Abstract
Solute carrier (SLC) proteins are pivotal for maintaining cellular homeostasis by transporting small molecules across cellular membranes. Recent discoveries have uncovered their involvement in modulating innate immunity, particularly within the cytosol. Here, we review emerging evidence that links SLC transporters to cytosolic innate immune recognition and highlight their role in regulating inflammation. We explore how SLC transporters influence the activation of endosomal Toll-like receptors, cytosolic NODs, and STING sensors. Understanding the contribution of SLCs in innate immune recognition provides insight into their fundamental biological functions and opens new avenues to develop possible therapeutic interventions for autoimmune and inflammatory diseases. This review aims to discuss current knowledge and identify key gaps in this rapidly evolving field.
Keywords: SLC15, SLC46, NOD receptors, STING agonists, Muropeptides, cGAMP
Solute carrier proteins at the interface of immunity and the environment
The human solute carrier (SLC) proteins include 66 families of multi-pass transmembrane transporters that facilitate the influx or efflux of various solutes across biological membranes in an ATP-independent manner [1]. This set of transporters includes at least three distinct structural superfamilies, each with unique molecular mechanisms, including the 12-transmembrane pass multi-facilitator superfamily (MFS), which uses a rocker-switch mechanism to toggle between outward- and inward-open confirmations to deliver cargo even against substrate gradients [1, 2]. The other two structural superfamilies include the gated pore and elevator mechanism transporters. Any given family of SLC transporters, denoted by a shared number (xx) in the SLCxx nomenclature, is defined by a high level of sequence similarity; SLCs within one family share a minimum of 20% identity across the entire protein sequence [3]. Compared to other common types of transporters that rely on the energy of ATP hydrolysis to pump ions (or solutes) and maintain gradients (e.g., ABC transporters), SLCs often use ion gradients to facilitate cargo movement as secondary active transporters [4]. Generally, transporters (see glossary) differ from channels, e.g., calcium-release activated channels, voltage-regulated anion channels (VRAC), or transient receptor potential channels, in that transporters do not create pores in the membrane but instead transport cargo by employing a cycle of conformational changes to move cargo across a membrane.
SLCs are known as “metabolic gatekeepers” for their role in transporting various metabolites, including nutrients, ions, and bioactive molecules, as well as some drugs, into or out of cells and organelles. These roles include important aspects of immune cell physiology and function [5, 6]. For example, SLC1A5 is a major glutamine transporter in T cells that supports T-cell differentiation and T-cell receptor (TCR) activation [7]. Recent studies have also linked several SLCs, as well as a few channel proteins, with the delivery of innate immune agonists, such as muropeptides (Box 1) and cyclic-dinucleotides (CDNs, Box 2), to their cytosolic innate immune receptors [8–10]. The same or closely related SLCs are also implicated in trafficking metabolites; this is interesting as it suggests previously underappreciated links between metabolites and innate immune activators. In this review, we focus on SLC trafficking of small molecule agonists of cytosolic muropeptide receptors, which are key regulators of inflammatory innate immune responses (Figure 1). We further discuss how some of the same SLCs have also been linked to endosomal Toll-like receptor (TLR) signaling through a mechanism that may not involve solute transport. This review also discusses the role of CDN transport via SLCs and other transmembrane carriers; indeed, this transport is an important consideration in the dissemination of autoinflammatory diseases and is key for strategies aiming to develop STING-targeted adjuvants, especially in the context of immuno-oncology [11, 12].
Box 1: Muropeptides (PGN fragments).
