Abstract
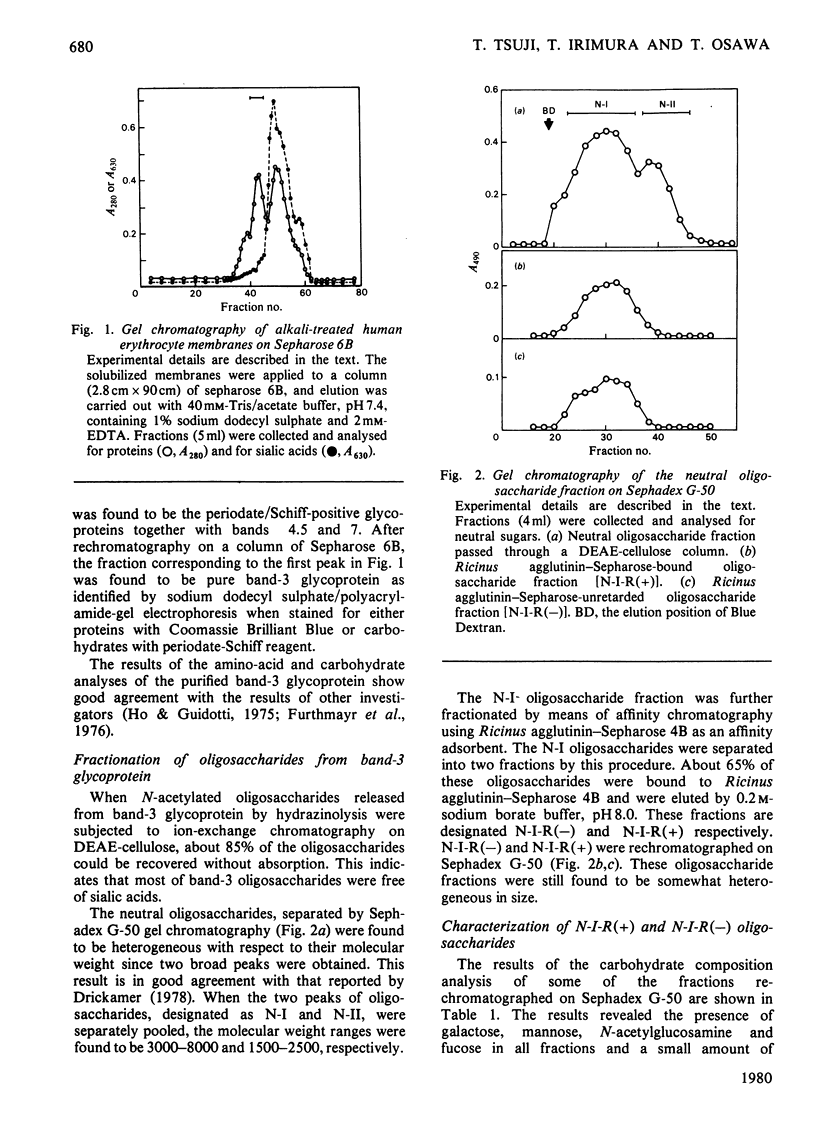
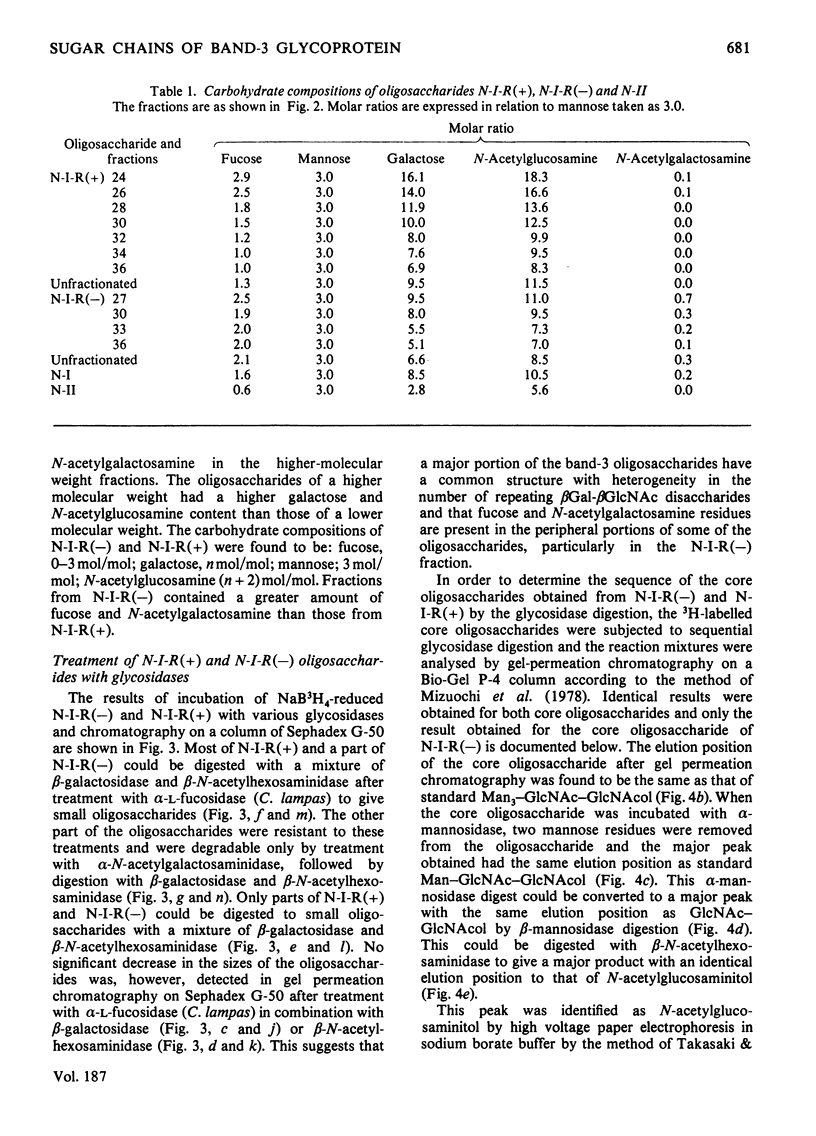
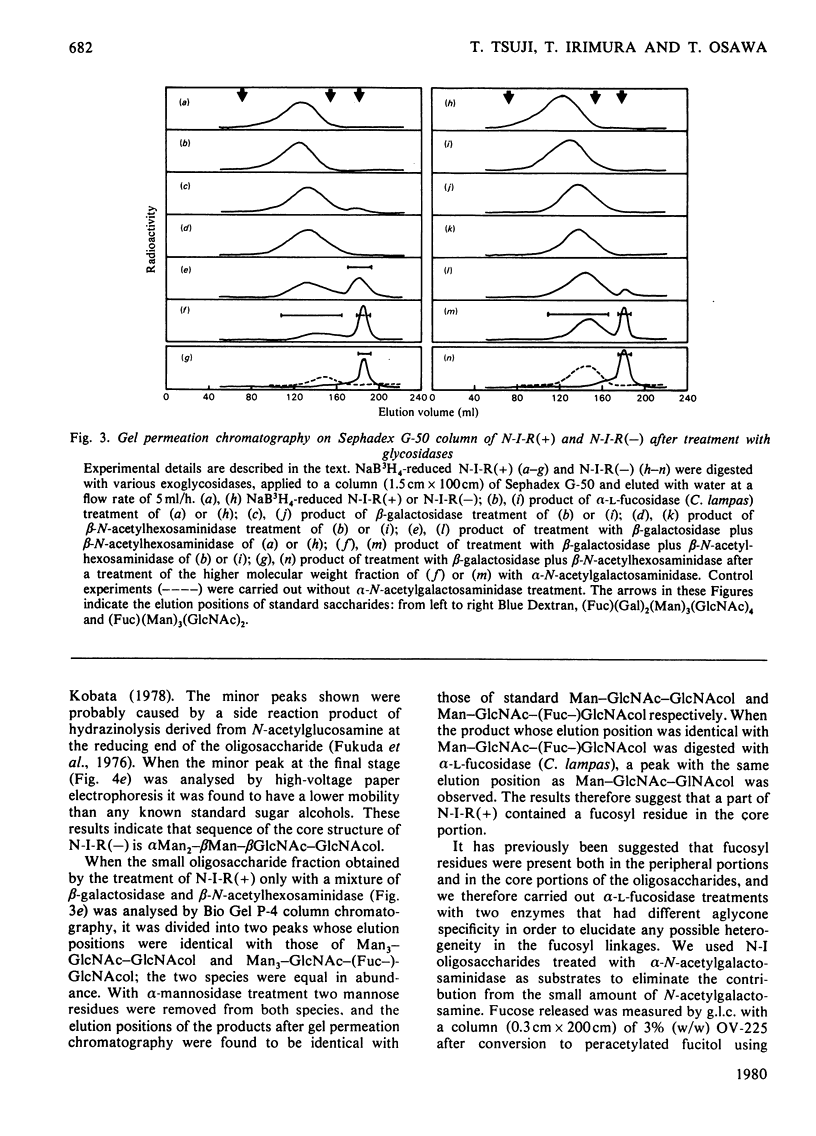
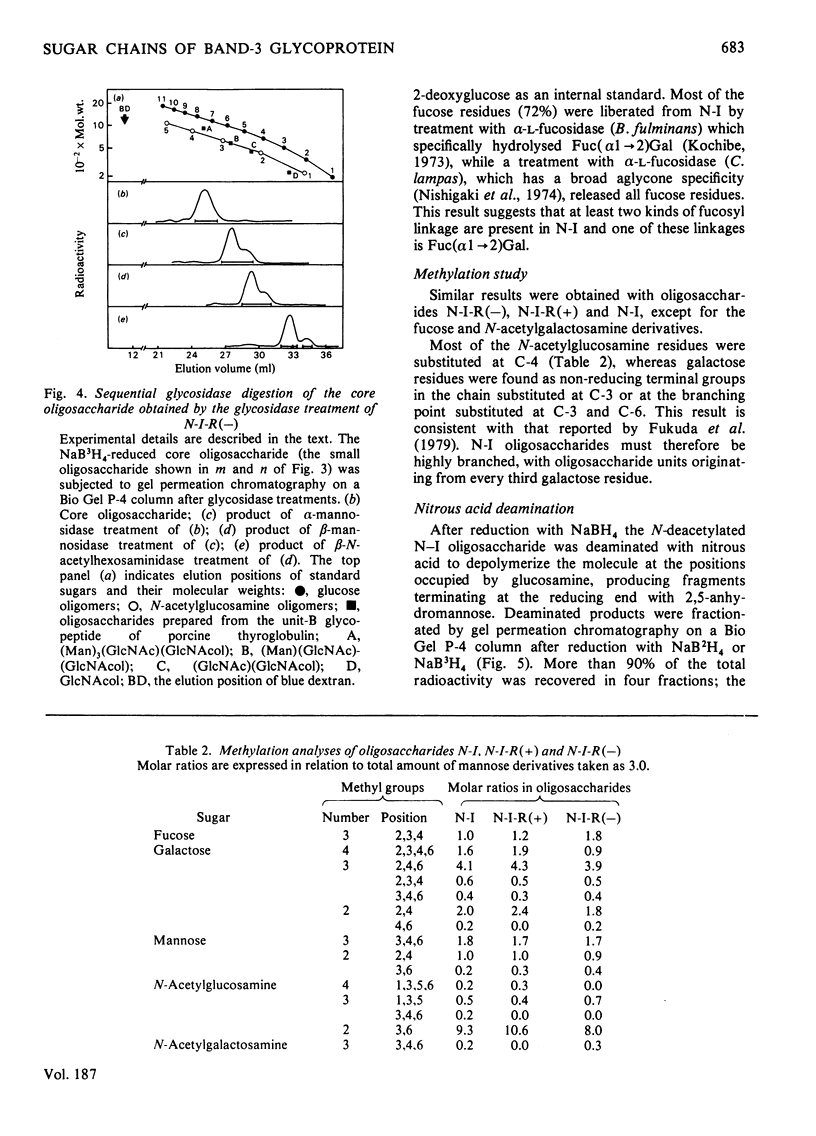
Band-3 glycoprotein was purified from human blood-group-A erythrocyte membranes by selective solubilization and gel chromatography on Sepharose 6B in the presence of sodium dodecyl sulphate. The purified glycoprotein was subjected to hydrazinolysis in order to release the carbohydrate moiety. The released oligosaccharides were N-acetylated and applied to a column of DEAE-cellulose. Most of the band-3 oligosaccharides obtained were found to be free of sialic acids. When this neutral fraction was subjected to gel chromatography on a column of Sephadex G-50, two broad peaks were observed indicating that the band-3 glycoprotein was heterogeneous in the size of the oligosaccharide moieties. All fractions from gel chromatography were found to contain galactose, mannose, N-acetylglucosamine and fucose. The higher-molecular-weight (mol.wt. 3000-8000) peak consisted of fucose, mannose, galactose, N-acetylglucosamine and N-acetylgalactosamine in a molar proportion of 1.6:3.0:8.4:10.5:0.2. Most of these oligosaccharides were digested with a mixture of beta-galactosidase and beta-N-acetylhexosaminidase after alpha-L-fucosidase treatment to give a small oligosaccharide with the structure alpha Man2-beta Man-beta GlcNAc-GlcNAc. Methylation studies and limited degradation by nitrous acid deamination showed that the oligosaccharides contained the repeating disaccharide Gal beta 1----4GlcNAc beta 1----3, with branching points at