Abstract
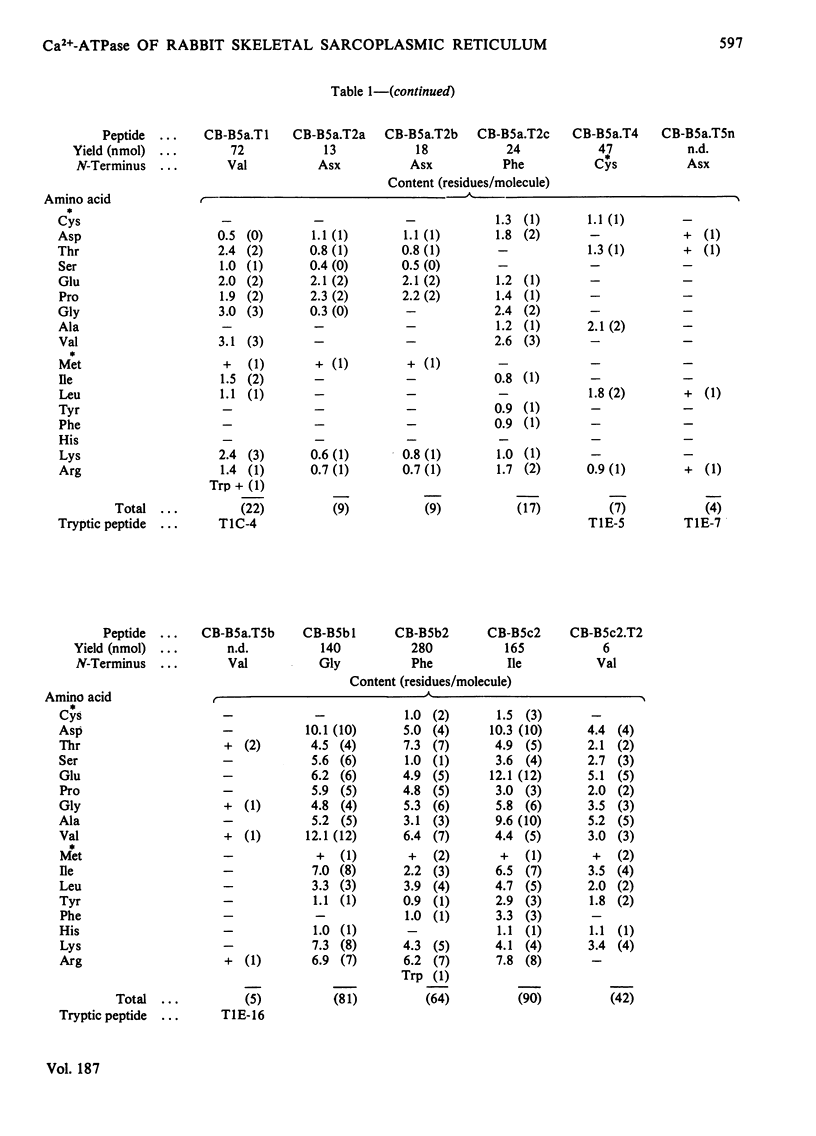
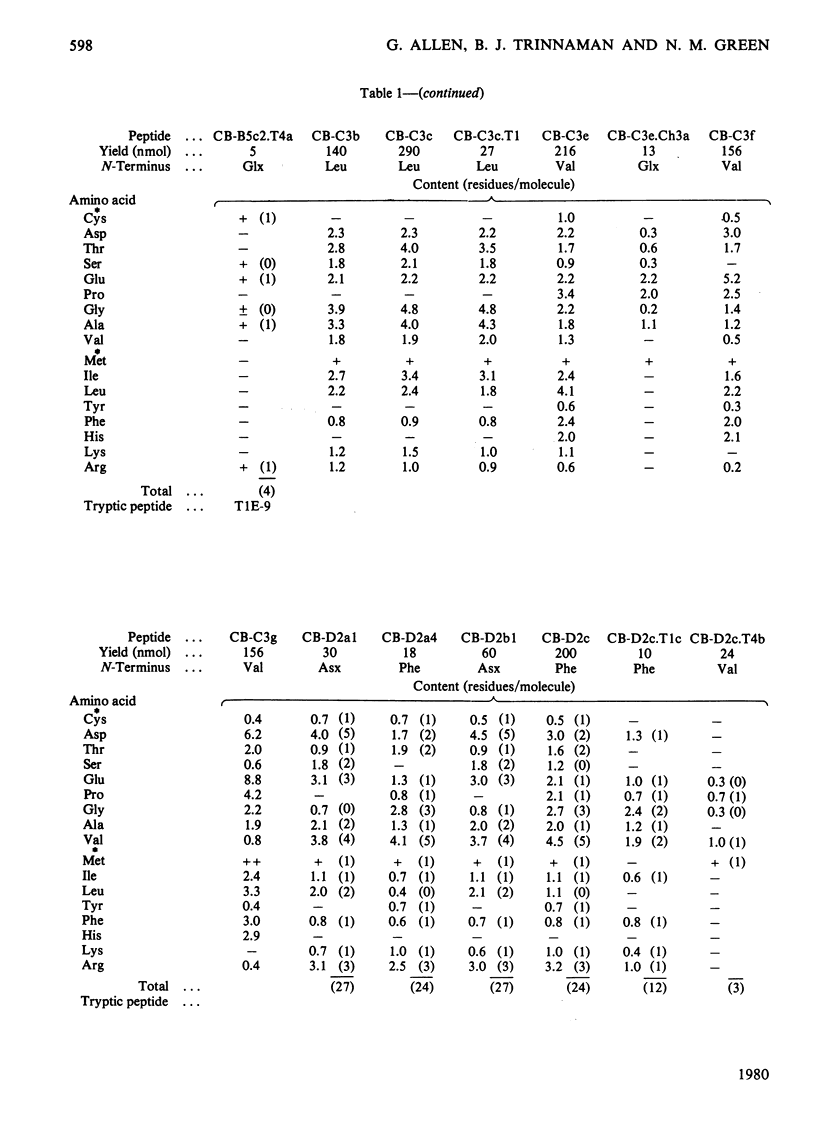
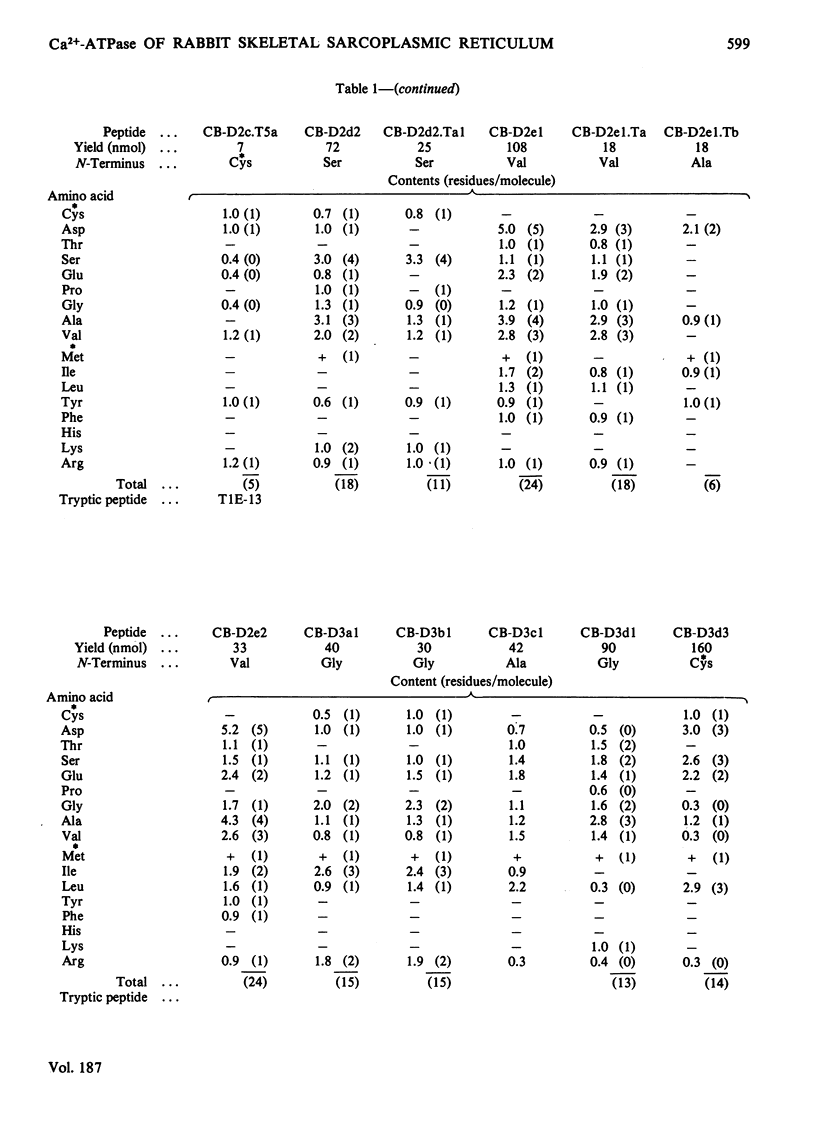
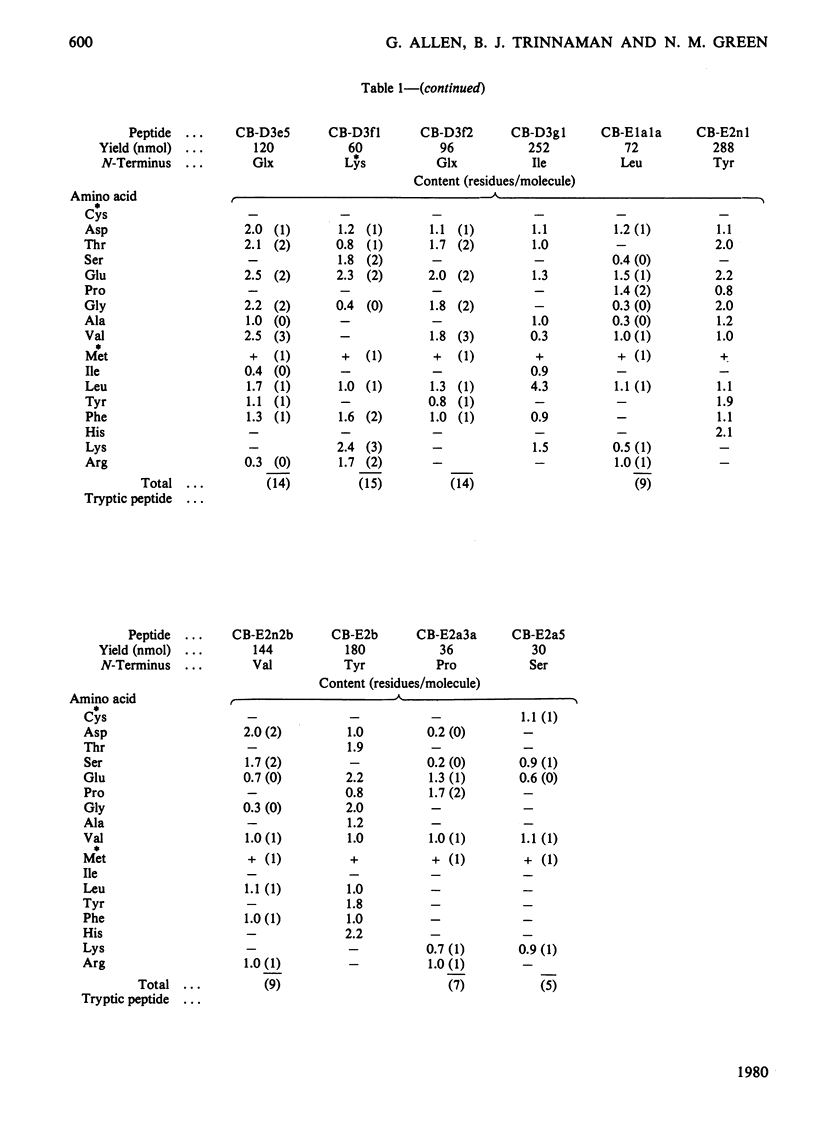
The isolation and characterization of the soluble peptides from the CNBr digest of the calcium ion-transporting adenosine triphosphatase protein of rabbit skeletal sarcoplasmic reticulum are described. The 562 unique residues of the protein were placed in sequences. The remaining part of the protein (about 500 residues) yielded long hydrophobic sequences that contained all but one of the tryptophan residues of the protein and that were probably derived largely from the intramembranous parts of the protein. Three long stretches of primary structure, constituting half of the protein, have been reconstructed from the information presented here together with the sequences found in peptides from other digests of the protein. The secondary structures of these sequences have been predicted. A model for the primary structure of the protein is presented and the implications discussed. Details of the isolation of peptides are contained in Supplementary Publication, SUP 50105 (29 pages), which has been deposited with the British Library Lending Division, Boston Spa, Wetherby, West Yorkshire LS23 7BQ, U.K., from whom copies can be obtained on the terms indicated in Biochem. J. (1978) 169, 5.
