Abstract
Spinal cord injuries have profound detrimental effects on individuals, regardless of whether they are caused by trauma or non-traumatic events. The compromised regeneration of the spinal cord is primarily attributed to damaged neurons, inhibitory molecules, dysfunctional immune response, and glial scarring. Unfortunately, currently, there are no effective treatments available that can fully repair the spinal cord and improve functional outcomes. Nevertheless, numerous pre-clinical approaches have been studied for spinal cord injury recovery, including using biomaterials, cells, drugs, or technological-based strategies. Combinatorial treatments, which target various aspects of spinal cord injury pathophysiology, have been extensively tested in the last decade. These approaches aim to synergistically enhance repair processes by addressing various obstacles faced during spinal cord regeneration. Thus, this review intends to provide scientists and clinicians with an overview of pre-clinical combinatorial approaches that have been developed toward the solution of spinal cord regeneration as well as update the current knowledge about spinal cord injury pathophysiology with an emphasis on the current clinical management.
Keywords: electric stimulation, neural tissue regeneration, neuroprotection, polytherapy, spinal cord injury
Introduction
Spinal cord injury (SCI) poses significant challenges with considerable socioeconomic implications. While current standards of care focus on surgical decompression and stabilization, the lack of effective treatments underscores the urgency of addressing this global health issue. With nearly 0.93 million new cases annually and approximately 27.04 million individuals affected worldwide (Alizadeh et al., 2019; Fan et al., 2022; Soendergaard et al., 2022). SCI is a matter of substantial concern. The majority of cases, over 90%, are traumatic in nature, with causes including vehicular accidents, falls, and sports injuries (Alizadeh et al., 2019). Clinical outcomes vary widely, from partial to complete sensory and/or motor function loss below the injury site, with cervical lesions often resulting in quadriplegia and thoracic lesions in paraplegia. Advances in medical interventions have improved survival rates, with tetraplegia and paraplegia patients showing high survival rates over decades (Alizadeh et al., 2019). However, SCI patients still face significant socioeconomic burdens, with lifetime costs estimated at $2.35 million per patient (Alizadeh et al., 2019). The purpose of this review is to summarize the research on combinatorial treatments for SCI, focusing on approaches that target multiple aspects of its pathophysiology. By addressing different barriers to spinal cord regeneration simultaneously, these treatments aim to boost repair processes synergistically.
Search Strategy
A review of the literature was conducted within the PubMed database in February 2023. The following terms were used: spinal cord injury AND combinatorial, spinal cord injury AND combined, spinal cord injury AND combination. Original research papers, published in English, mainly in the last five years, which had the keywords in the titles and/or abstracts were considered in the search. Both pre-clinical and clinical works were included. This search also retrieved a significant number of cancer-related works, which were excluded. The selected articles were categorized according to the type of intervention: Combination of Molecular Agents, Combination of Cells with Molecular Agents, Combination of Cells with Biomaterials, Combination of Biomaterials and Molecular Agents, Combination of Biomaterials, Cells and Molecular Agents, and Combination of Neuromodulation Technologies with Molecules or Cells. Two independent examiners conducted the search.
Pathophysiology of Spinal Cord Injury
An overview of primary injury
SCI typically results from vertebral fractures or dislocations, causing primary injury through mechanical forces impacting the spinal cord. Primary injury mechanisms include persistent compression, transient compression, distraction, and laceration or transection (Lima et al., 2022). These injuries damage ascending and descending pathways, disrupt the blood–spinal cord barrier, and trigger spinal shock, hypotension, and ischemia. Cervical injuries, the deadliest and most common, often lead to tetraplegia and can affect vital systems like respiration and cardiac function (Alizadeh et al., 2019; van Den Hauwe et al., 2020; Gee et al., 2022). Thoracic or lumbar injuries usually result in paraplegia and loss of bowel and bladder control, generally, the severity of primary injury determines the SCI outcome (Brouwers et al., 2020; Torregrossa et al., 2020).
Overview of secondary mechanisms of spinal cord injury
Secondary mechanisms of SCI encompass distinct phases, including acute, subacute, intermediate, and chronic stages. Following the initial insult, which triggers post-traumatic damage proportional to its severity, various pathophysiological changes occur. These include vascular alterations leading to hypoperfusion and ischemia, disruptions in ionic balance causing cell membrane depolarization, free radical-induced lipid peroxidation, glutamate excitotoxicity, a dysfunction inflammatory response, apoptosis of spinal cord cells, and the formation of the glial scar (Anjum et al., 2020; Lima et al., 2022). Then, in the chronic phase, which persists throughout the individual’s lifetime, the lesion stabilizes with cysts or cavities maturing. Wallerian degeneration of damaged axons continues, with the removal of severed axons and cell bodies taking years. These processes contribute to ongoing tissue damage and hinder functional recovery, highlighting the complexity of SCI pathology and the need for targeted therapeutic interventions to mitigate secondary injury cascades (Monteiro et al., 2020; Perrouin-Verbe et al., 2021; Alcántar-Garibay et al., 2022; Figure 1).
Figure 1.
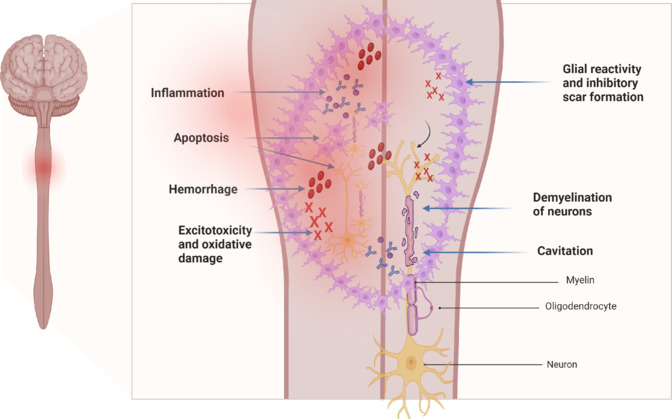
Representation of an injured spinal cord, with the respective pathophysiological events occurring after injury.
Created with BioRender.com.
Current Clinical Management
Current clinical management of SCI involves intensive care measures, early surgical decompression, and stabilization, followed by blood pressure augmentation to mitigate secondary injury. Studies have shown that early surgical intervention (< 24 hours post-injury) leads to better neurological outcomes, with patients undergoing early surgery demonstrating improved motor scores, additional neurological improvement, and shorter hospital stays compared to those undergoing delayed surgery (Badhiwala et al., 2021; Quddusi et al., 2023; Punjani et al., 2023). Recent research, including the Surgical Timing in Acute Spinal Cord Injury Study, supports the efficacy of early surgical intervention, with patients showing a greater likelihood of neurological improvement at 6-month follow-up (Sacino and Rosenblatt, 2019; Quddusi et al., 2023). Moreover, a previous study evaluating ultra-early decompression within 12 hours of injury has shown promising results in terms of neurological recovery in patients with cervical injury (Yousefifard et al., 2022). Maintaining cardiovascular stability is crucial during the acute phase of SCI to prevent neurogenic shock and spinal cord hypoperfusion, with guidelines recommending hemodynamic support to maintain mean arterial pressure above 85–90 mmHg (Sacino and Rosenblatt, 2019; Lee et al., 2021; Menacho and Floyd, 2021). While methylprednisolone was previously a standard treatment, its use is now controversial due to concerns about adverse effects and conflicting evidence regarding its efficacy (Canseco et al., 2021). Other drugs tested in clinical trials, such as thyrotropin-releasing hormone, naloxone, and GM1 (monosialotetrahexosylganglioside), have not shown significant benefits for SCI patients, highlighting the need for novel therapeutic approaches to improve outcomes in SCI management (Hawryluk et al., 2008).
Innovative Combinatorial Therapeutic Approaches
The lack of efficient treatment for SCI leads to the search for a variety of novel approaches to tackle SCI damaging outcomes. The primary emphasis of this review lies in the combinatorial therapeutic approaches that have been investigated over the past decade. Significant functional gains for individuals with SCI may require a combinatorial approach that addresses the multifactorial nature of SCI pathophysiology. In this sense, advances in combinatorial therapies, which can address different molecular and cellular secondary events of SCI, offer hope for spinal cord regeneration (Figure 2).
Figure 2.
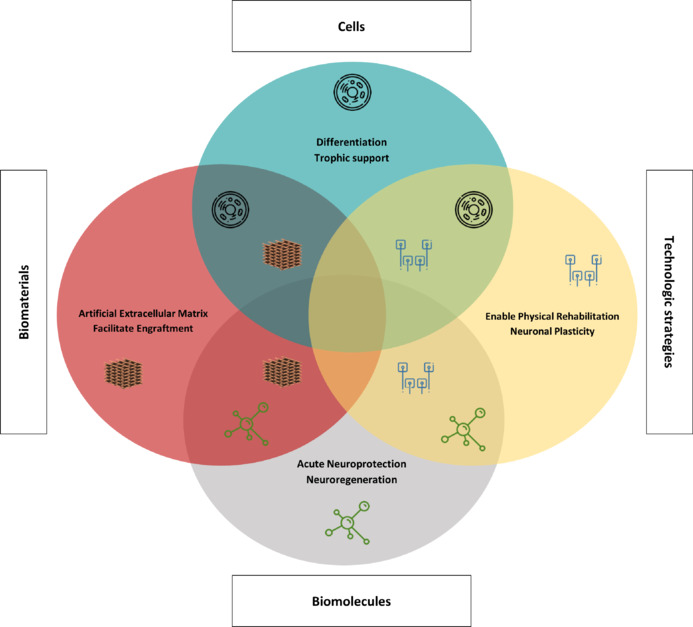
Concept of combined therapies for spinal cord injuries involves the integration of various approaches.
Cells and scaffolds may be utilized independently or in conjunction with pharmacological agents and additional biomolecules like growth factors and exosomes. Furthermore, technological methods can be synergistically employed with biomolecules or cells to address spinal cord injury. Created with Microsoft PowerPoint.
Combination of molecular agents
Several pharmacological treatments have already been combined. In 2013, the Martin Schwab group conducted tests on the anti-Nogo-A antibody and chondroitinase ABC (ChABC) enzyme, both of which showed to be promising treatments for enhancing functional recovery after SCI. They examined both treatments separately and in combination, determining that the synergistic effect between the two molecules resulted in superior outcomes, leading to enhanced sprouting and axon regeneration (Zhao et al., 2013). The ChABC also had been combined with other drugs, such as neurotrophin-3 (NT-3). A previous study had shown that NT-3 causes N-methyl-D-aspartate (NMDA) receptors to switch back to their immature form (expressing the NR2D subunit) and increases motoneuron’s plasticity. In a lateral T8 hemisection SCI model, Garcia et al. (2019) combined the use of ChABC, NT-3, and NR2D expression. Rats receiving the full combination group exhibited the most axonal sprouting and behavioral recovery in the Basso, Beattie, and Bresnahan (BBB) locomotor test, and in the case of electrical conductivity across the lesion, it was only restored in rats receiving the full combination (Garcia-Alias et al., 2011; Garcia-Alias and Fawcett, 2012). Another group tried to combine curcumin with epigallocatechin gallate in a rat model of acute compressive SCI. Both drugs are well known for their anti-inflammatory role potential and alone or together, they increased axonal sprouting, reduced glial scar formation, and decreased the levels of macrophage inflammatory protein 1-alpha, interleukin (IL)-1β, IL-4, and IL-6 cytokines (Ruzicka et al., 2018).
Interestingly, investigations have also explored the interaction between gabapentin and NMDA receptor antagonists, in particular dextromethorphan and MK-801, as a potential therapy for alleviating neuropathic pain-like symptoms in rats following SCI. With 15 or 30 mg/kg gabapentin combined with 10 mg/kg dextromethorphan proportionated a full alleviation of allodynia. MK-801 also augmented the effect of gabapentin in SCI rats (Shi et al., 2018).
Riluzole and magnesium were also combined to protect the spinal cord against glutamate excitotoxicity. Riluzole, acting as a sodium channel blocker, primarily exerts neuroprotective effects in SCI by inhibiting persistent sodium currents and decreasing presynaptic glutamate release, while enhancing glutamate uptake. Magnesium acts as an NMDA receptor blocker, counteracting excitotoxicity that results from excessive activation of this glutamate receptor. However, when tested together it was not observed any synergistic or cumulative effect, Riluzole was the only treatment that was able to achieve functional and histological improvements (Vasconcelos et al., 2016).
Due to the pivotal role neuronal apoptosis plays in secondary cellular events in SCI, Zhao et al. (2019) explored the potential of co-administering the PARP-1 inhibitor 3-aminobenzamide and the caspase-3 inhibitor z-DEVD-fmk to mitigate apoptosis in a rat SCI model. Their findings suggest that combination therapy proves beneficial for enhancing neuronal function recovery in rats afflicted with SCI.
In 2020, a group from China combined melatonin with methylprednisolone. Inhibition of axonal degeneration and reduction of nerve damage can be achieved with melatonin (Naseem and Parvez, 2014; Uyanikgil et al., 2017; Zhang et al., 2018) and this group tried to combine it with a lower dosage of methylprednisolone to ameliorate the side effects without a loss of effectiveness. They saw that melatonin could synergize with methylprednisolone to improve acute SCI through the PI3K-AKT1 pathway with fewer adverse reactions (Bi et al., 2021). Methylprednisolone also was combined with granulocyte colony-stimulating factor. Granulocyte colony-stimulating factor, a glycoprotein serving as a growth-stimulating factor for hematopoietic progenitor cells, when combined with methylprednisolone, demonstrated a synergistic effect, resulting in enhanced functional and histological improvements compared to the administration of either drug alone in the treatment of spinal cord contusion injuries in rats (Teixeira et al., 2018).
A combination of ceftriaxone with methylprednisolone was also done in a randomized, triple-blind clinical trial, where sixty individuals with acute traumatic SCI were to evaluate whether the combination therapy was superior to methylprednisolone alone. Results showed that the combination is responsible for improving inflammation and functional outcomes 6 months after the intervention (Mirzaei et al., 2020).
The combined action of methylprednisolone with recombinant human erythropoietin was also tested in a pilot double-blinded randomized controlled trial with acute cervical SCI patients. They were randomly assigned to either methylprednisolone alone or EPO plus methylprednisolone, but the group failed to prove that the combination therapy compared to methylprednisolone alone improves neurological functions (Ganjeifar et al., 2021). In another study, but using SCI rats, EPO was combined with IL-6. The administration of EPO following acute SCI led to enhanced functional recovery, whereas IL-6 alone did not produce any noticeable benefits. However, the combined use of both EPO and IL-6 showed advantages, albeit with outcomes less favorable than those observed with EPO alone (Barros et al., 2019).