C-6 of some of the galactose residues. These results indicate that a major portion of the band-3 oligosaccharide has a common core structure, with heterogeneity in the numbers of the repeating disaccharides, and contains fucose residues both in the peripheral portion and in the core portion. Haemagglutination tests were also carried out to determine the blood-group specificities of the glycoprotein and the results demonstrated the presence of both blood-group-H and I antigenic activities.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bretscher M. S. A major protein which spans the human erythrocyte membrane. J Mol Biol. 1971 Jul 28;59(2):351–357. doi: 10.1016/0022-2836(71)90055-6. [DOI] [PubMed] [Google Scholar]
- Brown P. A., Feinstein M. B., Sha'afi R. I. Membrane proteins related to water transport in human erythrocytes. Nature. 1975 Apr 10;254(5500):523–525. doi: 10.1038/254523a0. [DOI] [PubMed] [Google Scholar]
- Cabantchik Z. I., Rothstein A. Membrane proteins related to anion permeability of human red blood cells. I. Localization of disulfonic stilbene binding sites in proteins involved in permeation. J Membr Biol. 1974;15(3):207–226. doi: 10.1007/BF01870088. [DOI] [PubMed] [Google Scholar]
- Childs R. A., Feizi T., Fukuda M., Hakomori S. I. Blood-group-I activity associated with band 3, the major intrinsic membrane protein of human erythrocytes. Biochem J. 1978 Jul 1;173(1):333–336. doi: 10.1042/bj1730333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drickamer L. K. Orientation of the band 3 polypeptide from human erythrocyte membranes. Identification of NH2-terminal