Full text
PDF














Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen G., Bottomley R. C., Trinnaman B. J. Primary structure of the calcium ion-transporting adenosine triphosphatase from rabbit skeletal sarcoplasmic reticulum. Some peptic, thermolytic, tryptic and staphylococcal-proteinase peptides. Biochem J. 1980 Jun 1;187(3):577–589. doi: 10.1042/bj1870577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen G., Green N. M. A 31-residue tryptic peptide from the active site of the [Ca++]-transporting adenosine triphosphatase of rabbit sarcoplasmic reticulum. FEBS Lett. 1976 Mar 15;63(1):188–192. doi: 10.1016/0014-5793(76)80223-2. [DOI] [PubMed] [Google Scholar]
- Allen G., Green N. M. Primary structures of cysteine-containing peptides from the calcium ion-transporting adenosine triphosphatase of rabbit sarcoplasmic reticulum. Biochem J. 1978 Aug 1;173(2):393–402. doi: 10.1042/bj1730393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen G. Primary structure of the calcium ion-transporting adenosine triphosphatase of rabbit skeletal sarcoplasmic reticulum. Soluble peptides from the alpha-chymotryptic digest of the carboxymethylated protein. Biochem J. 1980 Jun 1;187(3):565–575. doi: 10.1042/bj1870565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capaldi R. A., Vanderkooi G. The low polarity of many membrane proteins. Proc Natl Acad Sci U S A. 1972 Apr;69(4):930–932. doi: 10.1073/pnas.69.4.930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardwicke P. M., Green N. M. The effect of delipidation on the adenosine triphosphatase of sarcoplasmic reticulum. Electron microscopy and physical properties. Eur J Biochem. 1974 Feb 15;42(1):183–193. doi: 10.1111/j.1432-1033.1974.tb03328.x. [DOI] [PubMed] [Google Scholar]
- KOSTKA V., CARPENTER F. H. INHIBITION OF CHYMOTRYPSIN ACTIVITY IN CRYSTALLINE TRYPSIN PREPARATIONS. J Biol Chem. 1964 Jun;239:1799–1803. [PubMed] [Google Scholar]
- Kretsinger R. H. Calcium-binding proteins. Annu Rev Biochem. 1976;45:239–266. doi: 10.1146/annurev.bi.45.070176.001323. [DOI] [PubMed] [Google Scholar]
- Levine M., Muirhead H., Stammers D. K., Stuart D. I. Structure of pyruvate kinase and similarities with other enzymes: possible implications for protein taxonomy and evolution. Nature. 1978 Feb 16;271(5646):626–630. doi: 10.1038/271626a0. [DOI] [PubMed] [Google Scholar]
- MacLennan D. H., Seeman P., Iles G. H., Yip C. C. Membrane formation by the adenosine triphosphatase of sarcoplasmic reticulum. J Biol Chem. 1971 Apr 25;246(8):2702–2710. [PubMed] [Google Scholar]
- Martonosi A., Halpin R. A. Sarcoplasmic reticulum. X. The protein composition of sarcoplasmic reticulum membranes. Arch Biochem Biophys. 1971 May;144(1):66–77. doi: 10.1016/0003-9861(71)90455-3. [DOI] [PubMed] [Google Scholar]
- Meissner G., Conner G. E., Fleischer S. Isolation of sarcoplasmic reticulum by zonal centrifugation and purification of Ca 2+ -pump and Ca 2+ -binding proteins. Biochim Biophys Acta. 1973 Mar 16;298(2):246–269. doi: 10.1016/0005-2736(73)90355-6. [DOI] [PubMed] [Google Scholar]