Another combined approach involved the utilization of a monoclonal antibody targeting high mobility group box-1 (anti-HMGB1 mAb) alongside epothilone B (Epo B). HMGB1, a damage-associated molecular pattern, plays a pivotal role in blood-spinal cord barrier disruption and inflammatory responses, significantly contributing to secondary damage subsequent to central nervous system injuries. Conversely, Epo B, an anticancer agent, enhances microtubule stability and promotes axon regeneration. While Epo B alone proved ineffective in promoting functional recovery, the combined administration of these compounds notably increased hindlimb movement compared to the administration of anti-HMGB1 mAb alone. The findings suggest that anti-HMGB1 mAb alone reduces lesion extent and enhances neuronal survival near the lesion, while Epo B facilitates axon outgrowth only when administered alongside anti-HMGB1 mAb, indicating that the therapeutic efficacy of Epo B is dependent on anti-HMGB1 mAb-mediated tissue preservation (Zhu et al., 2021).
From another perspective and to alleviate chronic pain after SCI, it was investigated the simultaneous intrathecal administration of gamma-aminobutyric acid agonist and endomorphin-1. The inhibitory gamma-aminobutyric acid system plays an important role in reducing neuropathic pain. Moreover, endomorphins have demonstrated efficacy in alleviating chronic pain triggered by nervous system injury. Consequently, this combination was investigated for its potential to reduce neuropathic pain within a rat model of SCI. Results indicated a significant enhancement in pain threshold compared to when endomorphin-1 or muscimol were administered individually. Additionally, this combination treatment led to increased expression of α2 subunits of gamma-aminobutyric acid receptors and NR1 subunits of NMDA receptors within the spinal cord (Hosseini et al., 2020).
In 2021, the combined effect of three Neurotrophins was studied to examine whether the combination applied after 90 or 120 minutes of the injury could attenuate cord pathology. Brain-derived neurotrophic factor (BDNF), ciliary neurotrophic factor, and glial cell line-derived neurotrophic factor were topically administrated over the injured spinal cord and significantly reduced the blood-spinal cord barrier breakdown, edema formation, cell injury, and overexpression of enzyme heme oxygenase-2 (HO-2) by downregulation of carbon monoxide production (Sharma et al., 2021).
Other combinations had been tried with steroids like, for example, thyrotropin-releasing hormone, GM-1 ganglioside, or naloxone. Compared with monotherapeutic treatment regimens, these combination therapies may have some benefits. However, it must be noted that the beneficial effects of this treatment were not observed in all forms of SCI patients, as in severe SCI patients (Winkler et al., 1998; Baptiste and Fehlings, 2007).
Combination of cells and molecular agents
Several authors tested the effect of combining cells with pharmacological agents to better integrate the cells into the neural tissue or to increase their survival upon transplantation. Synergistic interactions are observed with the co-administration of ChABC and neural stem precursor cells. By digesting CSPGs, ChABC mitigated the inhibitory effect of CSPGs on neural stem precursor cell migration and promoted neurite outgrowth after transplantation (Ikegami et al., 2005).
Combined with human umbilical cord mesenchymal stromal cells (hUCMSCs), Taxol, an anti-cancer drug known to inhibit scar formation and enhance axonal lengthening, was observed to enhance functional recovery post-SCI by increasing anti-inflammatory, anti-apoptotic, anti-astrogliosis, and axonal preservation mechanisms (Zhilai et al., 2012). In 2018, hUCMSCs were also combined with Quercitin. This drug is capable of reducing the inflammatory microenvironment after SCI. Several effects were observed following this conjugation, including neurological functional recovery, axonal preservation, macrophage polarization, reduction of cystic cavity size, and a reduction in proinflammatory cytokines. A number of anti-inflammatory cytokines were also increased, including IL-4, IL-10, and transforming growth factor-1 (Wang et al., 2018b).
In another study, methylprednisolone combined with amniotic membrane mesenchymal stem cells reduced secondary damage and enhanced functional recovery. When methylprednisolone (50 mg/kg) was combined with delayed amniotic membrane mesenchymal stem cell transplantation, myeloperoxidase activity, and proinflammatory cytokines were significantly reduced, as well as cell apoptosis. Furthermore, this combination also increased the levels of the anti-inflammatory cytokines and the survival rate of amniotic membrane mesenchymal stem cells in the injury site (Gao et al., 2014).
Another group investigated the integration of Schwann cells with a hormonal compound, 17β-estradiol. 17β-estradiol confers protection to a wide range of cell types and decreases tissue damage. Its combination with Schwann cells has been shown to decrease neurological and behavioral deficits, protect neurons and oligodendrocytes, and significantly decrease the number of Iba-1+ (microglia) and GFAP+ cells (astrocyte) in rats with SCI (Namjoo et al., 2018).
It was also found that combining olfactory ensheathing cells (OECs) and minocycline reduced levels of IL-1β, tumor necrosis factor-α, caspase-3, oxidative stress, astrogliosis, and cavitation while also improving axonal regeneration and functional recovery in a Wistar rat model (Pourkhodadad et al., 2019). OECs were additionally combined with ChABC in a rat SCI model. The group postulated that transplantation of canine OECs genetically modified by lentiviral transduction to express ChABC would offer a new delivery system of ChABC and potentiate a synergistic therapy. In this study, a modest functional improvement was observed in the combinational transplanted animals, who recovered greater forepaw reaching accuracy (Whishaw testing) and a normal gait when compared to the OECs transplanted group or controls (Prager et al., 2021).
Nori et al. (2018) explored the treatment of chronic SCI in an immunodeficient rat model by directing human NPCs toward an oligodendrogenic fate (oligodendrogenic neural progenitor cells, oNPCs) and employing sustained delivery of ChABC via a crosslinked methylcellulose biomaterial. This combined approach enhanced the long-term survival of oNPCs around the lesion center, promoting oligodendrocyte differentiation and remyelination of spared axons by engrafted oNPCs. Additionally, it led to improved neurobehavioral recovery.
The co-administration of human neural stem cells with lithium chloride (Li) was investigated to ascertain its potential to enhance cell proliferation, differentiation, and survival in a rat spinal contusion model, in comparison to individual treatments. Both the human neural stem cells + lithium chloride and lithium chloride alone groups demonstrated notable enhancements in locomotor scores and motor evoked potential compared to human neural stem cells alone (Mohammadshirazi et al., 2019).
In 2020, it was observed that NSCs loaded with tetrahedral framework nucleic acid exhibited similar neuroprotective effects. The combined outcome of this strategy resulted in a notable improvement in motor function recovery and tissue regeneration at SCI lesion sites (Ma et al., 2020). Another group evaluated the effect of epidermal neural crest stem cells combined with astaxanthin, a powerful antioxidant, on damage induced by SCI. Based on their results, astaxanthin plus epidermal neural crest stem cells yielded superior effects in terms of behavioral recovery, motor neuron preservation, and reduced demyelination, in comparison to either treatment administered alone (Mohaghegh Shalmani et al., 2020).
NSCs were also tested in conjunction with teramethylpyrazine. This combination improved functional outcomes in rats with SCI and offered spinal cord protection by increasing BDNF and decreasing Nogo-A levels, as well as restricting the JAK2/STAT3 signal transduction pathway. These findings suggest that teramethylpyrazine has potential therapeutic value in NSC transplantation for SCI treatment (Zhang et al., 2021).
It was also evaluated, in a rat model of contusive SCI, the combination of IPSC-NSCs and MSCs with a pH-responsive polyacetal-curcumin nanoconjugate (Bonilla et al., 2021). This allows curcumin to be released on a sustained basis. The intrathecal delivery of polyacetal-curcumin nanoconjugate in combination with stem cell transplantation did not result in significant enhancements in locomotor function. However, it did lead to notably smaller scars and promoted the preservation of β-III Tubulin-positive axons. Additionally, MSCs were co-administered with platelet-rich plasma, which comprises various growth factors. The findings revealed that this combined intervention improved axon regeneration, reduced cell apoptosis, enhanced locomotor function, and exhibited synergistic effects in SCI (Salarinia et al., 2020).
Zeraatpisheh et al. (2022) found that co-administration of FTY720 (an immunomodulatory drug) with NSPCs 7 days after SCI could enhance functional recovery and transplanted cells’ survival and migration.
In biological processes such as migration, proliferation, and cell survival, signaling molecules, such as growth factors, seem to play an essential role (Orazizadeh et al., 2009) and cellular transplantation can be further enhanced through the application of these neurotrophins. Thus, some groups tested this combination and showed that MSCs along with either nerve growth factor (NGF) (Huang et al., 2006) or basic fibroblast growth factor (Kim et al., 2006) can promote axonal regeneration, cell differentiation, and functional recovery in adult rats after SCI. One group from Korea tried to potentiate the function of adipose-derived mesenchymal stem cells by transfecting them with BDNF and heme oxygenase-1 (HO-1), to create either adipose-derived mesenchymal stem cells overexpressing BDNF (BDNF-MSCs) or HO-1 (HO-1-MSCs) (Khan et al., 2018). They injected BDNF-MSCs and HO-1-MSCs as a combination group, in SCI dogs, and compared to which one was injected individually. The combination group showed an increase in neuroregeneration and a significant improvement in hindlimb functions than in other groups.
Ana Alastrue-Agudo and her group tried to combine the transplantation of ependymal stem/progenitor cells of the spinal cord after injury with FM19G11 pharmacological treatment (an inhibitor of hypoxia-inducible factor). This combination improved certain locomotor functions when compared to the control group, although it did not yield a significant improvement in BBB scores compared to the individual treatments (Alastrue-Agudo et al., 2018).
Focusing on different aspects and to ameliorate spasticity, which is a frequent complication of SCI with no ideal treatment, Sun et al. (2021) combined sural nerve grafting with an infusion of acidic fibroblast growth factor in a monkey thoracic hemisection model. The combined treatment markedly reduced the spasticity in leg muscle tone, patella tendon reflex, and fanning of toes, and may be seen as a complementary approach to decreasing spasticity in patients with SCI.
Neural Tissue Engineering
Tissue engineering is a rapidly advancing field focused on repairing, replacing, or regenerating tissues or organs. This is achieved by applying fundamental principles from physics, chemistry, and biology to develop effective materials, devices, and clinical strategies (Di Silvio, 2007). It evolved from biomaterial development and is the process of creating functional tissues by combining scaffolds, cells, and biologically active molecules. Using tissue engineering, damaged tissues or whole organs can be restored, maintained, or improved.
Combination of cells and biomaterials
The inhibitory conditions resulting from SCI compromise cell survival. To address this challenge, biomaterials have been suggested to safeguard transplanted cells and modulate their behavior (Fathi et al., 2020; Narimanpour et al., 2020; Rezaei et al., 2020). Biomaterials can serve various roles in transplantation strategies for SCI: (A) three-dimensional microarchitectures can be engineered to facilitate cell seeding and guide axonal growth in a directed linear pattern, thereby promoting axonal regeneration across the lesion site (Gros et al., 2010; Gunther et al., 2015; Onuma-Ukegawa et al., 2015); (B) acts as a physical scaffold for cell adhesion, thereby augmenting the survival and retention of grafted cells at the lesion site (Hurtado et al., 2006; Olson et al., 2009; Bozkurt et al., 2010; Park et al., 2012); influences the migration of host cells (e.g., Schwann cells and astrocytes) (Suzuki et al., 2015); (C) impact the behavior and differentiation of grafted cells (Mekhail et al., 2015); and (D) regulate the release of encapsulated bioactive molecules (Mothe et al., 2013).
Multiple studies have demonstrated that incorporating cells into both natural and synthetic materials results in enhancements in cell survival, axonal regeneration, and functional recovery in pre-clinical SCI models (Table 1), and a recent systematic meta-analysis, from Michael G. Fehlings laboratory, demonstrated the superior efficacy of a combined administration of scaffolds and mesenchymal stem cells (MSCs) compared to the individual administration of each component. This was evidenced by enhanced improvements in motor function observed during interventions in the acute phase of SCI in animal studies (Yousefifard et al., 2019). Of note, one pre-clinical study combined MSC transplantation with a bioengineered nanofiber-hydrogel composite did not result in a significant effect on MSC transplant survival. Additionally, hind limb function was found to be similar across all experimental groups (Haggerty et al., 2022).
Table 1.