sequence and site of carbohydrate attachment. J Biol Chem. 1978 Oct 25;253(20):7242–7248. [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Findlay J. B. The receptor proteins for concanavalin A and Lens culinaris phytohemagglutinin in the membrane of the human erythrocyte. J Biol Chem. 1974 Jul 25;249(14):4398–4403. [PubMed] [Google Scholar]
- Fukuda M., Fukuda M. N., Hakomori S. Developmental change and genetic defect in the carbohydrate structure of band 3 glycoprotein of human erythrocyte membrane. J Biol Chem. 1979 May 25;254(10):3700–3703. [PubMed] [Google Scholar]
- Fukuda M., Kondo T., Osawa T. Studies on the hydrazinolysis of glycoproteins. Core structures of oligosaccharides obtained from Porcine thyroglobulin and pineapple stem bromelain. J Biochem. 1976 Dec;80(6):1223–1232. doi: 10.1093/oxfordjournals.jbchem.a131393. [DOI] [PubMed] [Google Scholar]
- Furthmayr H., Kahane I., Marchesi V. T. Isolation of the major intrinsic transmembrane protein of the human erythrocyte membrane. J Membr Biol. 1976 Mar 18;26(2-3):173–187. doi: 10.1007/BF01868872. [DOI] [PubMed] [Google Scholar]
- Gahmberg C. G., Myllyla G., Leikola J., Pirkola A., Nordling S. Absence of the major sialoglycoprotein in the membrane of human En(a--) erythrocytes and increased glycosylation of band 3. J Biol Chem. 1976 Oct 10;251(19):6108–6116. [PubMed] [Google Scholar]
- Goodwin T. W., Morton R. A. The spectrophotometric determination of tyrosine and tryptophan in proteins. Biochem J. 1946;40(5-6):628–632. doi: 10.1042/bj0400628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAKOMORI S. A RAPID PERMETHYLATION OF GLYCOLIPID, AND POLYSACCHARIDE CATALYZED BY METHYLSULFINYL CARBANION IN DIMETHYL SULFOXIDE. J Biochem. 1964 Feb;55:205–208. [PubMed] [Google Scholar]