Peptidoglycan (PGN) is an essential component of the bacterial cell wall that protects from stress and provides cell structure and integrity [89]. Gram-negative bacteria have a relatively thin layer of PGN that lies within the periplasm, between the inner and the outer lipid bilayer membranes. Gram-positive microbes lack an outer membrane but have a thick layer of PGN that can be exposed to the environment, or further encased by a chemically diverse capsule layer [90]. PGN consists of long polysaccharide strands, consisting of alternating N-acetyl glucosamine and N-acetyl muramic acid residues, with short peptide chains attached to the lactyl group of muramic acid. These stem peptides, in turn, are cross-linked to each other via various chemical linkages, creating the rigid meshwork of the bacterial sacculus or cell wall [91]. Across the bacterial kingdom, the diversity of PGN structures and modifications are highly variable but built upon this common theme [92]. During growth and division, as well as upon death, bacteria shed or release significant amounts of PGN fragments, often called muropeptides, into their environment [93]. These muropeptides can be recognized by various innate immune receptors, including cytosolic NOD1 and NOD2 in mammals or PGRP-LE in Drosophila sp.[94]. The minimal PGN-derived agonist of NOD1 is the dipeptide iE-DAP (iso-glutamate-diaminopimelic acid), and NOD1 recognition requires the presence of DAP, an amino acid unique to PGN from Gram-negative bacteria and Gram-positive bacilli [95, 96] (Figure 1). Larger DAP-containing muropeptides, such as the tripeptide Tri-DAP or the disaccharide-tetrapeptide TCT, are also NOD1 agonists [97]; the latter is also a potent agonist of the Drosophila Imd immune pathway via either the cell membrane receptor PGRP-LC or the cytosolic receptor PGRP-LE [9]. The minimal defined PGN-derived agonist for NOD2 is monosaccharide dipeptide MDP (muramyl dipeptide), which includes muramic acid and the first two amino acids from the stem peptide and may be derived from nearly any bacterial cell wall [94]. While MDP physically interacts with NOD2 [98], the molecular and biochemical mechanisms of NOD1 or NOD2 activation remain unclear and lack any structural data, although it is often assumed that NOD1 and 2 form an inflammasome-like ring structure much like other NOD-like receptors (NLRs) [99]. Of note, RIP2K is an adaptor-kinase that is essential for muropeptide-triggered NOD1 or NOD2 responses[100].
Box 2: Cyclic Dinucleotides (CDNs).
These second messengers consist of two nucleotides circularized through monophosphates with several possible chemical linkages. Bacterial species often generate di-cyclic-GMP, -AMP, or cGAMP, usually with 3’3’ linkages [101, 102]. In contrast, cGAMP and other CDNs are endogenously generated in animals by cyclic GMP-AMP synthases (cGAS and cGAS-like receptors [cGLRs]). In mammals, 2’3’-cGAMP is produced when cGAS detects cytoplasmic DNA, while in insects, CDNs are produced by cGLRs that respond to cytosolic RNA [103]. Drosophila cGLRs produce CDNs with a variety of different linkages and nucleotides (2’3’ cGAMP, 3’2’ cGAMP, and 2’3’ c-di-GMP) [104, 105]. CDNs bind STING, which then oligomerizes and translocates from the endoplasmic reticulum (ER) to the Golgi, leading to TANK-binding kinase 1 (TBK1) recruitment. TBK1 activates interferon regulatory factor 3 (IRF3) and induces type I interferons (IFNs) and other inflammatory cytokines [106, 107]. Dysregulation of the cGAMP-STING pathway has been implicated in various autoimmune diseases, such as SLE and rheumatoid arthritis, and in chronic inflammatory conditions, such as STING-associated vasculopathy with onset in infancy (SAVI), chronic kidney disease, and cancer [12, 108, 109]. Therefore, understanding the regulation of cGAMP transport and STING activation will be valuable for developing new therapies to tackle these diseases.
Figure 1: Cytosolic innate immune agonists.