- Migala A., Agostini B., Hasselbach W. Tryptic fragmentation of the calcium transport system in the sarcoplasmic reticulum. Z Naturforsch C. 1973 Mar-Apr;28(3):178–182. doi: 10.1515/znc-1973-3-414. [DOI] [PubMed] [Google Scholar]
- Murphy A. J. Sulfhydryl group modification of sarcoplasmic reticulum membranes. Biochemistry. 1976 Oct 5;15(20):4492–4496. doi: 10.1021/bi00665a025. [DOI] [PubMed] [Google Scholar]
- Offord R. E. Electrophoretic mobilities of peptides on paper and their use in the determination of amide groups. Nature. 1966 Aug 6;211(5049):591–593. doi: 10.1038/211591a0. [DOI] [PubMed] [Google Scholar]
- Ozols J., Gerard C. Covalent structure of the membranous segment of horse cytochrome b5. Chemical cleavage of the native hemoprotein. J Biol Chem. 1977 Dec 10;252(23):8549–8553. [PubMed] [Google Scholar]
- Piszkiewicz D., Landon M., Smith E. L. Anomalous cleavage of aspartyl-proline peptide bonds during amino acid sequence determinations. Biochem Biophys Res Commun. 1970 Sep 10;40(5):1173–1178. doi: 10.1016/0006-291x(70)90918-6. [DOI] [PubMed] [Google Scholar]
- Sarzala M. G., Michalak M. Studies on the heterogeneity of sarcoplasmic reticulum vesicles. Biochim Biophys Acta. 1978 Nov 2;513(2):221–235. doi: 10.1016/0005-2736(78)90175-x. [DOI] [PubMed] [Google Scholar]
- Stewart P. S., MacLennan D. H. Isolation and characterization of tryptic fragments of the adenosine triphosphatase of sarcoplasmic reticulum. J Biol Chem. 1976 Feb 10;251(3):712–719. [PubMed] [Google Scholar]
- Stewart P. S., MacLennan D. H. Surface particles of sarcoplasmic reticulum membranes. Structural features of the adenosine triphosphatase. J Biol Chem. 1974 Feb 10;249(3):985–993. [PubMed] [Google Scholar]
- Thorley-Lawson D. A., Green N. M. Separation and characterisation of tryptic fragments from the adenosine triphosphatase of sarcoplasmic reticulum. Eur J Biochem. 1975 Nov 1;59(1):193–200. doi: 10.1111/j.1432-1033.1975.tb02441.x. [DOI] [PubMed] [Google Scholar]
- Thorley-Lawson D. A., Green N. M. Studies on the location and orientation of proteins in the sarcoplasmic reticulum. Eur J Biochem. 1973 Dec 17;40(2):403–413. doi: 10.1111/j.1432-1033.1973.tb03209.x. [DOI] [PubMed] [Google Scholar]
- Thorley-Lawson D. A., Green N. M. The reactivity of the thiol groups of the adenosine triphosphatase of sarcoplasmic reticulum and their location on tryptic fragments of the molecule. Biochem J. 1977 Dec 1;167(3):739–748. doi: 10.1042/bj1670739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong S. W. The acetylated NH2-terminus of CaATPase from rabbit skeletal muscle sarcoplasmic reticulum: a commom NH2-terminal acetylated methionyl sequence. Biochem Biophys Res Commun. 1977 Feb 7;74(3):1242–1248. doi: 10.1016/0006-291x(77)91651-5. [DOI] [PubMed] [Google Scholar]