- Hase S., Matsushima Y. Amino sugar analysis by gas-liquid chromatography. J Biochem. 1969 Jul;66(1):57–62. doi: 10.1093/oxfordjournals.jbchem.a129120. [DOI] [PubMed] [Google Scholar]
- Ho M. K., Guidotti G. A membrane protein from human erythrocytes involved in anion exchange. J Biol Chem. 1975 Jan 25;250(2):675–683. [PubMed] [Google Scholar]
- Jenkins R. E., Tanner J. A. The major human erythrocyte membrane protein. Evidence for an S-shaped structure which traverses the membrane twice and contains a duplicated set of sites. Biochem J. 1975 Jun;147(3):393–399. doi: 10.1042/bj1470393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Järnefelt J., Rush J., Li Y. T., Laine R. A. Erythroglycan, a high molecular weight glycopeptide with the repeating structure [galactosyl-(1 leads to 4)-2-deoxy-2-acetamido-glucosyl(1 leads to 3)] comprising more than one-third of the protein-bound carbohydrate of human erythrocyte stroma. J Biol Chem. 1978 Nov 25;253(22):8006–8009. [PubMed] [Google Scholar]
- Kaifu R., Osawa T. Synthesis of O-beta-D-galactopyranosyl-(1 linked to 4)-O-(2acetamido-2-deoxy-beta-D-glucopyranosyl)-(1 linked to 2)-D-mannose and its interaction with various lectins. Carbohydr Res. 1976 Dec;52:179–185. doi: 10.1016/s0008-6215(00)85958-3. [DOI] [PubMed] [Google Scholar]
- Kennedy J. F., Rosevear A. An assessment of the fractionation of carbohydrates on concanavalin A-sepharose 4B by affinity chromatography. J Chem Soc Perkin 1. 1973;19:2041–2046. doi: 10.1039/p19730002041. [DOI] [PubMed] [Google Scholar]