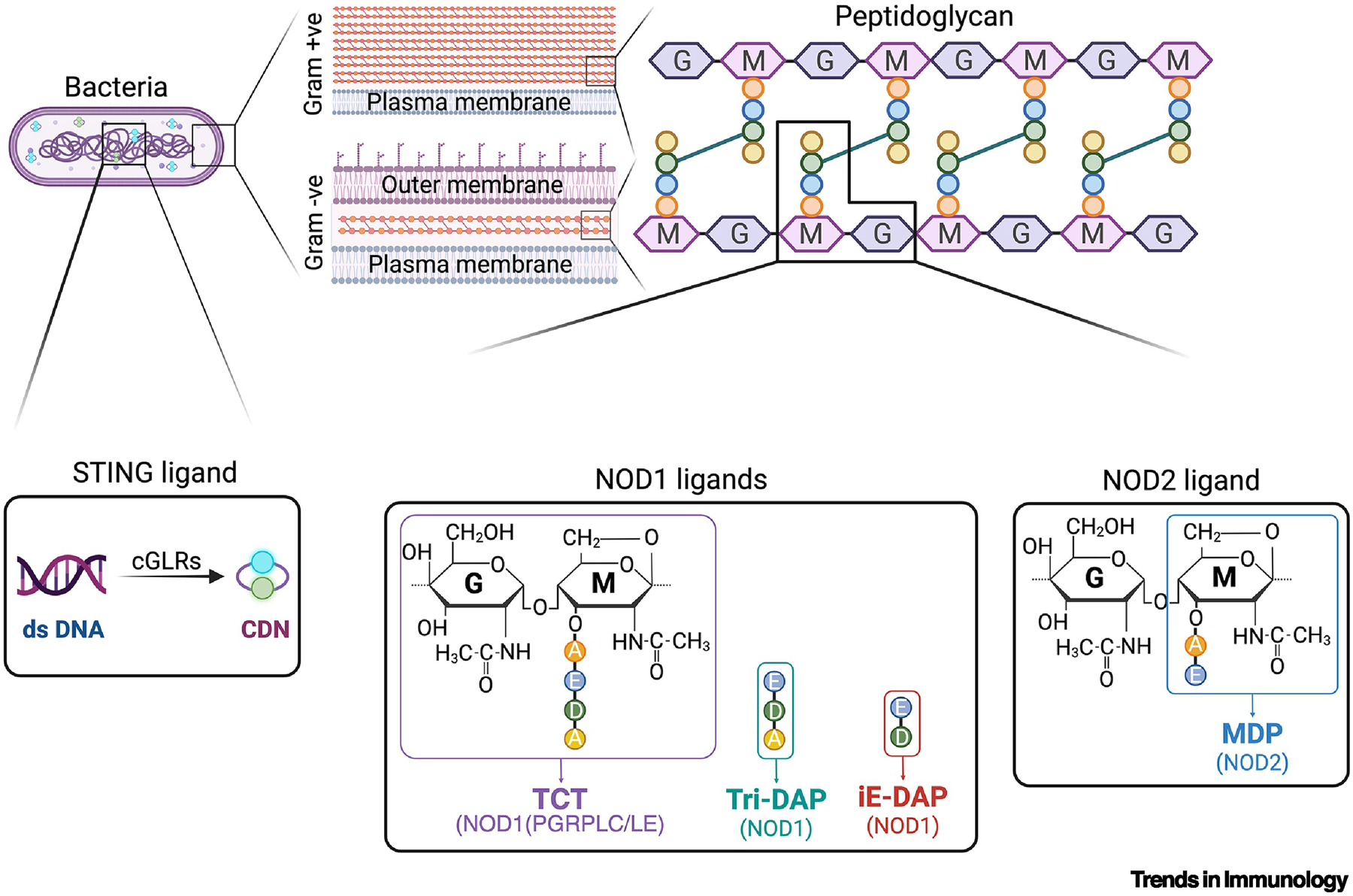
Fragments of the bacterial cell wall, peptidoglycan, are potent activators of cytosolic innate immunity in both insects and mammals [110, 111]. Various diaminopimelic acid (DAP)-containing fragments, derived from Gram-negative and Gram-positive bacilli, are potent activators of NOD1 in mammals, while the larger disaccharide DAP-tetrapeptide TCT is also a potent activator of PGRP-LC or PGRP-LE in Drosophila [9]. MDP, a monosaccharide dipeptide peptidoglycan fragment, is potentially derived from nearly any bacteria and is the minimal agonist of NOD2. Cyclic dinucleotides are ligands for STING and can be synthesized by animal cells via the action of cGAS-like receptors (cGLRs) that are triggered by cytosolic DNA (in mammals) or RNA (in Drosophila) and also generated by many bacterial species functioning as classical pathogen-associated molecular patterns (PAMPs) [103–105, 112]. Bacterial CDNs are typically dicyclic G-GMP or A-AMP, while animal cGLRs synthesize a mixed cGAMP [113].