Pre-clinical combinatorial approaches for SCI repair: cells and biomaterials (from 2013 to 2023)
| Model/Injury | Cells + Biomaterials | Outcomes | References |
|---|---|---|---|
| Rat/Contusion | MSCs + Nanofiber-hydrogel composite (NHC) | Did not find a significant effect of NHC on MSC transplant survival, and hind limb function was similar across all groups. | Haggerty et al., 2022 |
| Rat/Compression T2 | Adult rat SVZ-derived NSPCs + PDGF-A-conjugated HAMC | Enhanced sparing of host oligodendrocytes and neurons, increased fine motor (horizontal ladder) changes but not gross motor skills (BBB). | Mothe et al., 2013 |
| Rat/Hemisection T13-L2 | Neonatal NPCs + 4 mm porous collagen scaffold + neutralizing antibody of EGFR | Increased neuronal differentiation, increased BBB score, and angle on inclined plane. | Li et al., 2013 |
| Rat/Transection T9-10 | Co-culture SC-NT-3 and BMSC-TrkC + 2 mm 3D gelatin sponge scaffold | Enhanced axonal growth throughout the biomaterial, synaptic association of these cells with serotonergic neurons and some CST axons, upregulation of c-Fos in the grafted as well as host lumbar spinal cord cells, and improvement of BBB score. | Zeng et al., 2015 |
| Rat/Transection T3 | BMSCs + 2 mm multi-component fiber bundled agarose scaffolds | Increased growth of raphespinal, reticulospinal tracts and sensory fibers into but not exiting scaffold. | Gao et al., 2013 |
| Rat/Hemisection T9 | BMSCs + 3 mm honeycomb collagen scaffold | Rats implanted with HC scaffolds containing BMSCs displayed better motor and sensory recovery than those implanted with HC scaffolds only. | Onuma-Ukegawa et al., 2015 |
| Rat/Contusion T9 | DPSCs + Chitosan scaffolds | Higher cell viability and neural differentiation in the DPSC/chitosan-scaffold group and also a marked recovery of hind limb locomotor functions. | Zhang et al., 2016 |
| Rat/Hemisection T9 | Co-hEnSC + PCL/gelatin nanofibrous sc | Growth of neuronal cells was confirmed, and the recovery of sensory and motor functions was also confirmed in this group. | Babaloo et al., 2019 |
| Rat/Contusion T10-11 | hESC + HA-based hydrogel | Encapsulation of hESC-NS in HA-based hydrogel can increase differentiation of these cells into oligodendrocytes and improve locomotor function. | Zarei-Kheirabadi et al., 2020 |
| Rat/Excision T8-9 | hUC-MSCs + collagen scaffold [(NeuroRegen Scaffold (NRS)] | NRS and hUC-MSCs promoted locomotion in rats and improved cortical motor and somatosensory evoked potentials. | Wang et al., 2018a |
| Rat/Compression T10-12 | NSCs + porous collagen scaffolds | Enhancing NSC neuronal differentiation and functional integration in vivo, enabling robust axonal elongation, and reducing astrogliosis. | Kourgiantaki et al., 2020 |
| Rat/Contusion T10 | NSCs + Matrigel scaffold | SCI rats transplanted with NSCs in Matrigel showed improved behavioral recovery and neuronal and reactive astrocyte marker expression levels compared to PBS- or Matrigel-transplanted rats. | Wang et al., 2020 |
| Rat/Transection T10 | NSCs + Print 3D collagen/silk fibroin scaffold | 3D-CF + NSCs group with increased amplitude in motor evoked potential tests. The filling of the injury cavity was the best and glial scarring was reduced in the same group. | Jiang et al., 2020 |
| Rat/Transection T3 | NPCs + Print 3D biomimetic hydrogel scaffolds | Injured host axons regenerate into 3D biomimetic scaffolds and synapse onto NPCs implanted into the device and those implanted NPCs in turn extend axons out of the scaffold and into the host spinal cord below the injury. | Koffler et al., 2019 |
| Rat/Complete transection T9 | hbNSPCs and hscNSPCs + Aligned collagen sponge scaffold (ACSS) | Compared with the hbNSPC–ACSS, the hscNSPC–ACSS effectively promoted long-term cell survival and neuronal differentiation and reduced inflammation and glial scar formation. The transplanted hscNSPC–ACSS improved the recovery of locomotor functions. | Zou et al., 2020 |
| Rat/Transection T8 | Adult rat SCs + 4 mm PAN/PVC and fluid or pre-gelled Matrigel | Greater growth of virally traced brainstem-derived axons into and up to the caudal interface, formed synaptic junctions with the help of newly formed supportive astrocytic protrusions. | Williams et al., 2015 |
| Rat/Hemisection C5 | Adult GFP-SCs + 2 mm alginate-based anisotropic capillary hydrogel | Serotonergic growth through and caudal to the biomaterial up to the 1 mm SC injection site. | Liu et al., 2017b |
| Rat/Transection T8 | SCs + piezoelectric polyvinylidene fluoride trifluoroethylene (PVDF-TrFE) | Caused enhancements in axonal growth and formation of blood vessels following transection. | Lee et al., 2017 |
| Rat/Transection T9-10 | Activated SCs and/or rat BM-MSCs + 3 mm PLGA scaffold | Both exhibited significant recovery of nerve function as shown by the BBB score and electrophysiological test results. | Yang et al., 2017 |
| Rat/Hemisection T13 | OEC + 2 mm collagen-based multi-channel 3D matrices | No improvement in CatWalk gait analysis or alleviation of allodynia. | Deumens et al., 2013 |
| Rat/Transection T9-10 | OECs + 2 mm PLGA scaffolds | Increased BBB score and crossing of the inclined plane, increased axonal preservation, decreased astrogliosis. | Wang et al., 2017a |
| Rat/Hemisection C2 | ASCs and OECs + Gellan Gum (GG)-based hydrogel | Beneficial effects on both diaphragmatic recovery and sensory function. | Gomes et al., 2020 |
| Rat/Transection T9-10 | OECs + Spinal cord decellularized (DC) scaffolds | Better axon regeneration promoting protein expression than the SCI model and improve the proliferation and distribution of astrocytes at the site of injury. | Yu et al., 2021 |
| Mouse/Contusion T9 | NSPCs + FTY720-incorporated PLGA nanoparticles | Improvement in neurological functions and minimized tissue damage. Increased the survival of transplanted cells and promoted their differentiation towards more oligodendrocytic phenotype. | Zeraatpisheh et al., 2022 |
| Rat/Transection T9-10 | MSCs + Microhydrogel fibers | Significantly improved electrophysiological expression and re-established limb motor function. | Yao et al., 2020 |
For all the combinations of biomaterials and cells mentioned above, approximately 80% reported functional and histological recovery, while 12% showed only histological improvement with no functional recovery and 8% had no improvements at all. ASCs: Adipose tissue-derived stem/stromal cells; BBB: Basso, Beattie and Bresnahan motor scale; BM-MSCs: bone marrow-derived mesenchymal stem cells; BMSCs: bone marrow stromal cells; Co-hEnSC: co-cultured human endometrial stem cells; CST: corticospinal tract; DPSCs: human dental pulp stem cells; EGFR: epidermal growth factor receptor; FTY720: fingolimod; HA: hyaluronic acid; HAMC: hyaluronan/methylcellulose; hbNSPCs: human fetal brain derived NSPCs; hESC: human embryonic stem cell; hESC-NS: human embryonic stem cell derived-neural stem cells; hscNSPCs: human spinal cord derived NSPCs; hUC-MSCs: human umbilical cord-derived mesenchymal stem cells; MSCs: mesenchymal stem cells; NHC: nanofiber-hydrogel composite; NSCs: neural stem cells; NSPCs: neural stem/progenitor cells; NT-3: neurotrophin-3; OEC: olfactory ensheathing cell; PAN/PVC: polyacrylonitrile/polyvinyl chloride; PCL: poly E-caprolactone; PDGF-A: platelet derived growth factor subunit A; PLGA: poly(lactide-co-glycolide; SCs: Schwann cells; SVZ: subventricular zone; TrkC: tropomyosin receptor kinase C.
In 2013, it was seen that the use of nerve guidance scaffolds (templated agarose scaffolds), filled with genetically engineered bone marrow stromal cells for the secretion of BDNF could imply improvements in the growth of axons as well as motor functions even during severe SCI (Gao et al., 2013).
In 2016, Zhang et al. utilized chitosan scaffolds to establish a conducive microenvironment, promoting enhanced survival of human dental pulp stem cells (DPSCs) and facilitating improved neural differentiation. The study showed that chitosan scaffolds enhanced cell viability and promoted neural differentiation of DPSCs in vitro. The DPSCs incubated with chitosan scaffolds also exhibited augmented secretion of neurotrophic factors, such as glial cell line-derived neurotrophic factor, BDNF, b-NGF, and NT-3. The Wnt/β-catenin signaling pathway was identified as pivotal in the neural differentiation of DPSCs in conjunction with chitosan scaffolds. Moreover, the co-transplantation of DPSCs and chitosan scaffolds into an animal model of SCI led to substantial restoration of hind limb locomotor functions. The findings suggest that chitosan scaffolds provide a supportive microenvironment for DPSC survival and neural differentiation (Zhang et al., 2016).
Another study used a 3D gelatin sponge infused with genetically modified BM-MSCs and Schwann cells in the transected spinal cord of rats, resulting in a marked enhancement of hindlimb function and cortical motor evoked potential (Zeng et al., 2015). Furthermore, Babaloo et al. (2019) investigated the effect of differentiated human endometrial stem cells/Schwann cells on poly ε-caprolactone/gelatin scaffolds in a hemisected SCI rat model. They observed that this combinatorial approach leads to improvement in both sensory and motor functions.
Human embryonic stem cells derived-NSCs encapsulated in hyaluronic acid hydrogel and delivered via injection into the lesion site, potentiated the differentiation of these cells in oligodendrocytes, leading to enhanced behavioral recovery (Zarei-Kheirabadi et al., 2020). Also, important to mention, that combined applications of NSCs with collagen (Kourgiantaki et al., 2020), Matrigel (Wang et al., 2020), and collagen/silk fibroin bio-printed (Jiang et al., 2020) scaffolds have been shown to promote spinal cord repair.
Another therapeutic strategy tested for SCI repair is the application of a 3D silk fibrous scaffold joined with dermal fibroblast-reprogrammed neurons, which is shown to improve functional recovery (Koffler et al., 2019). A study by Yu et al. (2021) found that combining decellularized spinal cord scaffolds with OECs resulted in enhancements in cell proliferation and axon regeneration. Similarly, according to Lee and collaborators, the integration of polyvinylidene fluoride trifluoroethylene conduits with Schwann cells led to increased axonal growth and formation of blood vessels following transection (Lee et al., 2017). Furthermore, the co-administration of activated Schwann cells and BM-MSCs via a multichannel poly(lactide-co-glycolide) (PLGA) scaffold fully transected spinal cords, demonstrated enhancements in functional recovery (Yang et al., 2017).
Even though NPC transplantation is beneficial, its low survival and differentiation rates pose several significant limitations. One group from Spain tried a new approach of using a combination of electric and magnetic stimulation to induce proliferation and further neuronal differentiation (Cuenca-Ortola et al., 2022). Thus, they used both wireless electrical and magnetic stimulation to condition in vitro NPCs seeded on alignments of poly (lactic acid) nanofibrous scaffolds prior to transplantation. A continuous stimulation pattern and an intermittent stimulation pattern were compared. As a result of continuous stimulation, NPCs proliferate and differentiate into oligodendrocytic and neuronal lineages preferentially. In contrast, intermittent stimulation patterns did not affect NPC proliferation with detrimental effects on neural differentiation (Cuenca-Ortola et al., 2022).
Gomes et al. (2020) investigated the therapeutic potential of a hydrogel made from gellan gum modified with GRGDS peptide, combined with ASCs and OECs, for cervical SCI in rats. The study found that 5 weeks post-injury, the group receiving the combinatorial therapy of hydrogel and cells showed increased inspiratory bursting of the affected hemidiaphragm compared to untreated animals, particularly at the ventral portion of the muscle. Additionally, this group showed a significant reduction in mechanical allodynia, as assessed by Von Frey testing, indicating a potential improvement in sensory function. No differences were observed in forelimb motor function or markers for axonal regrowth, astrogliosis, and inflammatory cells (Gomes et al., 2020).
Based on these previous positive results, several clinical trials combining cells with scaffolds were initiated (Table 2).
Table 2.
Ongoing clinical trials investigating the use of scaffolds with cells for SCI treatment
| ClinicalTrials. gov identifier | Cells + Biomaterials | Injury (ASIA) | Number of patients | Timing | Primary outcomes |
|---|---|---|---|---|---|
| NCT03933072 | Autologous OECs, ONFs + collagen scaffold | Complete SCI between C5–T10 (A) | 2 | > 8 months | ASIA scale; Berg Balance scale; Deep sensation; Vibration; Joint position sense |
| NCT02688049 | MSCs, NSCs + NeuroRegen scaffold | Complete SCI between C5–T12 (A) | 30 | Chronic SCI | ASIA scale; SSEP; MEP |
| NCT02688062 | BMMCs + NeuroRegen scaffold | Complete SCI-thoracic (A) | 22 | Chronic SCI | ASIA scale; SSEP; MEP; FIM |
| NCT02510365 | Autologous BMMCs + NeuroRegen scaffolds | Complete SCI-thoracic (A) | 7 | < 4 months | ASIA scale; SSEP; MEP |
ASIA: American Spinal Injury Association Impairment Scale; BMMCs: bone marrow mononuclear cells; FIM: functional independence measure; MEP: motor evoked potential; MSCs: mesenchymal stem cells; NSCs: neural stem cells; OECs: olfactory ensheathing cells; ONFs: olfactory nerve fibroblasts; SCI: spinal cord injury; SSEP: somatosensory evoked potential.
Combination of biomaterials and molecular agents
Currently, there is a preference for localized delivery over systemic delivery, as it has been shown to yield more efficient outcomes in terms of mitigating detrimental side effects (Zustiak et al., 2020). It is important to note that systemic delivery of an agent commonly requires high doses of the drug, which increases the risk of negative side effects (Chvatal et al., 2008). However, controlled drug delivery greatly reduces the risk of these side effects. The most common way to deliver drugs locally is to use hydrogels, fibers, microparticles, and nanoparticles, either implanted or injected, or a combination thereof. For instance, the difference between systemic and localized/controlled delivery of methylprednisolone shows that only the delivery using nanoparticles of PLGA embedded in agarose hydrogels reduces lesion volume and downregulates pro-inflammatory proteins (Chvatal et al., 2008). Similarly, PLGA/F-127 gels can also be used to locally deliver hirudin, a thrombin inhibitor, with an improved motor function and a reduction of activated macrophages in patients with SCI (Sellers et al., 2014).
Cerqueira et al. (2013) investigated the potential of dendrimer nanoparticles loaded with methylprednisolone as a therapeutic approach for SCI. The authors report that these nanoparticles can effectively penetrate the blood-brain barrier and reduce microglial activation, which is an important factor in SCI pathology. The study also demonstrates that the dendrimer nanoparticles are biocompatible and have a low toxicity profile, indicating their potential as a safe therapeutic option.
Polyethylene glycol formulations containing magnesium were developed to increase the effectiveness of tolerable magnesium doses (Kwon et al., 2009). SCI rats with this combination demonstrated enhanced tissue sparing and functional recovery (Kaptanoglu et al., 2003).
In 2013, a work from China found that sustained delivery of dibutyryl cyclic adenosine monophosphate, an analog of cAMP, loaded in poly(propylene carbonate) fibers, into the spinal cord, can promote functional recovery, axonal regenerative sprouting, stimulate axonal outgrowth and reduce astrogliosis after spinal cord hemisection (Xia et al., 2013). Furthermore, Papa et al. (2014) showed that after SCI, To-Pro3-loaded poly(methyl methacrylate) nanoparticles embedded in agarose carbomer hydrogel could be a promising drug carrier for the selective administration in activated microglia/macrophages and thus potentially able to counteract relevant secondary inflammatory events in SCI.
Another work has shown that Cabazitaxel (a microtubule inhibitor) micelles (Cab-M) embedded in a P10R5-LA copolymer hydrogel reduce scar formation and axon inhibitory molecules (Li et al., 2019). Additionally, this hydrogel helped sustain the Cab-M release (Liu et al., 2017a). Another group combined Cetuximab with Taxol co-modified collagen scaffolds and showed significantly increased neural regeneration. Furthermore, scaffold transplantation decreased the deposition of scar-related inhibitors within the lesion in a rat severe SCI model (Fan et al., 2018).
