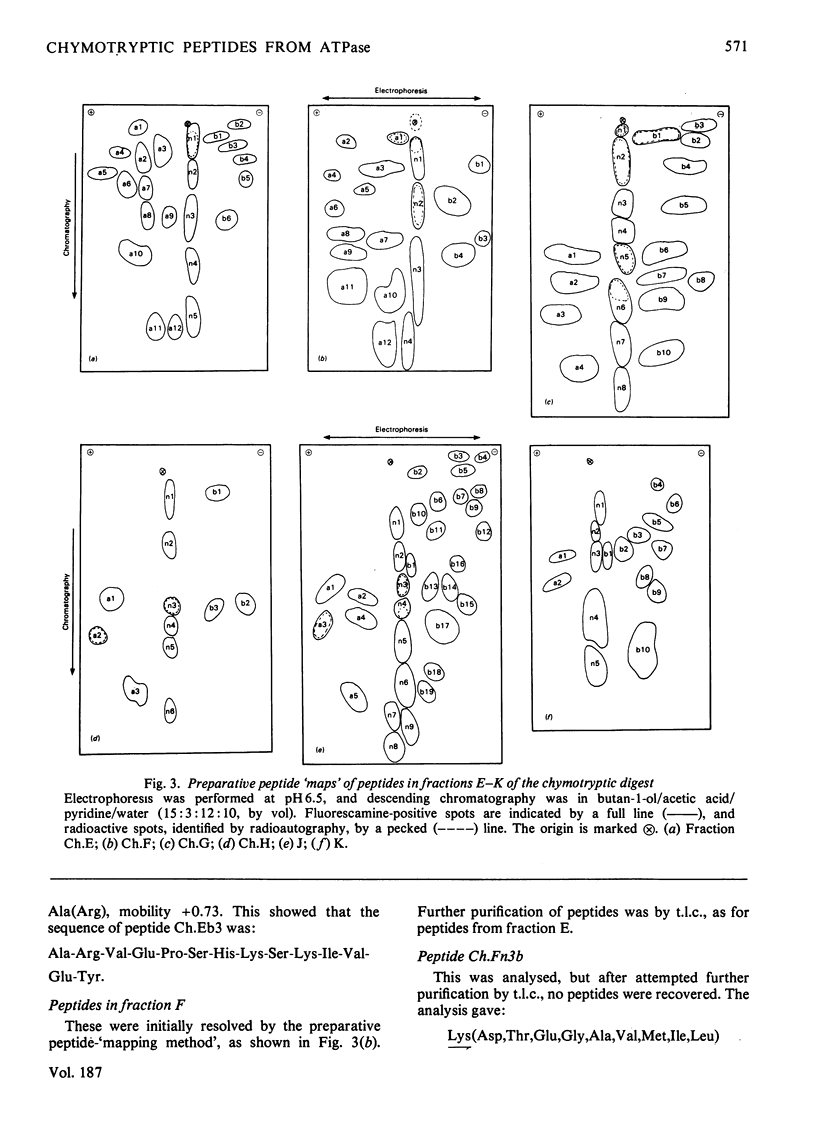
The isolation of the soluble peptides from the chymotryptic digest of the calcium-transporting ATPase of rabbit skeletal sarcoplasmic reticulum is described. These peptides were partially sequenced and the information obtained was used to align tryptic peptides of the protein and to confirm sequences within the tryptic peptides. Details of the isolation of some peptides and the amino acid analyses of the peptides are given in Supplementary Publication SUP 50103 (10 pages), which has been deposited with the British Library Lending Division, Boston Spa, Wetherby, West Yorkshire LS23 7BQ, U.K., from whom copies can be obtained on the terms indicated in Biochem. J. (1978) 169, 5.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen G., Green N. M. A 31-residue tryptic peptide from the active site of the [Ca++]-transporting adenosine triphosphatase of rabbit sarcoplasmic reticulum. FEBS Lett. 1976 Mar 15;63(1):188–192. doi: 10.1016/0014-5793(76)80223-2. [DOI] [PubMed] [Google Scholar]
- Allen G., Green N. M. Primary structures of cysteine-containing peptides from the calcium ion-transporting adenosine triphosphatase of rabbit sarcoplasmic reticulum. Biochem J. 1978 Aug 1;173(2):393–402. doi: 10.1042/bj1730393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen G. The primary structure of the calcium-transporting adenosine triphosphatase of rabbit skeletal sarcoplasmic reticulum. Soluble tryptic peptides from the succinylated carboxymethylated protein. Biochem J. 1980 Jun 1;187(3):545–563. doi: 10.1042/bj1870545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen G., Trinnaman B. J., Green N. M. The primary structure of the calcium ion-transporting adenosine triphosphatase protein of rabbit skeletal sarcoplasmic reticulum. Peptides derived from digestion with cyanogen bromide, and the sequences of three long extramembranous segments. Biochem J. 1980 Jun 1;187(3):591–616. doi: 10.1042/bj1870591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- INGRAM V. M. A specific chemical difference between the globins of normal human and sickle-cell anaemia haemoglobin. Nature. 1956 Oct 13;178(4537):792–794. doi: 10.1038/178792a0. [DOI] [PubMed] [Google Scholar]
- KATZ A. M., DREYER W. J., ANFINSEN C. B. Peptide separation by two-dimensional chromatography and electrophoresis. J Biol Chem. 1959 Nov;234:2897–2900. [PubMed] [Google Scholar]
- Offord R. E. Electrophoretic mobilities of peptides on paper and their use in the determination of amide groups. Nature. 1966 Aug 6;211(5049):591–593. doi: 10.1038/211591a0. [DOI] [PubMed] [Google Scholar]
- Thorley-Lawson D. A., Green N. M. Studies on the location and orientation of proteins in the sarcoplasmic reticulum. Eur J Biochem. 1973 Dec 17;40(2):403–413. doi: 10.1111/j.1432-1033.1973.tb03209.x. [DOI] [PubMed] [Google Scholar]