SLCs and muropeptides
Almost all bacteria produce peptidoglycan (PGN) as the major structural component of their cell wall. Small fragments of PGN, often referred to as muropeptides, are agonists of NOD1 and NOD2, which are mammalian cytosolic innate immune sensors. Peptidoglycan recognition protein-LE (PGRP-LE) is a cytosolic innate sensor in Drosophila (Box 1). Exogenously provided muropeptides access these cytosolic receptors in both mammals (in vivo and in vitro cell cultures) and insects [9, 13], yet the underlying molecular and cellular mechanisms involved in delivering these inflammatory elicitors to the cytosol have yet to be fully elucidated (Figure 2). Several studies have implicated the SLC15 family in the delivery of muropeptides, while more recent reports argue that the SLC46 family of transporters is vitally important for this function. However, it remains unclear whether both of these SLC families are involved in muropeptide delivery, for example, in different steps in a single pathway, through redundant functions, or in a cell-type-specific manner. A better understanding of the mechanisms by which these transporters regulate immunity and inflammation, maintain immune homeostasis, and contribute to inflammatory disease processes is needed. Deciphering the distinct roles of these different SLCs should provide insights into the mechanisms underlying NOD1/2 activation and possibly inform the development of targeted therapies for conditions such as inflammatory bowel disease (IBD) or psoriasis.
Figure 2: Transport of muropeptides to cytosolic NOD1/2 receptors in mammalian cells.
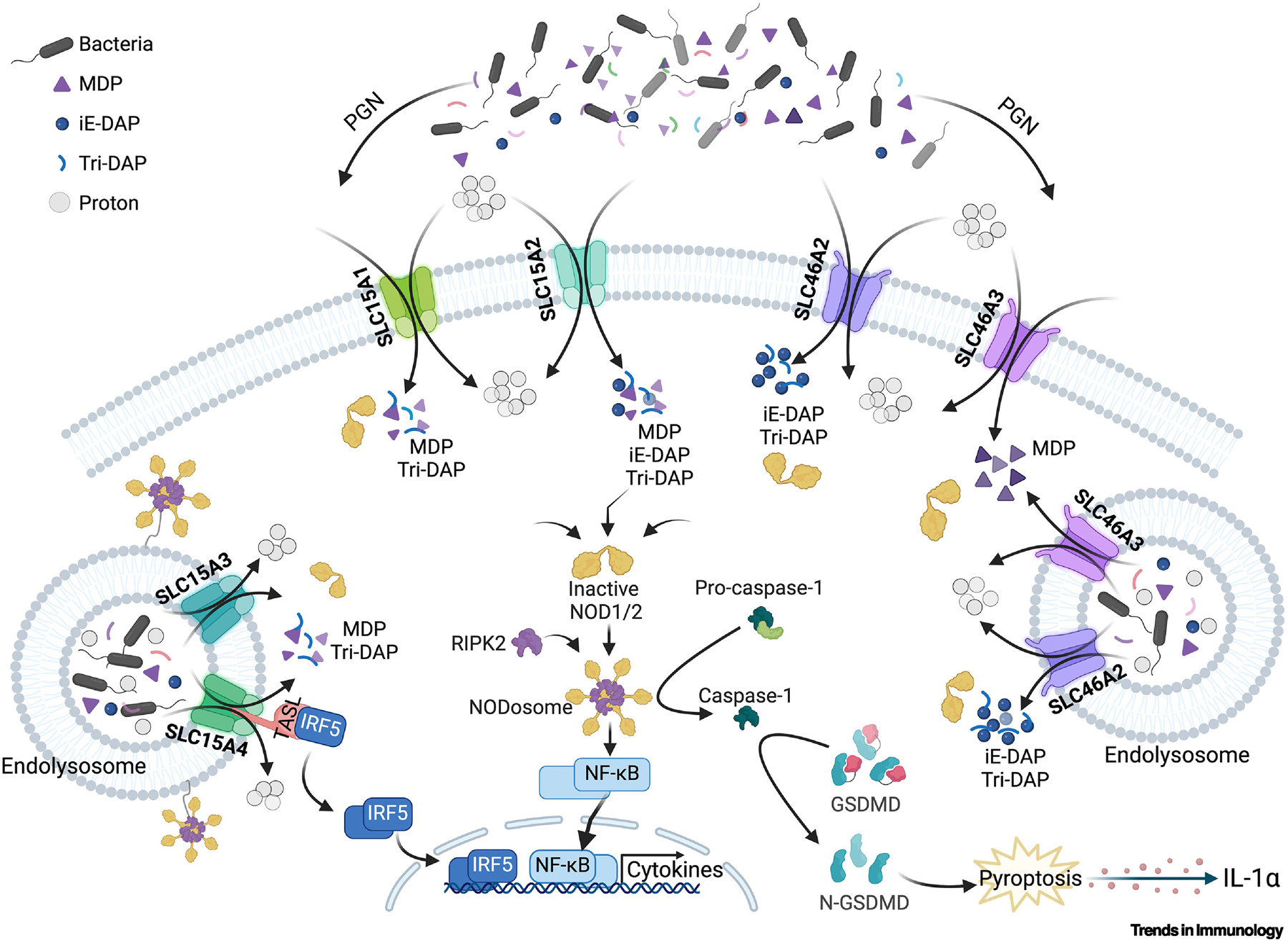
Muropeptides can enter cells, such as epithelial cells, keratinocytes, macrophages, and DCs, directly across the plasma membrane, where SLC15A1 and A2 localize, or via the endolysosomal vesicle, where SLC15A3 and A4 localize [114]. SLC46A2 and A3 are likely also endolysosomal, although plasma membrane localization and function are possible [8, 10]. All of these SLCs are proton-coupled symporters and rely on a proton gradient to move cargo [1]. Once within the cytosol, these muropeptides activate NOD1 or NOD2 and drive classic NF-κB cytokine responses in myeloid cells, while in primary keratinocytes [8, 115], these same innate receptors drive a pyroptotic-like cell permeabilization that releases IL-1α [8].
SLC15s and muropeptide transport
The SLC15 family (also known as the proton-coupled oligopeptide transporter family) is characterized as di/tripeptide transporters in humans and mice [14]. At least four of the five SLC15s have additionally been linked to muropeptide transport.