In a different approach, a group in 2021, tried to implant Taxol-modified linear ordered collagen scaffold after a scar tissue removal surgery (one or two surgeries) in the canine SCI model. They saw that the Taxol-linear ordered collagen scaffold combination induced axonal regeneration, neurogenesis, and electrophysiological and functional recovery after one scar tissue removal surgery. The researchers also discovered that one scar tissue removal was associated with more activation of endogenous NSCs in the injured rather than the second procedure (Yin et al., 2021).
Bighinati et al. (2020) developed an implantable polymeric delivery system and implanted a biomaterial (electrospun poly (l-lactic acid) [PLLA]) loaded with ibuprofen and triiodothyronine (T3) to reduce inflammation in a rat model of T8 contusion SCI. PLLA + ibuprofen + T3 improved endogenous regeneration and functional locomotion by reducing glutamate release at 24 hours and 8 days post-injury compared to PLLA-implanted rats. The treatment also reduced the lesion volume at 60 days post-injury.
Serp-1, an immunomodulatory biologic drug, has the capacity to reduce inflammation and protect neurons in SCI therapy. In animal models of SCI, a combination of chitosan and stable collagen hydrogel has been developed to create a highly biodegradable material with low antigenicity and favorable biocompatibility. Consequently, the delivery of Serp-1 via chitosan-collagen hydrogel was observed to sustain therapeutic effects and enhance functional recovery in SCI rats (Kwiecien et al., 2020).
Recently, Nazemi et al. (2020) demonstrated an alternative co-delivery system. In this approach, paclitaxel is encapsulated in PLGA microspheres. The encapsulated product is then injected into a minocycline-loaded hydrogel and placed into the lesion site. As a result, there was an increase in neuronal regeneration, a reduction in inflammatory cell activity, and a decrease in fibrotic scar size (Nazemi et al., 2020). Additionally, the co-delivery system also avoids the toxicity of paclitaxel on peripheral nerve tissue (Nazemi et al., 2020).
Another study combined small molecules known for increasing neuronal differentiation of rat spinal cord NSCs, such as LDN193189 (a potent inhibitor of the bone morphogenetic pathway, inhibiting ALK1, ALK2, ALK3, and ALK6), SB431542 (potent and selective inhibitor of the transforming growth factor-β type I), CHIR99021 (potent glycogen synthase kinase 3 inhibitor) and P7C3-A20 (an aminopropyl carbazole that elevates cellular nicotinamide adenine dinucleotide), loaded in a collagen hydrogel. The authors showed that this combination induced neurogenesis and inhibited astrogliogenesis of endogenous NSCs in the lesion site (Yang et al., 2021).
Biomaterials had also been combined with growth factors and extracellular vesicles (Table 3). As both growth factors and extracellular vesicles have low stability, short half-lives, and rapid inactivation by enzymes, biomaterials can prolong and sustain their delivery, thereby increasing overall effectiveness (Wang et al., 2017b).
Table 3.
Pre-clinical combinatorial approaches for SCI repair: biomaterials and small molecules
| Models | Molecules + Biomaterials | Outcomes | References |
|---|---|---|---|
| Mice/Contusion T9-10 | Shh + PLGA microspheres | Sprouting of nerve fibers, the proliferation of oligodendrocytes, the decrease of gliosis, and behavioral recovery after SCI. | Lowry et al., 2012 |
| Rat/Compression T2 | FGF2 + PLGA nanoparticles | Achieved higher blood vessel density in the dorsal horns, due to either greater angiogenesis near the epicenter of the injury or vasoprotection acutely after spinal cord injury. | Kang et al., 2013 |
| Rat/Compression T1-2 | NT-3 + PLGA nanoparticles | Significant axon growth with no effect on the astroglial response to injury and functional recovery in comparison with vehicle and injury controls. | Elliott Donaghue et al., 2015 |
| Rat/Contusion T9 | VEGF, angiopoietin-1 (Ang-1), and bFGF + PLGA microspheres | Stimulates neoangiogenesis and neurogenesis, accelerating recovery of neurologic function. | Yu et al., 2016 |
| Rat/Compression T9 | NGF + GNLs | Enhanced the neuroprotection effect in SCI rats compared with NGF alone. This combination has higher monodispersity, half-life, and stability. | Zhu et al., 2016 |
| Rat/Transection T9 | NT-3 + poly(propylene fumarate)-collagen scaffold | Improves the inhibitory microenvironment, facilitates axonal and neuronal regeneration, survival of various types of functional neurons, and remyelination and synapse formation of regenerated axons. | Chen et al., 2018 |
| Rat/Transection T9-10 | Exosomes derived from hMSCs + Peptide-modified adhesive hydrogel | Significant nerve recovery and urinary tissue preservation by effectively mitigating inflammation and oxidation. | Li et al., 2020 |
| Rat/Transection T2 | BDNF + hydrogel - HA and MC | Enhancing axonal growth and promoting neural stem cell formation. | Khaing et al., 2016 |
| Rat/Compression T9 | HGF + gelatin-FA hydrogel | HGF combined with gelatin-FA hydrogel promoted endogenous repair and recovery more effectively than HGF with hydrogel. | Yamane et al., 2018 |
For all the combinations of biomaterials and small molecules mentioned above, approximately 78% reported functional and histological recovery and 22% showed histological recovery with no functional improvements. BDNF: Brain-derived neurotrophic factor; bFGF: basic fibroblast growth factor; FA: furfurylamine; FGF2: fibroblast growth factor-2; GNLs: gelatin nanostructured lipid carriers; HA: hyaluronic acid; HGF: hepatocyte growth factor; hMSCs: human placenta amniotic membrane mesenchymal stem cell; MC: methylcellulose; NGF: nerve growth factor; NT-3: neurotrophin-3; PLGA: poly(lactide-co-glycolide; Shh: sonic hedgehog; VEGF: vascular endothelial growth factor.
According to one study from 2012, PLGA microspheres can be used to deliver Sonic Hedgehog (Lowry et al., 2012). Sonic Hedgehog is a multifunctional growth factor that has a critical role in spinal cord formation, acting as an axon guidance molecule and promoting the formation of motor neurons and oligodendrocytes from ventral cord progenitor cells. Sustained Sonic Hedgehog delivery by PLGA microspheres led to nerve fiber sprouting, oligodendrocyte proliferation, and decreased gliosis (Lowry et al., 2012).
Fibroblast growth factor 2, which plays an important role in the regulation of cell survival, cell division, cell differentiation, and cell migration (Ornitz et al., 1996), and NT-3, which promotes the survival and differentiation of existing neurons and the growth and differentiation of new neurons (Kakizawa, 2021), were combined with PLGA nanoparticles. This combination demonstrated prolonged, but localized release when dispersed in hyaluronan-methylcellulose hydrogels (Kang et al., 2013; Elliott Donaghue et al., 2015). Another study tested the sustained delivery of vascular endothelial growth factor, angiopoietin-1, and basic fibroblast growth factor using PLGA microspheres. Results demonstrated increased miR-210 expression, angiogenesis, suppressed Eph-A3, higher recruiting of neural precursors, and an increased spared white matter (Yu et al., 2016).
The utilization of gelatin nanostructured lipid carriers was also employed to extend the release of NGF and augment neuroprotection and recovery subsequent to SCI by suppressing the expression of endoplasmic reticulum stress-induced apoptosis proteins (Zhu et al., 2016). The poly (propylene fumarate)-collagen scaffold coated with NT-3 was also demonstrated to promote the regeneration of neural tissues, leading to functional recovery (Chen et al., 2018). Moreover, a recent study conducted by Li et al. (2020) utilized exosomes derived from hMSCs, immobilized in peptide-modified adhesive hydrogel, for the treatment of injured nerve tissues. This combination facilitated significant nerve recovery and preservation of urinary tissue by effectively attenuating inflammation and oxidative stress.
Combination of biomaterials, cells, and biomolecules
In 2020, Rodríguez-Barrera et al. explored an alternative approach for SCI treatment. They combined immunization with neural-derived peptides (INDP), scar removal, and the use of a fibrin glue matrix with MSCs. This combination aimed to modify the non-permissive microenvironment post-SCI. However, it did not elicit proper axonal regeneration or neurogenesis when compared to treatment with INDP alone. Notably, INDP alone significantly enhanced motor recovery, anti-inflammatory cytokines, regeneration-associated molecules, axonal regeneration, and neurogenesis compared to other treatment groups (Rodríguez-Barrera et al., 2020).
Another group pursued a combined approach to achieve neuroprotection and promote neuroregeneration. They combined the use of INDP, inhibition of glial scar formation (dipyridyl: DPY), and a biocompatible matrix (fibrin glue FG) impregnated with bone marrow MSCs. The combination therapy (INDP + DPY + FG + MSCs) emerged as the most effective strategy for promoting recovery of motor and sensory function. Additionally, the combination therapy group exhibited a significant increase in axonal density and tissue preservation (Garcia et al., 2019).
Nori et al. (2018) described a study that evaluated the therapeutic potential of combining human oNPCs with sustained delivery of ChABC using an innovative affinity release strategy in a crosslinked methylcellulose hydrogel for the treatment of chronic SCI in a thoracic compression rat model. The researchers transplanted oNPCs into rats with chronic SCI and administered ChABC to enhance the survival and differentiation of the transplanted cells. The study found that the combination therapy improved motor function in the rats, as well as increased myelin thickness and decreased the formation of glial scars. The findings of this study highlight the potential of using stem cell-based therapies and ChABC for the treatment of chronic SCI.
In another study, it was hypothesized that combining human dental stem cells of the apical papilla (SCAP) with pharmacologically active microcarriers (PAMs) releasing BDNF would improve locomotor function in rats through immunomodulation and neuroprotection. SCAP BDNF-PAMs were administered in a rat contusion model of SCI. The treatment enhanced rat BBB scoring, decreased the expression of inducible nitric oxide synthase, and increased the expression of βIII tubulin, growth-associated protein 43, and 5-hydroxytryptamine (5-HT) (Kandalam et al., 2020).
Combination of neuromodulation technologies with molecules or cells
It was hypothesized that combinations of electrical stimulation with pharmacological or/and cell transplantation may cooperate synergistically to activate and functionally remodel spinal locomotor circuits. This cooperative effect might enable the coordinated and context-dependent function of paralyzed hindlimbs in rats following complete SCI.
Courtine et al. (2009) tested a combination of 5-HT2A and 5-HT1A/7 serotonin agonists and epidural electrical stimulation at two positions distal to the lesion and they found that this combination allows rats with SCI to produce full weight-bearing bipedal treadmill locomotion that is almost undifferentiable from voluntary stepping before the injury. Additionally, the group demonstrated that sensory input allows adaptive motor patterns to develop without supraspinal influences. Other groups tested similar combinations with similarly encouraging results (Landry et al., 2006; Gerasimenko et al., 2007; Yao et al., 2021).
From a different perspective, others have tried to see the effects of the combination of transcranial magnetic stimulation with cells, specifically BMSC transplantation, or a Raf inhibitor on SCI in rats (Feng et al., 2021). According to their findings, combination therapy mitigated spinal cord lesions and neuronal apoptosis induced by SCI. It also elevated the expression levels of growth-associated protein 43, NGF, and BDNF, while suppressing GFAP expression, and inhibited the activation of the Raf/MEK/ERK signaling pathway in rats with SCI compared to monotherapy.
Sinopoulou et al. (2022) employed a multifaceted approach involving cervical contused rats. This approach entailed intraspinal injections of a neuroplasticity-promoting mediator (lentiviral-ChABC), along with 11 weeks of cortical epidural electrical stimulation administered for 3 hours daily, 5 days per week, and behavioral rehabilitation sessions lasting 15 minutes daily, 5 days per week. With this combination of treatments, there was a significant increase in muscle activity and upper limb dexterity compared to either single treatment or no treatment.
To bypass an extrinsic factor that inhibits axon elongation, repulsive guidance molecule-a, which is upregulated around spinal cord lesion sites, Yamanaka et al. (2021) used a combinational approach with an antibody anti-repulsive guidance molecule-a in a monkey model to promotes axon sprouting, regeneration, and motor recovery after SCI and a repetitive transcranial magnetic stimulation. Dexterous hand movements were restored more effectively with the combination of antibody and repetitive transcranial magnetic stimulation.
Another neuromodulatory technology that is being used is photobiomodulation. A non-invasive light-driven intervention, or low-level laser/light therapy (Cardoso et al., 2022a), that uses red and near-infrared light to stimulate healing processes, reduce pain, and reduce inflammation in several tissues, including the nervous system (Rojas and Gonzalez-Lima, 2011, 2013; de Almeida et al., 2013; Arany, 2016; Hamblin, 2017; Cardoso et al., 2021; Cardoso et al., 2022b). The mechanism is not fully understood, however, the absorption of light by cytochrome c oxidase accelerates electron reactions in mammalian mitochondria. Additionally, it increases energy metabolism and ATP production and prevents NO-induced cell death (Hassan et al., 2021).
Chen et al. (2021) conjugated hUCMSCs and photobiomodulation with a laser in a rat SCI model. In this study, the animals were distributed into SCI, hUCMSCs, laser, and combination treatments (hUCMSCs + laser) groups. They saw that the combined treatment significantly reduced secondary damage and promoted functional recovery compared to either monotherapy (Chen et al., 2021). The photobiomodulation therapy was also combined with ChABC to decrease neuropathic pain, a frequent complication after SCI. With this attempt, there was a reduction in allodynia and thermal hyperalgesia with photobiomodulation, ChABC, and the combination, but it did not reduce mechanical hyperalgesia (Janzadeh et al., 2020). Evidence has suggested that photobiomodulation and human adipose-derived stem cells have neuroprotective effects after SCI. The combined effect of the human adipose-derived stem cells and laser therapy significantly improved motor function and alleviated hyperalgesia and allodynia associated with SCI (Sarveazad et al., 2019).
Conclusion
Spinal cord regeneration is a complex physiological process, and no optimal treatment has yet been developed for fully repairing the spinal cord. Several approaches such as biomolecules, stem cells, and 3D scaffolds are being explored to regenerate the spinal cord. Despite evidence suggesting that these strategies can be useful for spinal cord reconstruction, they do not appear to adequately improve clinical outcomes. So, combining these strategies may be more effective than using them alone.