- Kochibe N. Purification and properties of alpha-L-fucosidase from Bacillus fulminans. J Biochem. 1973 Dec;74(6):1141–1149. doi: 10.1093/oxfordjournals.jbchem.a130341. [DOI] [PubMed] [Google Scholar]
- Koeltzow D. E., Epley J. D., Conrad H. E. The lipopolysaccharides of Aerobacter aerogenes strains A3(S1) and NCTC 243. Biochemistry. 1968 Aug;7(8):2920–2928. doi: 10.1021/bi00848a032. [DOI] [PubMed] [Google Scholar]
- Krusius T., Finne J., Rauvala H. The poly(glycosyl) chains of glycoproteins. Characterisation of a novel type of glycoprotein saccharides from human erythrocyte membrane. Eur J Biochem. 1978 Dec 1;92(1):289–300. doi: 10.1111/j.1432-1033.1978.tb12747.x. [DOI] [PubMed] [Google Scholar]
- Matsumoto I., Osawa T. The specific purification of various carbohydrate-binding hemagglutinins. Biochem Biophys Res Commun. 1972 Mar 10;46(5):1810–1815. doi: 10.1016/0006-291x(72)90055-1. [DOI] [PubMed] [Google Scholar]
- Mizuochi T., Yonemasu K., Yamashita K., Kobata A. The asparagine-linked sugar chains of subcomponent C1q of the first component of human complement. J Biol Chem. 1978 Oct 25;253(20):7404–7409. [PubMed] [Google Scholar]
- Nishigaki M., Muramatsu T., Kobata A., Maeyama K. The broad aglycon specificity of alpha-l-fucosidase from marine gastropods. J Biochem. 1974 Mar;75(3):509–517. doi: 10.1093/oxfordjournals.jbchem.a130419. [DOI] [PubMed] [Google Scholar]