SLC15A1 (or PepT1) is one of the first transporters implicated in the transport of bacterial muropeptides (determined via transfection-based assays) [15]. SLC15A1 is prominently expressed in enteric epithelial cells of the small intestines in humans, mice, rats, and rabbits, as well as in several myeloid cell types in humans and mice [16, 17]. In addition to transporting a wide variety of di/tripeptides, SLC15A1 transports peptide-like drugs, including β-lactam antibiotics and angiotensin-converting enzyme (ACE) inhibitors [18]. SLC15A1 is thought to transport several bacterial muropeptides, including muramyl dipeptide (MDP) and Tri-DAP (Box 1) [15, 19], as well as N-formylmethionyl-leucyl-phenylalanine, which constitutes another inflammatory trigger [20]. Slc15a1 expression is upregulated in human intestinal inflammatory conditions such as IBD, and in the mouse colon during infection with pathogenic bacteria such as enteropathogenic Escherichia coli and Citrobacter rodentium [17, 21]; however, the literature is inconsistent. For example, reduced PEPT1 (SLC15A1) expression was reported in the colons of IBD patients but was not associated with the s2297322 single-nucleotide polymorphism in SLC15A1 loci, or with IBD susceptibility [19]. In a mouse model of colitis (induced by dextran sodium sulfate [DSS]), reducing the gut microbiota with an antibiotic cocktail abolished Slc15a1 upregulation, indicating that SLC15A1 may play a role in exacerbating inflammation during microbiome-dependent models of colitis [18]. While Slc15a1 overexpression in mouse intestinal epithelial cells (IECs) aggravated DSS-induced but not TNBS-induced colitis, this phenotype did not require Nod2 [18]. Moreover, the knockout mouse phenotype did not support an essential role for Slc15a1 in MDP responses because Nod2-driven cytokine production in bone marrow-derived macrophages (BMDMs) was unaffected [22]. Likewise, MDP-induced cytokine responses were reported to be independent of Slc15a1 expression in the gut, and these knockout mice were as susceptible to colitis as wild-type mice [19]. Further evidence against a role for SLC15A1 in the NOD2 pathway in humans comes from a clinical study using colon biopsy samples. In this study, samples derived from Swedish and Finnish IBD patients and controls showed an association between IBD and a coding polymorphism in SLC15A1; however, the directionality of this association was opposite in the two cohorts examined (Swedish vs. Finnish) arguing against a mechanistic link [19, 21]. Thus, these findings, from mouse models and patient cohorts, raise significant doubts about the possible connection between SLC15A1 and MDP delivery to NOD2.