Theoretically, combinatorial strategies have the highest chance of creating an environment that mimics a healthy spinal cord’s niche with potentially better results. For the injured spinal cord to be restored structurally and functionally, a combination of neuroprotective and neuroregenerative agents may need to be used simultaneously. In our analysis, the vast majority of the works reported functional and/or histological improvements when using a combinatorial approach. However, it is important to be aware of the publication bias, when only positive results are published, it can create a skewed view of the scientific evidence and lead to incorrect conclusions. Publication bias can lead to wasted resources and missed opportunities to learn from unsuccessful experiments. Moreover, combinatorial trials also have many challenges. The combination may cause toxic effects or block the effects of one or more therapeutic agents. For all studies (pre-clinical or clinical trials), a large number of animals/individuals are needed since controls should be included for all groups and combinations. Moreover, different time points of administration, dosages, or routes of administration can produce different results. Therefore, identifying the most effective combinatorial therapies for SCI is complex, time-consuming, and expensive; however, it might be crucial to find a successful clinical treatment.
The lack of significant success in human clinical trials for SCI represents a challenge in biomedical research. Despite advancements in understanding the complexities of SCI and the development of potential therapeutic interventions, translating promising preclinical results into meaningful outcomes for patients has remained elusive. If this has been true for single therapies, thus doing it for combinatorial therapies will be even more complex. Factors such as anatomical and physiological differences between species, as well as the limitations of experimental models in recapitulating the complexity of human SCI, contribute to the translational gap. Moreover, human SCI encompasses a spectrum of injuries, ranging from incomplete to complete lesions, affecting different levels of the spinal cord. This heterogeneity makes it challenging to translate the uniform treatment strategies usually developed and tested in pre-clinical trials. It is also important to mention that experimental therapies and interventions tested in animal models may not always replicate the same efficacy or safety profile when translated to humans. Factors such as pharmacokinetics, dosing, and immune responses may differ between species, influencing the outcomes of therapeutic trials. Thus, the design of pre-clinical trials needs to be more rigorous and better mimic the clinical situation. For instance, researchers may use a range of animal models that better represent the different aspects of human SCI, including species-specific differences and injury mechanisms, use models with diverse injury severities, use a comprehensive battery of outcome measures to better capture the complexity of SCI recovery, and also increase the use of standardized protocols, reporting guidelines and open databases to enhance reproducibility and comparability across pre-clinical studies. Additionally, the design of clinical trials also owns some challenges. Issues such as patient selection criteria, outcome measures, and the duration of follow-up periods can significantly impact the ability of the trial to detect meaningful treatment effects. Additionally, the relatively small patient population and the need for long-term assessments further complicate the design and execution of clinical trials in this field. In the case of combinatorial therapies, the design of clinical trials will have to consider the statistical power needed to detect treatment effects between single and combined agents, this may reveal to be cost-prohibitive and pose significant logistical questions. Despite these challenges, ongoing research efforts continue to explore innovative approaches for SCI treatment, including cell-based therapies, neuroprotective agents, biomaterial scaffolds, and neuromodulation techniques. While animal models play a key role in SCI research by providing insights into pathophysiological mechanisms and testing new therapies, it is also essential to recognize their limitations and consider the differences between animal experiments and human injuries when interpreting research findings and planning translational studies. Integrating findings from both animal and human studies, along with advances in computational modeling and non-animal in vitro models, can help bridge the gap between preclinical research and clinical applications in the pursuit of effective treatments for SCI. Collaborative efforts between researchers, clinicians, industry partners, and patient advocacy groups are essential for advancing the field and improving outcomes for individuals living with SCI.
Funding Statement
Funding: This work has been funded by National funds, through the Foundation for Science and Technology (FCT) - project UIDB/50026/2020 (DOI 10.54499/UIDB/50026/2020), UIDP/50026/2020 (DOI 10.54499/UIDP/50026/2020) and LA/P/0050/2020 (DOI 10.54499/LA/P/0050/2020) (to NAS). Financial support was also provided by Prémios Santa Casa Neurociências–Prize Melo e Castro for Spinal Cord Injury Research (MC-18-2021) and by Wings for Life Spinal Cord Research Foundation (WFL-PT-14/23) (to NAS).
Footnotes
Conflicts of interest: The authors declare no conflicts of interest.
C-Editors: Zhao M, Liu WJ, Qiu Y; T-Editor: Jia Y
Data availability statement:
Not applicable.
References
- Alastrue-Agudo A, Rodriguez-Jimenez FJ, Mocholi EL, De Giorgio F, Erceg S, Moreno-Manzano V. FM19G11 and ependymal progenitor/stem cell combinatory treatment enhances neuronal preservation and oligodendrogenesis after severe spinal cord injury. Int J Mol Sci. 2018;19:200. doi: 10.3390/ijms19010200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alcántar-Garibay OV, Incontri-Abraham D, Ibarra A. Spinal cord injury-induced cognitive impairment: a narrative review. Neural Regen Res. 2022;17:2649–2654. doi: 10.4103/1673-5374.339475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alizadeh A, Dyck SM, Karimi-Abdolrezaee S. Traumatic spinal cord injury: an overview of pathophysiology, models and acute injury mechanisms. Front Neurol. 2019;10:282. doi: 10.3389/fneur.2019.00282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anjum A, Yazid MD, Fauzi Daud M, Idris J, Ng AMH, Selvi Naicker A, Ismail OHR, Athi Kumar RK, Lokanathan Y. Spinal cord injury: pathophysiology, multimolecular interactions, and underlying recovery mechanisms. Int J Mol Sci. 2020;21:7533. doi: 10.3390/ijms21207533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arany PR. Craniofacial wound healing with photobiomodulation therapy: new insights and current challenges. J Dent Res. 2016;95:977–984. doi: 10.1177/0022034516648939. [DOI] [PubMed] [Google Scholar]
- Babaloo H, Ebrahimi-Barough S, Derakhshan MA, Yazdankhah M, Lotfibakhshaiesh N, Soleimani M, Joghataei MT, Ai J. PCL/gelatin nanofibrous scaffolds with human endometrial stem cells/Schwann cells facilitate axon regeneration in spinal cord injury. J Cell Physiol. 2019;234:11060–11069. doi: 10.1002/jcp.27936. [DOI] [PubMed] [Google Scholar]
- Badhiwala JH, Wilson JR, Witiw CD, Harrop JS, Vaccaro AR, Aarabi B, Grossman RG, Geisler FH, Fehlings MG. The influence of timing of surgical decompression for acute spinal cord injury: a pooled analysis of individual patient data. Lancet Neurol. 2021;20:117–126. doi: 10.1016/S1474-4422(20)30406-3. [DOI] [PubMed] [Google Scholar]
- Baptiste DC, Fehlings MG. Update on the treatment of spinal cord injury. Prog Brain Res. 2007;161:217–233. doi: 10.1016/S0079-6123(06)61015-7. [DOI] [PubMed] [Google Scholar]
- Barros AGC, Cristante AF, Santos GBD, Natalino RJM, Ferreira RJR, Barros-Filho TEP. Evaluation of the effects of erythropoietin and interleukin-6 in rats submitted to acute spinal cord injury. Clinics (Sao Paulo) 2019;74:e674. doi: 10.6061/clinics/2019/e674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi J, Sun P, Feng E, Shen J, Chen C, Tan H, Li Z, Lin Y. Melatonin synergizes with methylprednisolone to ameliorate acute spinal cord injury. Front Pharmacol. 2021;12:723913. doi: 10.3389/fphar.2021.723913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bighinati A, Focarete ML, Gualandi C, Pannella M, Giuliani A, Beggiato S, Ferraro L, Lorenzini L, Giardino L, Calza L. Improved functional recovery in rat spinal cord injury induced by a drug combination administered with an implantable polymeric delivery system. J Neurotrauma. 2020;37:1708–1719. doi: 10.1089/neu.2019.6949. [DOI] [PubMed] [Google Scholar]
- Bonilla P, Hernandez J, Giraldo E, Gonzalez-Perez MA, Alastrue-Agudo A, Elkhenany H, Vicent MJ, Navarro X, Edel M, Moreno-Manzano V. Human-induced neural and mesenchymal stem cell therapy combined with a curcumin nanoconjugate as a spinal cord injury treatment. Int J Mol Sci. 2021;22:5966. doi: 10.3390/ijms22115966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozkurt G, Mothe AJ, Zahir T, Kim H, Shoichet MS, Tator CH. Chitosan channels containing spinal cord-derived stem/progenitor cells for repair of subacute spinal cord injury in the rat. Neurosurgery. 2010;67:1733–1744. doi: 10.1227/NEU.0b013e3181f9af35. [DOI] [PubMed] [Google Scholar]
- Brouwers EMJR, et al. Recovery after traumatic thoracic- and lumbar spinal cord injury: the neurological level of injury matters. Spinal Cord. 2020;58:980–987. doi: 10.1038/s41393-020-0463-1. [DOI] [PubMed] [Google Scholar]
- Canseco JA, Karamian BA, Bowles DR, Markowitz MP, DiMaria SL, Semenza NC, Leibensperger MR, Smith ML, Vaccaro AR. Updated review: the steroid controversy for management of spinal cord injury. World Neurosurg. 2021;150:1–8. doi: 10.1016/j.wneu.2021.02.116. [DOI] [PubMed] [Google Scholar]
- Cardoso FDS, Gonzalez-Lima F, Gomes da Silva S. Photobiomodulation for the aging brain. Ageing Res Rev. 2021;70:101415. doi: 10.1016/j.arr.2021.101415. [DOI] [PubMed] [Google Scholar]
- Cardoso FDS, Salehpour F, Coimbra NC, Gonzalez-Lima F, Gomes da Silva S. Photobiomodulation for the treatment of neuroinflammation: a systematic review of controlled laboratory animal studies. Front Neurosci. 2022;16:1006031. doi: 10.3389/fnins.2022.1006031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardoso FDS, de Souza Oliveira Tavares C, Araujo BHS, Mansur F, Lopes-Martins RÁB, Gomes da Silva S. Improved spatial memory and neuroinflammatory profile changes in aged rats submitted to photobiomodulation therapy. Cell Mol Neurobiol. 2022;42:1875–1886. doi: 10.1007/s10571-021-01069-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerqueira SR, Oliveira JM, Silva NA, Leite-Almeida H, Ribeiro-Samy S, Almeida A, Mano JF, Sousa N, Salgado AJ, Reis RL. Microglia response and in vivo therapeutic potential of methylprednisolone-loaded dendrimer nanoparticles in spinal cord injury. Small. 2013;9:738–749. doi: 10.1002/smll.201201888. [DOI] [PubMed] [Google Scholar]
- Chen H, Wang Y, Tu W, Wang H, Yin H, Sha H, Li Y. Effects of photobiomodulation combined with MSCs transplantation on the repair of spinal cord injury in rat. J Cell Physiol. 2021;236:921–930. doi: 10.1002/jcp.29902. [DOI] [PubMed] [Google Scholar]
- Chen X, Zhao Y, Li X, Xiao Z, Yao Y, Chu Y, Farkas B, Romano I, Brandi F, Dai J. Functional multichannel poly(propylene fumarate)-collagen scaffold with collagen-binding neurotrophic factor 3 promotes neural regeneration after transected spinal cord injury. Adv Healthc Mater. 2018;7:e1800315. doi: 10.1002/adhm.201800315. [DOI] [PubMed] [Google Scholar]
- Chvatal SA, Kim YT, Bratt-Leal AM, Lee H, Bellamkonda RV. Spatial distribution and acute anti-inflammatory effects of methylprednisolone after sustained local delivery to the contused spinal cord. Biomaterials. 2008;29:1967–1975. doi: 10.1016/j.biomaterials.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtine G, Gerasimenko Y, van den Brand R, Yew A, Musienko P, Zhong H, Song B, Ao Y, Ichiyama RM, Lavrov I, Roy RR, Sofroniew MV, Edgerton VR. Transformation of nonfunctional spinal circuits into functional states after the loss of brain input. Nat Neurosci. 2009;12:1333–1342. doi: 10.1038/nn.2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuenca-Ortola I, Martinez-Rojas B, Moreno-Manzano V, Garcia Castello M, Monleon Pradas M, Martinez-Ramos C, Mas Estelles J. A strategy for magnetic and electric stimulation to enhance proliferation and differentiation of NPCs seeded over PLA electrospun membranes. Biomedicines. 2022;10:2736. doi: 10.3390/biomedicines10112736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Almeida P, Lopes-Martins RA, Tomazoni SS, Albuquerque-Pontes GM, Santos LA, Vanin AA, Frigo L, Vieira RP, Albertini R, de Carvalho Pde T, Leal-Junior EC. Low-level laser therapy and sodium diclofenac in acute inflammatory response induced by skeletal muscle trauma: effects in muscle morphology and mRNA gene expression of inflammatory markers. Photochem Photobiol. 2013;89:501–507. doi: 10.1111/j.1751-1097.2012.01232.x. [DOI] [PubMed] [Google Scholar]