- Stellner K., Saito H., Hakomori S. I. Determination of aminosugar linkages in glycolipids by methylation. Aminosugar linkages of ceramide pentasaccharides of rabbit erythrocytes and of Forssman antigen. Arch Biochem Biophys. 1973 Apr;155(2):464–472. doi: 10.1016/0003-9861(73)90138-0. [DOI] [PubMed] [Google Scholar]
- Svensson S., Hammarström S. G., Kabat E. A. The effect of borate on polysaccharide-protein and antigen-antibody reactions and its use for the purification and fractionation of crossreacting antibodies. Immunochemistry. 1970 May;7(5):413–422. doi: 10.1016/0019-2791(70)90223-5. [DOI] [PubMed] [Google Scholar]
- Takasaki S., Kobata A. Microdetermination of sugar composition by radioisotope labeling. Methods Enzymol. 1978;50:50–54. doi: 10.1016/0076-6879(78)50006-2. [DOI] [PubMed] [Google Scholar]
- Tomita M., Kurokawa T., Onozaki K., Ichiki N., Osawa T., Ukita T. Purification of galactose-binding phytoagglutinins and phytotoxin by affinity column chromatography using sepharose. Experientia. 1972 Jan 15;28(1):84–85. doi: 10.1007/BF01928278. [DOI] [PubMed] [Google Scholar]
- Watanabe K., Hakomori S. I., Childs R. A., Feizi T. Characterization of a blood group I-active ganglioside. Structural requirements for I and i specificities. J Biol Chem. 1979 May 10;254(9):3221–3228. [PubMed] [Google Scholar]
- Yokoyama K., Terao T., Osawa T. Carbohydrate-binding specificity of pokeweed mitogens. Biochim Biophys Acta. 1978 Jan 18;538(2):384–396. doi: 10.1016/0304-4165(78)90366-5. [DOI] [PubMed] [Google Scholar]
- Yu J., Steck T. L. Isolation and characterization of band 3, the predominant polypeptide of the human erythrocyte membrane. J Biol Chem. 1975 Dec 10;250(23):9170–9175. [PubMed] [Google Scholar]