SLC15A2 (or PepT2), is expressed in a wide variety of tissues but is predominantly found in the kidneys of humans, mice, and rats, where it plays a crucial role in the reabsorption of dietary peptides [23, 24]. SLC15A2 is also expressed in human and mouse immune cells, such as macrophages, monocytes, and splenocytes. It has been argued that SLC15A2 in these cells transports bacterial muropeptides such as MDP, iE-DAP, and Tri-DAP into the cytosol [24–27]. In mouse BMDMs, SLC15A2 is expressed on the plasma membrane and is required for the import of rhodamine-labeled MDP [28]; however, rhodamine is nearly the same molecular weight as MDP, making these results challenging to interpret. Additionally, Slc15a2-deficient mouse BMDMs displayed only modestly reduced (≤2-fold) cytokine (interleukin-6 [IL-6] and tumor necrosis factor-α [TNF-α]) induction following muropeptide/lipopolysaccharide (LPS) stimulation compared with LPS alone. In contrast, muropeptide did not inhibit the import of a model SLC15 dipeptide cargo (GlySar), arguing for only a minor role for this transporter in delivering either Tri-DAP or MDP to cytosolic NOD1 or NOD2, respectively [28]. SLC15A1 and A2 are also expressed in human skin epidermal keratinocytes, where they are thought to transport MDP for NOD2 activation, although the effects of SLC15 inhibitors or siRNA have been modest, suggesting only a minor role for Slc15a2 in bacterial muropeptide delivery in the skin [26, 27]. The Drosophila SLC15A homolog, Yin, was also reported to transport MDP into the cytosol when expressed in human gut epithelial cell lines [25]; however, loss-of-function studies in flies with SLC15 family homologs such as Yin, CG2930, and/or CG9444 failed to implicate these transporters in the delivery of the muropeptide TCT to the Drosophila cytosolic PGN sensor PGRP-LE [29, 30], further raising doubts about the role of SLC15s in muropeptide transport.
SLC15A3, also known as PHT2, localizes to the lysosome and transports histidine and lymphatic peptides [29]. It is expressed in a variety of immune cells, such as human and mouse macrophages and dendritic cells (DCs) [10, 24, 31, 32], and its expression is stimulated by TLR activation as well as in colitis and peritonitis, as examined by mRNA expression in mouse models [24, 32–34]. When expressed by transfection and re-routed to the plasma membrane, SLC15A3 transports bacterial muropeptides, such as MDP and Tri-DAP, in the mouse colon and macrophage cell lines, as shown by mass spectrometry [34]. SLC15A3 was also linked to the delivery of MDP-coated beads to NOD2 associated with tubule endosomal compartments in bone marrow-derived DCs [31]. However, Slc15a3 knockout BMDMs only had a small effect on the induction of interleukin-1β (IL-1β) production relative to wild-type BMDMs, further suggesting that SLC15A3 has a modest role in NOD2 signaling. Beyond its potential role in transporting bacterial muropeptides, SLC15A3 may also function as a first-line defense against viral infections. Specifically, overexpressing SLC15A3 protected cells from viral infections by inhibiting viral DNA replication, as observed in a herpes simplex virus-1 (HSV-1) infection model; the mechanism of protection seemed to involve a SLC15A3 interaction with MAVS and STING to drive type I interferon (IFN) expression in human monocytes, as evidenced from protein and mRNA expression [35].