- Deumens R, Van Gorp SF, Bozkurt A, Beckmann C, Fuhrmann T, Montzka K, Tolba R, Kobayashi E, Heschel I, Weis J, Brook GA. Motor outcome and allodynia are largely unaffected by novel olfactory ensheathing cell grafts to repair low-thoracic lesion gaps in the adult rat spinal cord. Behav Brain Res. 2013;237:185–189. doi: 10.1016/j.bbr.2012.09.036. [DOI] [PubMed] [Google Scholar]
- Di Silvio L. 15 - Bone tissue engineering and biomineralization. In: Boccaccini AR, Gough JE, editors. Tissue engineering using ceramics and polymers. Cambridge: Woodhead Publishing; 2007. pp. 319–331. [Google Scholar]
- Elliott Donaghue I, Tator CH, Shoichet MS. Sustained delivery of bioactive neurotrophin-3 to the injured spinal cord. Biomater Sci. 2015;3:65–72. doi: 10.1039/c4bm00311j. [DOI] [PubMed] [Google Scholar]
- Fan B, Wei Z, Feng S. Progression in translational research on spinal cord injury based on microenvironment imbalance. Bone Research. 2022;10:35. doi: 10.1038/s41413-022-00199-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan C, Li X, Zhao Y, Xiao Z, Xue W, Sun J, Li X, Zhuang Y, Chen Y, Dai J. Cetuximab and taxol co-modified collagen scaffolds show combination effects for the repair of acute spinal cord injury. Biomater Sci. 2018;6:1723–1734. doi: 10.1039/c8bm00363g. [DOI] [PubMed] [Google Scholar]
- Fathi A, Khanmohammadi M, Goodarzi A, Foroutani L, Mobarakeh ZT, Saremi J, Arabpour Z, Ai J. Fabrication of chitosan-polyvinyl alcohol and silk electrospun fiber seeded with differentiated keratinocyte for skin tissue regeneration in animal wound model. J Biol Eng. 2020;14:27. doi: 10.1186/s13036-020-00249-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng S, Wang S, Sun S, Su H, Zhang L. Effects of combination treatment with transcranial magnetic stimulation and bone marrow mesenchymal stem cell transplantation or Raf inhibition on spinal cord injury in rats. Mol Med Rep. 2021;23:294. doi: 10.3892/mmr.2021.11934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganjeifar B, Rezaee H, Keykhosravi E, Tavallaii A, Bahadorkhan G, Nakhaei M, Abouei Mehrizi MA. The effect of combination therapy with erythropoietin and methylprednisolone in patients with traumatic cervical spinal cord injury: a pilot randomized controlled trial. Spinal Cord. 2021;59:347–353. doi: 10.1038/s41393-020-00604-2. [DOI] [PubMed] [Google Scholar]
- Gao M, Lu P, Bednark B, Lynam D, Conner JM, Sakamoto J, Tuszynski MH. Templated agarose scaffolds for the support of motor axon regeneration into sites of complete spinal cord transection. Biomaterials. 2013;34:1529–1536. doi: 10.1016/j.biomaterials.2012.10.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao S, Ding J, Xiao HJ, Li ZQ, Chen Y, Zhou XS, Wang JE, Wu J, Shi WZ. Anti-inflammatory and anti-apoptotic effect of combined treatment with methylprednisolone and amniotic membrane mesenchymal stem cells after spinal cord injury in rats. Neurochem Res. 2014;39:1544–1552. doi: 10.1007/s11064-014-1344-9. [DOI] [PubMed] [Google Scholar]
- Garcia E, Rodriguez-Barrera R, Buzoianu-Anguiano V, Flores-Romero A, Malagon-Axotla E, Guerrero-Godinez M, De la Cruz-Castillo E, Castillo-Carvajal L, Rivas-Gonzalez M, Santiago-Tovar P, Morales I, Borlongan C, Ibarra A. Use of a combination strategy to improve neuroprotection and neuroregeneration in a rat model of acute spinal cord injury. Neural Regen Res. 2019;14:1060–1068. doi: 10.4103/1673-5374.250627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Alias G, Petrosyan HA, Schnell L, Horner PJ, Bowers WJ, Mendell LM, Fawcett JW, Arvanian VL. Chondroitinase ABC combined with neurotrophin NT-3 secretion and NR2D expression promotes axonal plasticity and functional recovery in rats with lateral hemisection of the spinal cord. J Neurosci. 2011;31:17788–17799. doi: 10.1523/JNEUROSCI.4308-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Alias G, Fawcett JW. Training and anti-CSPG combination therapy for spinal cord injury. Exp Neurol. 2012;235:26–32. doi: 10.1016/j.expneurol.2011.09.009. [DOI] [PubMed] [Google Scholar]
- Gee CM, Williams AM, Peters CM, Eves ND, Sheel AW, West CR. Influence of respiratory loading on left-ventricular function in cervical spinal cord injury. J Physiol. 2022;600:4105–4118. doi: 10.1113/JP282717. [DOI] [PubMed] [Google Scholar]
- Gerasimenko YP, Ichiyama RM, Lavrov IA, Courtine G, Cai L, Zhong H, Roy RR, Edgerton VR. Epidural spinal cord stimulation plus quipazine administration enable stepping in complete spinal adult rats. J Neurophysiol. 2007;98:2525–2536. doi: 10.1152/jn.00836.2007. [DOI] [PubMed] [Google Scholar]
- Gomes ED, Ghosh B, Lima R, Goulao M, Moreira-Gomes T, Martins-Macedo J, Urban MW, Wright MC, Gimble JM, Sousa N, Silva NA, Lepore AC, Salgado AJ. Combination of a gellan gum-based hydrogel with cell therapy for the treatment of cervical spinal cord injury. Front Bioeng Biotechnol. 2020;8:984. doi: 10.3389/fbioe.2020.00984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gros T, Sakamoto JS, Blesch A, Havton LA, Tuszynski MH. Regeneration of long-tract axons through sites of spinal cord injury using templated agarose scaffolds. Biomaterials. 2010;31:6719–6729. doi: 10.1016/j.biomaterials.2010.04.035. [DOI] [PubMed] [Google Scholar]
- Gunther MI, Gunther M, Schneiders M, Rupp R, Blesch A. AngleJ: a new tool for the automated measurement of neurite growth orientation in tissue sections. J Neurosci Methods. 2015;251:143–150. doi: 10.1016/j.jneumeth.2015.05.021. [DOI] [PubMed] [Google Scholar]
- Haggerty AE, Maldonado-Lasuncion I, Nitobe Y, Yamane K, Marlow MM, You H, Zhang C, Cho B, Li X, Reddy S, Mao HQ, Oudega M. The effects of the combination of mesenchymal stromal cells and nanofiber-hydrogel composite on repair of the contused spinal cord. Cells. 2022;11:1137. doi: 10.3390/cells11071137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamblin MR. Mechanisms and applications of the anti-inflammatory effects of photobiomodulation. AIMS Biophys. 2017;4:337–361. doi: 10.3934/biophy.2017.3.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan MP, Abdollahifar MA, Aliaghaei A, Tabeie F, Vafaei-Nezhad S, Norouzian M, Abbaszadeh HA. Photobiomodulation therapy improved functional recovery and overexpression of interleukins-10 after contusion spinal cord injury in rats. J Chem Neuroanat. 2021;117:102010. doi: 10.1016/j.jchemneu.2021.102010. [DOI] [PubMed] [Google Scholar]
- Hawryluk GW, Rowland J, Kwon BK, Fehlings MG. Protection and repair of the injured spinal cord: a review of completed, ongoing, and planned clinical trials for acute spinal cord injury. Neurosurg Focus. 2008;25:E14. doi: 10.3171/FOC.2008.25.11.E14. [DOI] [PubMed] [Google Scholar]
- Hosseini M, Karami Z, Yousefifard M, Janzadeh A, Zamani E, Nasirinezhad F. Simultaneous intrathecal injection of muscimol and endomorphin-1 alleviates neuropathic pain in rat model of spinal cord injury. Brain Behav. 2020;10:e01576. doi: 10.1002/brb3.1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang F, Wang J, Chen A. Effects of co-grafts mesenchymal stem cells and nerve growth factor suspension in the repair of spinal cord injury. J Huazhong Univ Sci Technolog Med Sci. 2006;26:206–210. doi: 10.1007/BF02895817. [DOI] [PubMed] [Google Scholar]
- Hurtado A, Moon LD, Maquet V, Blits B, Jerome R, Oudega M. Poly (D,L-lactic acid) macroporous guidance scaffolds seeded with Schwann cells genetically modified to secrete a bi-functional neurotrophin implanted in the completely transected adult rat thoracic spinal cord. Biomaterials. 2006;27:430–442. doi: 10.1016/j.biomaterials.2005.07.014. [DOI] [PubMed] [Google Scholar]
- Ikegami T, Nakamura M, Yamane J, Katoh H, Okada S, Iwanami A, Watanabe K, Ishii K, Kato F, Fujita H, Takahashi T, Okano HJ, Toyama Y, Okano H. Chondroitinase ABC combined with neural stem/progenitor cell transplantation enhances graft cell migration and outgrowth of growth-associated protein-43-positive fibers after rat spinal cord injury. Eur J Neurosci. 2005;22:3036–3046. doi: 10.1111/j.1460-9568.2005.04492.x. [DOI] [PubMed] [Google Scholar]
- Janzadeh A, Sarveazad A, Hamblin MR, Teheripak G, Kookli K, Nasirinezhad F. The effect of chondroitinase ABC and photobiomodulation therapy on neuropathic pain after spinal cord injury in adult male rats. Physiol Behav. 2020;227:113141. doi: 10.1016/j.physbeh.2020.113141. [DOI] [PubMed] [Google Scholar]
- Jiang JP, Liu XY, Zhao F, Zhu X, Li XY, Niu XG, Yao ZT, Dai C, Xu HY, Ma K, Chen XY, Zhang S. Three-dimensional bioprinting collagen/silk fibroin scaffold combined with neural stem cells promotes nerve regeneration after spinal cord injury. Neural Regen Res. 2020;15:959–968. doi: 10.4103/1673-5374.268974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakizawa S. Subchapter 41C - neurotrophin-3. In: Ando H, Ukena K, Nagata S, editors. Handbook of hormones (Second edition) San Diego: Academic Press; 2021. pp. 483–485. [Google Scholar]
- Kandalam S, De Berdt P, Ucakar B, Vanvarenberg K, Bouzin C, Gratpain V, Diogenes A, Montero-Menei CN, des Rieux A. Human dental stem cells of the apical papilla associated to BDNF-loaded pharmacologically active microcarriers (PAMs) enhance locomotor function after spinal cord injury. Int J Pharm. 2020;587:119685. doi: 10.1016/j.ijpharm.2020.119685. [DOI] [PubMed] [Google Scholar]
- Kang CE, Baumann MD, Tator CH, Shoichet MS. Localized and sustained delivery of fibroblast growth factor-2 from a nanoparticle-hydrogel composite for treatment of spinal cord injury. Cells Tissues Organs. 2013;197:55–63. doi: 10.1159/000339589. [DOI] [PubMed] [Google Scholar]
- Kaptanoglu E, Beskonakli E, Solaroglu I, Kilinc A, Taskin Y. Magnesium sulfate treatment in experimental spinal cord injury: emphasis on vascular changes and early clinical results. Neurosurg Rev. 2003;26:283–287. doi: 10.1007/s10143-003-0272-y. [DOI] [PubMed] [Google Scholar]
- Khaing ZZ, Agrawal NK, Park JH, Xin S, Plumton GC, Lee KH, Huang YJ, Niemerski AL, Schmidt CE, Grau JW. Localized and sustained release of brain-derived neurotrophic factor from injectable hydrogel/microparticle composites fosters spinal learning after spinal cord injury. J Mater Chem B. 2016;4:7560–7571. doi: 10.1039/c6tb01602b. [DOI] [PubMed] [Google Scholar]
- Khan IU, Yoon Y, Kim A, Jo KR, Choi KU, Jung T, Kim N, Son Y, Kim WH, Kweon OK. Improved healing after the co-transplantation of HO-1 and BDNF overexpressed mesenchymal stem cells in the subacute spinal cord injury of dogs. Cell Transplant. 2018;27:1140–1153. doi: 10.1177/0963689718779766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KN, Oh SH, Lee KH, Yoon DH. Effect of human mesenchymal stem cell transplantation combined with growth factor infusion in the repair of injured spinal cord. Acta Neurochir Suppl. 2006;99:133–136. doi: 10.1007/978-3-211-35205-2_25. [DOI] [PubMed] [Google Scholar]
- Koffler J, Zhu W, Qu X, Platoshyn O, Dulin JN, Brock J, Graham L, Lu P, Sakamoto J, Marsala M, Chen S, Tuszynski MH. Biomimetic 3D-printed scaffolds for spinal cord injury repair. Nat Med. 2019;25:263–269. doi: 10.1038/s41591-018-0296-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kourgiantaki A, Tzeranis DS, Karali K, Georgelou K, Bampoula E, Psilodimitrakopoulos S, Yannas IV, Stratakis E, Sidiropoulou K, Charalampopoulos I, Gravanis A. Neural stem cell delivery via porous collagen scaffolds promotes neuronal differentiation and locomotion recovery in spinal cord injury. NPJ Regen Med. 2020;5:12. doi: 10.1038/s41536-020-0097-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwiecien JM, Zhang L, Yaron JR, Schutz LN, Kwiecien-Delaney CJ, Awo EA, Burgin M, Dabrowski W, Lucas AR. Local serpin treatment via chitosan-collagen hydrogel after spinal cord injury reduces tissue damage and improves neurologic function. J Clin Med. 2020;9:1221. doi: 10.3390/jcm9041221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon BK, Roy J, Lee JH, Okon E, Zhang H, Marx JC, Kindy MS. Magnesium chloride in a polyethylene glycol formulation as a neuroprotective therapy for acute spinal cord injury: preclinical refinement and optimization. J Neurotrauma. 2009;26:1379–1393. doi: 10.1089/neu.2009.0884. [DOI] [PubMed] [Google Scholar]
- Landry ES, Lapointe NP, Rouillard C, Levesque D, Hedlund PB, Guertin PA. Contribution of spinal 5-HT1A and 5-HT7 receptors to locomotor-like movement induced by 8-OH-DPAT in spinal cord-transected mice. Eur J Neurosci. 2006;24:535–546. doi: 10.1111/j.1460-9568.2006.04917.x. [DOI] [PubMed] [Google Scholar]
- Lee YS, Wu S, Arinzeh TL, Bunge MB. Enhanced noradrenergic axon regeneration into schwann cell-filled PVDF-TrFE conduits after complete spinal cord transection. Biotechnol Bioeng. 2017;114:444–456. doi: 10.1002/bit.26088. [DOI] [PubMed] [Google Scholar]
- Lee YS, Kim KT, Kwon BK. Hemodynamic management of acute spinal cord injury: a literature review. Neurospine. 2021;18:7–14. doi: 10.14245/ns.2040144.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Zhang Y, Mu J, Chen J, Zhang C, Cao H, Gao J. Transplantation of human mesenchymal stem-cell-derived exosomes immobilized in an adhesive hydrogel for effective treatment of spinal cord injury. Nano Lett. 2020;20:4298–4305. doi: 10.1021/acs.nanolett.0c00929. [DOI] [PubMed] [Google Scholar]
- Li X, Xiao Z, Han J, Chen L, Xiao H, Ma F, Hou X, Li X, Sun J, Ding W, Zhao Y, Chen B, Dai J. Promotion of neuronal differentiation of neural progenitor cells by using EGFR antibody functionalized collagen scaffolds for spinal cord injury repair. Biomaterials. 2013;34:5107–5116. doi: 10.1016/j.biomaterials.2013.03.062. [DOI] [PubMed] [Google Scholar]
- Li X, Wu M, Gu L, Ren Y, Mu M, Wang Y, Gao X, Li J, Tong A, Zhu H, Zhou L, Chen H, Guo G. A single dose of thermal-sensitive biodegradable hybrid hydrogel promotes functional recovery after spinal cord injury. Appl Mater Today. 2019;14:66–75. [Google Scholar]
- Lima R, Monteiro A, Salgado AJ, Monteiro S, Silva NA. Pathophysiology and therapeutic approaches for spinal cord injury. Int J Mol Sci. 2022;23:13833. doi: 10.3390/ijms232213833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Schackel T, Weidner N, Puttagunta R. Biomaterial-supported cell transplantation treatments for spinal cord injury: challenges and perspectives. Front Cell Neurosci. 2017;11:430. doi: 10.3389/fncel.2017.00430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Sandner B, Schackel T, Nicholson L, Chtarto A, Tenenbaum L, Puttagunta R, Muller R, Weidner N, Blesch A. Regulated viral BDNF delivery in combination with Schwann cells promotes axonal regeneration through capillary alginate hydrogels after spinal cord injury. Acta Biomater. 2017;60:167–180. doi: 10.1016/j.actbio.2017.07.024. [DOI] [PubMed] [Google Scholar]
- Lowry N, Goderie SK, Lederman P, Charniga C, Gooch MR, Gracey KD, Banerjee A, Punyani S, Silver J, Kane RS, Stern JH, Temple S. The effect of long-term release of Shh from implanted biodegradable microspheres on recovery from spinal cord injury in mice. Biomaterials. 2012;33:2892–2901. doi: 10.1016/j.biomaterials.2011.12.048. [DOI] [PubMed] [Google Scholar]
- Ma W, Zhan Y, Zhang Y, Xie X, Mao C, Lin Y. Enhanced neural regeneration with a concomitant treatment of framework nucleic acid and stem cells in spinal cord injury. ACS Appl Mater Interfaces. 2020;12:2095–2106. doi: 10.1021/acsami.9b19079. [DOI] [PubMed] [Google Scholar]
- Mekhail M, Almazan G, Tabrizian M. Purine-crosslinked injectable chitosan sponges promote oligodendrocyte progenitor cells’ attachment and differentiation. Biomater Sci. 2015;3:279–287. doi: 10.1039/c4bm00215f. [DOI] [PubMed] [Google Scholar]
- Menacho ST, Floyd C. Current practices and goals for mean arterial pressure and spinal cord perfusion pressure in acute traumatic spinal cord injury: defining the gaps in knowledge. J Spinal Cord Med. 2021;44:350–356. doi: 10.1080/10790268.2019.1660840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirzaei F, Meshkini A, Habibi B, Salehpour F, Rafei E, Fathi W, Alavi SHN, Majdi A, Rahigh Aghasan S, Naseri Alavi SA. Ceftriaxone plus methylprednisolone combination therapy versus methylprednisolone monotherapy in patients with acute spinal cord injury: a randomized, triple-blind clinical trial. Int J Spine Surg. 2020;14:706–712. doi: 10.14444/7102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohaghegh Shalmani L, Valian N, Pournajaf S, Abbaszadeh F, Dargahi L, Jorjani M. Combination therapy with astaxanthin and epidermal neural crest stem cells improves motor impairments and activates mitochondrial biogenesis in a rat model of spinal cord injury. Mitochondrion. 2020;52:125–134. doi: 10.1016/j.mito.2020.03.002. [DOI] [PubMed] [Google Scholar]
- Mohammadshirazi A, Sadrosadat H, Jaberi R, Zareikheirabadi M, Mirsadeghi S, Naghdabadi Z, Ghaneezabadi M, Fardmanesh M, Baharvand H, Kiani S. Combinational therapy of lithium and human neural stem cells in rat spinal cord contusion model. J Cell Physiol. 2019;234:20742–20754. doi: 10.1002/jcp.28680. [DOI] [PubMed] [Google Scholar]
- Monteiro S, Pinho AG, Macieira M, Serre-Miranda C, Cibrao JR, Lima R, Soares-Cunha C, Vasconcelos NL, Lentilhas-Graca J, Duarte-Silva S, Miranda A, Correia-Neves M, Salgado AJ, Silva NA. Splenic sympathetic signaling contributes to acute neutrophil infiltration of the injured spinal cord. J Neuroinflammation. 2020;17:282. doi: 10.1186/s12974-020-01945-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mothe AJ, Tam RY, Zahir T, Tator CH, Shoichet MS. Repair of the injured spinal cord by transplantation of neural stem cells in a hyaluronan-based hydrogel. Biomaterials. 2013;34:3775–3783. doi: 10.1016/j.biomaterials.2013.02.002. [DOI] [PubMed] [Google Scholar]
- Namjoo Z, Moradi F, Aryanpour R, Piryaei A, Joghataei MT, Abbasi Y, Hosseini A, Hassanzadeh S, Taklimie FR, Beyer C, Zendedel A. Combined effects of rat Schwann cells and 17beta-estradiol in a spinal cord injury model. Metab Brain Dis. 2018;33:1229–1242. doi: 10.1007/s11011-018-0220-8. [DOI] [PubMed] [Google Scholar]
- Narimanpour Z, Nazm Bojnordi M, Somayeh EB, Elham V, Jamileh S, Ghasemi H. Silk nanofibrous electrospun scaffold amplifies proliferation and stemness profile of mouse spermatogonial stem cells. Regen Eng Transl Med. 2020;8:86–93. [Google Scholar]
- Naseem M, Parvez S. Role of melatonin in traumatic brain injury and spinal cord injury. ScientificWorldJournal. 2014;2014:586270. doi: 10.1155/2014/586270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nazemi Z, Nourbakhsh MS, Kiani S, Heydari Y, Ashtiani MK, Daemi H, Baharvand H. Co-delivery of minocycline and paclitaxel from injectable hydrogel for treatment of spinal cord injury. J Control Release. 2020;321:145–158. doi: 10.1016/j.jconrel.2020.02.009. [DOI] [PubMed] [Google Scholar]
- Nori S, Khazaei M, Ahuja CS, Yokota K, Ahlfors JE, Liu Y, Wang J, Shibata S, Chio J, Hettiaratchi MH, Fuhrmann T, Shoichet MS, Fehlings MG. Human oligodendrogenic neural progenitor cells delivered with chondroitinase ABC facilitate functional repair of chronic spinal cord injury. Stem Cell Reports. 2018;11:1433–1448. doi: 10.1016/j.stemcr.2018.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson HE, Rooney GE, Gross L, Nesbitt JJ, Galvin KE, Knight A, Chen B, Yaszemski MJ, Windebank AJ. Neural stem cell- and Schwann cell-loaded biodegradable polymer scaffolds support axonal regeneration in the transected spinal cord. Tissue Eng Part A. 2009;15:1797–1805. doi: 10.1089/ten.tea.2008.0364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onuma-Ukegawa M, Bhatt K, Hirai T, Kaburagi H, Sotome S, Wakabayashi Y, Ichinose S, Shinomiya K, Okawa A, Enomoto M. Bone marrow stromal cells combined with a honeycomb collagen sponge facilitate neurite elongation in vitro and neural restoration in the hemisected rat spinal cord. Cell Transplant. 2015;24:1283–1297. doi: 10.3727/096368914X682134. [DOI] [PubMed] [Google Scholar]
- Orazizadeh M, Rashidi I, Saremi J, Latifi M. Focal adhesion kinase (FAK) involvement in human endometrial remodeling during the menstrual cycle. Iran Biomed J. 2009;13:95–101. [PubMed] [Google Scholar]
- Ornitz DM, Xu J, Colvin JS, McEwen DG, MacArthur CA, Coulier F, Gao G, Goldfarb M. Receptor specificity of the fibroblast growth factor family. J Biol Chem. 1996;271:15292–15297. doi: 10.1074/jbc.271.25.15292. [DOI] [PubMed] [Google Scholar]
- Papa S, Ferrari R, De Paola M, Rossi F, Mariani A, Caron I, Sammali E, Peviani M, Dell’Oro V, Colombo C, Morbidelli M, Forloni G, Perale G, Moscatelli D, Veglianese P. Polymeric nanoparticle system to target activated microglia/macrophages in spinal cord injury. J Control Release. 2014;174:15–26. doi: 10.1016/j.jconrel.2013.11.001. [DOI] [PubMed] [Google Scholar]
- Park SS, Lee YJ, Lee SH, Lee D, Choi K, Kim WH, Kweon OK, Han HJ. Functional recovery after spinal cord injury in dogs treated with a combination of Matrigel and neural-induced adipose-derived mesenchymal Stem cells. Cytotherapy. 2012;14:584–597. doi: 10.3109/14653249.2012.658913. [DOI] [PubMed] [Google Scholar]
- Perrouin-Verbe B, Lefevre C, Kieny P, Gross R, Reiss B, Le Fort M. Spinal cord injury: a multisystem physiological impairment/dysfunction. Rev Neurol (Paris) 2021;177:594–605. doi: 10.1016/j.neurol.2021.02.385. [DOI] [PubMed] [Google Scholar]
- Pourkhodadad S, Oryan SH, Kaka G, Sadraie SH. Neuroprotective effects of combined treatment with minocycline and olfactory ensheathing cells transplantation against inflammation and oxidative stress after spinal cord injury. Cell J. 2019;21:220–228. doi: 10.22074/cellj.2019.6126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prager J, Ito D, Carwardine DR, Jiju P, Chari DM, Granger N, Wong LF. Delivery of chondroitinase by canine mucosal olfactory ensheathing cells alongside rehabilitation enhances recovery after spinal cord injury. Exp Neurol. 2021;340:113660. doi: 10.1016/j.expneurol.2021.113660. [DOI] [PubMed] [Google Scholar]
- Punjani N, Deska-Gauthier D, Hachem LD, Abramian M, Fehlings MG. Neuroplasticity and regeneration after spinal cord injury. N Am Spine Soc J. 2023;15:100235. doi: 10.1016/j.xnsj.2023.100235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quddusi A, Pedro KM, Alvi MA, Hejrati N, Fehlings MAO. Early surgical intervention for acute spinal cord injury: time is spine. Acta Neurochir (Wien) 2023;165:2665–2674. doi: 10.1007/s00701-023-05698-0. [DOI] [PubMed] [Google Scholar]
- Rezaei N, Bojnordi MN, Ghasemi Hamidabadi H. Differentiation of bone marrow stromal stem cells seeded on silk scaffold to mature oligodendrocyte using cerebrospinal fluid. J Chem Neuroanat. 2020;106:101790. doi: 10.1016/j.jchemneu.2020.101790. [DOI] [PubMed] [Google Scholar]
- Rodríguez-Barrera R, Flores-Romero A, Buzoianu-Anguiano V, Garcia E, Soria-Zavala K, Incontri-Abraham D, Garibay-López M, Juárez-Vignon Whaley JJ, Ibarra A. Use of a combination strategy to improve morphological and functional recovery in rats with chronic spinal cord injury. Front Neurol. 2020;11:189. doi: 10.3389/fneur.2020.00189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojas JC, Gonzalez-Lima F. Low-level light therapy of the eye and brain. Eye Brain. 2011;3:49–67. doi: 10.2147/EB.S21391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojas JC, Gonzalez-Lima F. Neurological and psychological applications of transcranial lasers and LEDs. Biochem Pharmacol. 2013;86:447–457. doi: 10.1016/j.bcp.2013.06.012. [DOI] [PubMed] [Google Scholar]
- Ruzicka J, Urdzikova LM, Svobodova B, Amin AG, Karova K, Dubisova J, Zaviskova K, Kubinova S, Schmidt M, Jhanwar-Uniyal M, Jendelova P. Does combined therapy of curcumin and epigallocatechin gallate have a synergistic neuroprotective effect against spinal cord injury? Neural Regen Res. 2018;13:119–127. doi: 10.4103/1673-5374.224379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacino A, Rosenblatt K. Early management of acute spinal cord injury-part I: initial injury to surgery. J Neuroanaesth Crit Care. 2019;6:213–221. doi: 10.1055/s-0039-1694688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salarinia R, Hosseini M, Mohamadi Y, Ghorbani A, Alamdari DH, Mafinezhad A, Sadeghnia H. Combined use of platelet-rich plasma and adipose tissue-derived mesenchymal stem cells shows a synergistic effect in experimental spinal cord injury. J Chem Neuroanat. 2020;110:101870. doi: 10.1016/j.jchemneu.2020.101870. [DOI] [PubMed] [Google Scholar]
- Sarveazad A, Janzadeh A, Taheripak G, Dameni S, Yousefifard M, Nasirinezhad F. Co-administration of human adipose-derived stem cells and low-level laser to alleviate neuropathic pain after experimental spinal cord injury. Stem Cell Res Ther. 2019;10:183. doi: 10.1186/s13287-019-1269-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellers DL, Kim TH, Mount CW, Pun SH, Horner PJ. Poly(lactic-co-glycolic) acid microspheres encapsulated in Pluronic F-127 prolong hirudin delivery and improve functional recovery from a demyelination lesion. Biomaterials. 2014;35:8895–8902. doi: 10.1016/j.biomaterials.2014.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma A, Feng L, Muresanu DF, Huang H, Menon PK, Sahib S, Ryan Tian Z, Lafuente JV, Buzoianu AD, Castellani RJ, Nozari A, Wiklund L, Sharma HS. Topical application of CNTF, GDNF and BDNF in combination attenuates blood-spinal cord barrier permeability, edema formation, hemeoxygenase-2 upregulation, and cord pathology. Prog Brain Res. 2021;266:357–376. doi: 10.1016/bs.pbr.2021.06.013. [DOI] [PubMed] [Google Scholar]
- Shi T, Hao JX, Wiesenfeld-Hallin Z, Xu XJ. Gabapentin and NMDA receptor antagonists interacts synergistically to alleviate allodynia in two rat models of neuropathic pain. Scand J Pain. 2018;18:687–693. doi: 10.1515/sjpain-2018-0083. [DOI] [PubMed] [Google Scholar]