Like SLC15A3, SLC15A4 (or PHT1) localizes to the lysosome and transports histidine and di/tripeptides [29]. SLC15A4 is highly conserved among mammals and is expressed in many cell types, including monocytes, macrophages, and certain tumor cells, such as prostate cancer cells [31, 36]. SLC15A4 has high substrate affinity for short peptides at a low pH (6.5), aligning with its expression on endosome/lysosome membranes; it has also been suggested to act as a muropeptide transporter, based on transfection and knockout studies [29, 37]. SLC15A4 expression is upregulated after MDP challenge in human peripheral blood mononuclear cells (PBMCs) [36], in inflamed colon tissues from ulcerative colitis patients [38], and in the PBMCs of systemic lupus erythematosus (SLE) patients [39]. However, Slc15a4 expression is down-regulated in DSS-induced colitis in mice, suggesting that it may be regulated by signals from the microbiome [34]. However, in contrast to Nod2 knockout mice, Slc15a4-deficient mice are less susceptible to DSS-induced colitis compared to wild-type mice, suggesting that SLC15A4 is not a key component of the NOD2 pathway [40]. SLC15A4 is also genetically linked to SLE, based on genome-wide association studies, and its expression significantly increases in patient PBMCs [41]. Moreover, Slc15a4-deficient murine DCs accumulate high amounts of histidine in the lysosomal compartment and display markedly impaired endolysosomal TLR responses compared with wild-type DCs [42]. This linkage to TLR signaling is presumably unconnected to any possible function in the NOD1/2 pathways. While it has been argued that the elevated lysosomal histidine may inhibit the proteolytic activities of cathepsins B and L, and thus endosomal TLR processing [40], this TLR signaling defect has recently been linked to cytosolic signal transduction via TASL (‘TLR adaptor interacting with SLC15A4 on the lysosome’). This is relevant because TASL physically interacts with SLC15A4 on the cytosolic side of the endolysosomal membrane and recruits and activates IRF5 via a pLxIS motif (similar to motifs found in STING and MAVS). Based on TASL knockout and point mutations in the pLxIS motif studies, the TASL-SLC15A4 interaction drives TLR7, TLR8, and TLR9-mediated IFN-I signaling in primary and transformed cell lines, e.g., human macrophages and DCs [43, 44]. Based on the Cryo-EM structure, the N-terminal helix of TASL inserts into the cargo-binding pocket of SLC15A4, presumably preventing any potential transport activity [42, 45]. In addition to defects in endosomal TLR signaling, one report also suggests that Slc15a4-deficient mice have defective responses to intraperitoneal challenge with Tri-DAP (a NOD1 agonist), although much of these cytokine induction data lacked statistical significance, and the response to MDP was completely normal [40], arguing against a key role for SLC15A4 in NOD1 or NOD2 pathways.
In summary, while several SLC15s have been implicated in muropeptide delivery and NOD1 or 2 activation (mostly via overexpression assays), knockout phenotypes do not (yet) support a key role for these transporters in modulating NOD1 or NOD2 signaling pathways, at least in the various cell types examined. SLC15s might also serve redundant functions as muropeptide transporters with cell-type specific roles. By contrast, SLC15A4, via its interaction with TASL, seems to be crucial for endosomal TLR-mediated induction of type I IFNs, at least in DCs, which is known to be a key inflammatory response in the pathogenesis of SLE.
SLC46s and muropeptide transport
The SLC46 family also belongs to the MFS and includes three members sharing ~30% identity in human and mouse [10]. SLC46A1 is best characterized as the proton-coupled folate transporter (PCFT) and is essential for folate and anti-folate absorption in the small intestine and across the choroid plexus in humans. Patients carrying loss-of-function mutations in SLC46A1 display an autosomal recessive disorder of hereditary folate malabsorption, characterized by severe folate deficiency in circulation and in the central nervous system [46]. Distinct from its role in folate metabolism, overexpression of mouse SLC46A1 in U937 human monocytic cell lines modestly increased cGAMP-related signaling, as evidenced by IRF3 phosphorylation in cell lysates, suggesting it may also weakly transport cGAMP [47] (see below for a discussion of cGAMP transport).
SLC46A2 (TSCOT, Ly110) is prominently expressed in mouse and human skin keratinocytes, neonatal thymus, and human lung epithelial cells but not in most classic immune cells [48], although a recent report showed expression in primary human monocytes [47]. Recent research highlights the role of SLC46A2 in transporting NOD1-activating/DAP-type muropeptides, such as iE-DAP and tracheal cytotoxin (TCT) [10, 49]. In mouse and human primary skin keratinocytes, Slc46a2 is essential for the response to DAP-muropeptides but is not involved in the response to MDP. Novel methods to track muropeptide trafficking used click-chemistry-modified DAP-muropeptides (which adds only a small 5-carbon “alkyne handle” relative to native iE-DAP); the findings demonstrated that Slc46a2 is essential for intracellular delivery of DAP-type muropeptides, because iE-DAP-Alk was readily detected by click-addition of a fluor in primary keratinocytes from wild-type or Nod2 knockout mice, but not Slc46a2 knockout mice [8]. In a complementary approach, the Caspase-1-Gasdermin D-mediated pyroptotic pathway was also examined in mouse primary keratinocytes. This pathway results in the Gasdermin-D-dependent release of pre-formed IL-α, a damage-associated molecular pattern (DAMP) in the skin [8]. In these keratinocytes, Slc46a2-dependent activation of NOD1-triggered the Caspase-1-Gasdermin D-mediated pyroptotic pathway with no evidence of NF-κB-mediated cytokine induction, distinct from any NOD1/2 response pathway reported in macrophages [8]. In human skin organoids, iE-DAP triggered IL-1RA-sensitive signaling from epidermal keratinocytes that was sensed by the underlying dermal fibroblasts, leading to CXCL8 secretion, driving robust recruitment of neutrophils to the site, as observed in the bacterial muropeptide challenge mouse model [8].