- Sinopoulou E, Spejo AB, Roopnarine N, Burnside ER, Bartus K, De Winter F, McMahon SB, Bradbury EJ. Chronic muscle recordings reveal recovery of forelimb function in spinal injured female rats after cortical epidural stimulation combined with rehabilitation and chondroitinase ABC. J Neurosci Res. 2022;100:2055–2076. doi: 10.1002/jnr.25111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soendergaard PL, Norup A, Kruse M, Biering-Sørensen F. Socioeconomic consequences of traumatic and non-traumatic spinal cord injuries: a Danish nationwide register-based study. Spinal Cord. 2022;60:647–654. doi: 10.1038/s41393-021-00724-3. [DOI] [PubMed] [Google Scholar]
- Sun WM, Ma CL, Xu J, He JP. Reduction in post-spinal cord injury spasticity by combination of peripheral nerve grafting and acidic fibroblast growth factor infusion in monkeys. J Int Med Res. 2021;49:3000605211022294. doi: 10.1177/03000605211022294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki H, Kanchiku T, Imajo Y, Yoshida Y, Nishida N, Gondo T, Yoshii S, Taguchi T. Artificial collagen-filament scaffold promotes axon regeneration and long tract reconstruction in a rat model of spinal cord transection. Med Mol Morphol. 2015;48:214–224. doi: 10.1007/s00795-015-0104-5. [DOI] [PubMed] [Google Scholar]
- Teixeira WGJ, Cristante AF, Marcon RM, Bispo G, Ferreira R, de Barros-Filho TEP. Granulocyte colony-stimulating factor combined with methylprednisolone improves functional outcomes in rats with experimental acute spinal cord injury. Clinics (Sao Paulo) 2018;73:e235. doi: 10.6061/clinics/2018/e235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torregrossa F, Sallì M, Grasso G. Emerging therapeutic strategies for traumatic spinal cord injury. World Neurosurg. 2020;140:591–601. doi: 10.1016/j.wneu.2020.03.199. [DOI] [PubMed] [Google Scholar]
- Uyanikgil Y, Cavusoglu T, Kilic KD, Yigitturk G, Celik S, Tubbs RS, Turgut M. Useful effects of melatonin in peripheral nerve injury and development of the nervous system. J Brachial Plex Peripher Nerve Inj. 2017;12:e1–6. doi: 10.1055/s-0036-1597838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Den Hauwe L, Sundgren PC, Flanders AE. Spinal trauma and spinal cord injury (SCI) In: Hodler J, Kubik-Huch RA, von Schulthess GK, editors. Diseases of the brain, head and neck, spine 2020–2023: diagnostic imaging. Cham: Springer International Publishing; 2020. pp. 231–240. [PubMed] [Google Scholar]
- Vasconcelos NL, Gomes ED, Oliveira EP, Silva CJ, Lima R, Sousa N, Salgado AJ, Silva NA. Combining neuroprotective agents: effect of riluzole and magnesium in a rat model of thoracic spinal cord injury. Spine J. 2016;16:1015–1024. doi: 10.1016/j.spinee.2016.04.013. [DOI] [PubMed] [Google Scholar]
- Wang C, Sun C, Hu Z, Huo X, Yang Y, Liu X, Botchway BOA, Davies H, Fang M. Improved neural regeneration with olfactory ensheathing cell inoculated PLGA scaffolds in spinal cord injury adult rats. Neurosignals. 2017;25:1–14. doi: 10.1159/000471828. [DOI] [PubMed] [Google Scholar]
- Wang J, Chu R, Ni N, Nan G. The effect of Matrigel as scaffold material for neural stem cell transplantation for treating spinal cord injury. Sci Rep. 2020;10:2576. doi: 10.1038/s41598-020-59148-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang N, Xiao Z, Zhao Y, Wang B, Li X, Li J, Dai J. Collagen scaffold combined with human umbilical cord-derived mesenchymal stem cells promote functional recovery after scar resection in rats with chronic spinal cord injury. J Tissue Eng Regen Med. 2018;12:e1154–1163. doi: 10.1002/term.2450. [DOI] [PubMed] [Google Scholar]
- Wang X, Wang YY, Zhang LL, Li GT, Zhang HT. Combinatory effect of mesenchymal stromal cells transplantation and quercetin after spinal cord injury in rat. Eur Rev Med Pharmacol Sci. 2018;22:2876–2887. doi: 10.26355/eurrev_201805_14990. [DOI] [PubMed] [Google Scholar]
- Wang Z, Wang Z, Lu WW, Zhen W, Yang D, Peng S. Novel biomaterial strategies for controlled growth factor delivery for biomedical applications. NPG Asia Materials. 2017;9:e435. [Google Scholar]
- Williams RR, Henao M, Pearse DD, Bunge MB. Permissive Schwann cell graft/spinal cord interfaces for axon regeneration. Cell Transplant. 2015;24:115–131. doi: 10.3727/096368913X674657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler T, Sharma HS, Stålberg E, Westman J. Spinal cord bioelectrical activity, edema and cell injury following a focal trauma to the rat spinal cord. An experimental study using pharmacological and morphological approaches. In: Stålberg E, Sharma HS, Olsson Y, editors. Spinal cord monitoring: basic principles, regeneration, pathophysiology, and clinical aspects. Vienna: Springer Vienna; 1998. pp. 283–363. [Google Scholar]
- Xia T, Ni S, Li X, Yao J, Qi H, Fan X, Wang J. Sustained delivery of dbcAMP by poly(propylene carbonate) micron fibers promotes axonal regenerative sprouting and functional recovery after spinal cord hemisection. Brain Res. 2013;1538:41–50. doi: 10.1016/j.brainres.2013.09.027. [DOI] [PubMed] [Google Scholar]
- Yamanaka H, Takata Y, Nakagawa H, Isosaka-Yamanaka T, Yamashita T, Takada M. An enhanced therapeutic effect of repetitive transcranial magnetic stimulation combined with antibody treatment in a primate model of spinal cord injury. PLoS One. 2021;16:e0252023. doi: 10.1371/journal.pone.0252023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamane K, Mazaki T, Shiozaki Y, Yoshida A, Shinohara K, Nakamura M, Yoshida Y, Zhou D, Kitajima T, Tanaka M, Ito Y, Ozaki T, Matsukawa A. Collagen-binding hepatocyte growth factor (HGF) alone or with a Gelatin- furfurylamine hydrogel enhances functional recovery in mice after spinal cord injury. Sci Rep. 2018;8:917. doi: 10.1038/s41598-018-19316-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang EZ, Zhang GW, Xu JG, Chen S, Wang H, Cao LL, Liang B, Lian XF. Multichannel polymer scaffold seeded with activated Schwann cells and bone mesenchymal stem cells improves axonal regeneration and functional recovery after rat spinal cord injury. Acta Pharmacol Sin. 2017;38:623–637. doi: 10.1038/aps.2017.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Fan Y, Zhang H, Zhang Q, Zhao Y, Xiao Z, Liu W, Chen B, Gao L, Sun Z, Xue X, Shu M, Dai J. Small molecules combined with collagen hydrogel direct neurogenesis and migration of neural stem cells after spinal cord injury. Biomaterials. 2021;269:120479. doi: 10.1016/j.biomaterials.2020.120479. [DOI] [PubMed] [Google Scholar]
- Yao Q, Guan J, Ma L, Cheng L, Duan F, Xu F, Zhao W, Duan W, Wu H, Chen Z, Jian F. Wireless epidural electrical stimulation in combination with serotonin agonists improves intraspinal metabolism in spinal cord injury rats. Neuromodulation. 2021;24:416–426. doi: 10.1111/ner.13344. [DOI] [PubMed] [Google Scholar]
- Yao S, He F, Cao Z, Sun Z, Chen Y, Zhao H, Yu X, Wang X, Yang Y, Rosei F, Wang LN. Mesenchymal stem cell-laden hydrogel microfibers for promoting nerve fiber regeneration in long-distance spinal cord transection injury. ACS Biomater Sci Eng. 2020;6:1165–1175. doi: 10.1021/acsbiomaterials.9b01557. [DOI] [PubMed] [Google Scholar]
- Yin W, Xue W, Zhu H, Shen H, Xiao Z, Wu S, Zhao Y, Cao Y, Tan J, Li J, Liu W, Wang L, Meng L, Chen B, Zhao M, Jiang X, Li X, Ren C, Dai J. Scar tissue removal-activated endogenous neural stem cells aid Taxol-modified collagen scaffolds in repairing chronic long-distance transected spinal cord injury. Biomater Sci. 2021;9:4778–4792. doi: 10.1039/d1bm00449b. [DOI] [PubMed] [Google Scholar]
- Yousefifard M, Nasseri Maleki S, Askarian-Amiri S, Vaccaro AR, Chapman JR, Fehlings MG, Hosseini M, Rahimi-Movaghar V. A combination of mesenchymal stem cells and scaffolds promotes motor functional recovery in spinal cord injury: a systematic review and meta-analysis. J Neurosurg Spine. 2019;32:269–284. doi: 10.3171/2019.8.SPINE19201. [DOI] [PubMed] [Google Scholar]
- Yousefifard M, Hashemi B, Forouzanfar MM, Khatamian Oskooi R, Madani Neishaboori A, Jalili Khoshnoud R. Ultra-early spinal decompression surgery can improve neurological outcome of complete cervical spinal cord injury; a systematic review and meta-analysis. Arch Acad Emerg Med. 2022;10:e11. doi: 10.22037/aaem.v10i1.1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu F, Li P, Du S, Lui KW, Lin Y, Chen L, Ren Q, Wang J, Mei J, Xiao J, Zhu J. Olfactory ensheathing cells seeded decellularized scaffold promotes axonal regeneration in spinal cord injury rats. J Biomed Mater Res A. 2021;109:779–787. doi: 10.1002/jbm.a.37066. [DOI] [PubMed] [Google Scholar]
- Yu S, Yao S, Wen Y, Wang Y, Wang H, Xu Q. Angiogenic microspheres promote neural regeneration and motor function recovery after spinal cord injury in rats. Sci Rep. 2016;6:33428. doi: 10.1038/srep33428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarei-Kheirabadi M, Sadrosadat H, Mohammadshirazi A, Jaberi R, Sorouri F, Khayyatan F, Kiani S. Human embryonic stem cell-derived neural stem cells encapsulated in hyaluronic acid promotes regeneration in a contusion spinal cord injured rat. Int J Biol Macromol. 2020;148:1118–1129. doi: 10.1016/j.ijbiomac.2020.01.219. [DOI] [PubMed] [Google Scholar]
- Zeng X, Qiu XC, Ma YH, Duan JJ, Chen YF, Gu HY, Wang JM, Ling EA, Wu JL, Wu W, Zeng YS. Integration of donor mesenchymal stem cell-derived neuron-like cells into host neural network after rat spinal cord transection. Biomaterials. 2015;53:184–201. doi: 10.1016/j.biomaterials.2015.02.073. [DOI] [PubMed] [Google Scholar]
- Zeraatpisheh Z, Shamsi F, Sarkoohi P, Torabi S, Alipour H, Aligholi H. Effects of FTY720 on neural cell behavior in two and three-dimensional culture and in compression spinal cord injury. Cell Mol Bioeng. 2022;15:331–340. doi: 10.1007/s12195-022-00724-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Huang Z, Guo M, Meng L, Piao M, Zhang M, Yu H. Effect of combination therapy with neural stem cell transplantation and teramethylpyrazine in rats following acute spinal cord injury. Neuroreport. 2021;32:1311–1319. doi: 10.1097/WNR.0000000000001725. [DOI] [PubMed] [Google Scholar]
- Zhang J, Lu X, Feng G, Gu Z, Sun Y, Bao G, Xu G, Lu Y, Chen J, Xu L, Feng X, Cui Z. Chitosan scaffolds induce human dental pulp stem cells to neural differentiation: potential roles for spinal cord injury therapy. Cell Tissue Res. 2016;366:129–142. doi: 10.1007/s00441-016-2402-1. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Zhang WX, Zhang YJ, Liu YD, Liu ZJ, Wu QC, Guan Y, Chen XM. Melatonin for the treatment of spinal cord injury. Neural Regen Res. 2018;13:1685–1692. doi: 10.4103/1673-5374.238603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao RR, Andrews MR, Wang D, Warren P, Gullo M, Schnell L, Schwab ME, Fawcett JW. Combination treatment with anti-Nogo-A and chondroitinase ABC is more effective than single treatments at enhancing functional recovery after spinal cord injury. Eur J Neurosci. 2013;38:2946–2961. doi: 10.1111/ejn.12276. [DOI] [PubMed] [Google Scholar]
- Zhao W, Li H, Hou Y, Jin Y, Zhang L. Combined administration of poly-ADP-ribose polymerase-1 and caspase-3 inhibitors alleviates neuronal apoptosis after spinal cord injury in rats. World Neurosurg. 2019;127:e346–352. doi: 10.1016/j.wneu.2019.03.116. [DOI] [PubMed] [Google Scholar]
- Zhilai Z, Hui Z, Anmin J, Shaoxiong M, Bo Y, Yinhai C. A combination of taxol infusion and human umbilical cord mesenchymal stem cells transplantation for the treatment of rat spinal cord injury. Brain Res. 2012;1481:79–89. doi: 10.1016/j.brainres.2012.08.051. [DOI] [PubMed] [Google Scholar]
- Zhu SP, Wang ZG, Zhao YZ, Wu J, Shi HX, Ye LB, Wu FZ, Cheng Y, Zhang HY, He S, Wei X, Fu XB, Li XK, Xu HZ, Xiao J. Gelatin nanostructured lipid carriers incorporating nerve growth factor inhibit endoplasmic reticulum stress-induced apoptosis and improve recovery in spinal cord injury. Mol Neurobiol. 2016;53:4375–4386. doi: 10.1007/s12035-015-9372-2. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Uezono N, Yasui T, Nakajo M, Nagai T, Wang D, Nishibori M, Nakashima K. Combinatrial treatment of anti-High Mobility Group Box-1 monoclonal antibody and epothilone B improves functional recovery after spinal cord contusion injury. Neurosci Res. 2021;172:13–25. doi: 10.1016/j.neures.2021.04.002. [DOI] [PubMed] [Google Scholar]
- Zou Y, Ma D, Shen H, Zhao Y, Xu B, Fan Y, Sun Z, Chen B, Xue W, Shi Y, Xiao Z, Gu R, Dai J. Aligned collagen scaffold combination with human spinal cord-derived neural stem cells to improve spinal cord injury repair. Biomater Sci. 2020;8:5145–5156. doi: 10.1039/d0bm00431f. [DOI] [PubMed] [Google Scholar]
- Zustiak SP, Sheth S, Imaninezhad M. Chapter 12 - Pharmacological therapies and factors delivery for spinal cord injury regeneration. In: Perale G, Rossi F, editors. Spinal cord injury (SCI) repair strategies. Cambridge: Woodhead Publishing; 2020. pp. 223–248. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.