Given the sequence similarity between SLC46A2 and SLC46A1 (PCFT), the effect of methotrexate (MTX), a commonly used anti-inflammatory anti-folate drug, as a possible muropeptide transport competitor, was also examined. Excess MTX inhibited the transport of iE-DAP into mouse keratinocytes, which was quantified by direct click-iE-DAP assays and by monitoring IL-1α release and pyroptosis in primary keratinocytes [8]. In vivo, topical MTX treatment phenocopied Slc46a2 or Nod1-deficiency, showing reduced skin inflammation in a mouse model of psoriasis, while these same knockouts were unresponsive to MTX [8]. It is intriguing that SLC46A2 has also been identified as a potential tumor suppressor in lung cancer given that its mRNA expression is markedly reduced in various lung cancer types compared to healthy controls; however, whether this downregulation relates to the inflammatory response in the tumor, is unknown at this time [50].
The third member of this family, SLC46A3, is a widely expressed proton-coupled transporter known for its role in delivering NOD2-activating bacterial muropeptides [8, 10, 48, 49] as well as a variety of other unrelated molecules, to the cytosol in human and mouse cells [51–54]. Slc46a3-deficient mice exhibit a marked decrease in neutrophil recruitment upon intraperitoneal (i.p.) or intradermal MDP challenge, while MDP transport, monitored with a click-chemistry approach, required Slc46a3 in primary mouse keratinocytes, suggesting its crucial role in transporting MDP [8]. However, the response to NOD1 activators was normal in Slc46a3 knockout mice and keratinocytes, suggesting distinct muropeptide selectivity for SLC46A2 and SLC46A3. In addition to its role in cytosolic innate immune sensing, SLC46A3 has been linked to the transport of a variety of other molecules, including bile acids, steroid conjugates, fluorescein derivatives, and anti-cancer antibody-drug conjugates (ADCs), such as trastuzumab emtansine and pyrrolobenzodiazepine dimers [8, 52, 54]. Of note, SLC46A3 expression inversely correlates to lipid-based nanoparticle (LNP) delivery in various cell lines, perhaps due to changes in the lysosomal pH [52]. Thus, the potential role of SLC46A3 in preventing/mitigating LNP delivery might offer a promising avenue for modulating drug delivery and enhancing the therapeutic index of LNP-chemotherapeutic agents, although this remains to be thoroughly investigated. Recent studies also highlight the potential of SLC46A3 in cancer therapy following its connection to ADC transport as in the case of trastuzumab emtansine [54]. This suggests that SLC46A3 is a putative biomarker for treatment responsiveness in certain cancers [51]. Overall, these findings highlight the versatile functions of SLC46A3 in both immune signaling and the transport of diverse molecules, underscoring its potential as a candidate therapeutic target for various diseases. However, questions remain regarding the selectivity and specificity of this transporter.
As mentioned above, studies in Slc46a2 knockout and Slc46a3 knockout mice demonstrate a strong link to the transport of muropeptides for NOD1/NOD2 activation. The restricted expression pattern of Slc46a2 argues for restricted access to cytosolic NOD1, for example, in the skin, but perhaps not the gut, while the broader expression of Slc46a3, e.g., in immune cells and epithelial cell types, suggests more frequent access to cytosolic NOD2 in epithelial surfaces and hematopoietic cells. However, this remains conjectural. Nevertheless, the selective requirement for Slc46a2 in NOD1 activation by DAP-muropeptides, and Slc46a3 in NOD2 activation by MDP, predicts preferential binding of these agonists to their cognate SLC46 transporter — a prediction that requires further experimentation, as does the characterization of SLC46s’ function in myeloid cells. Of note, the potential role of MTX as a competitive inhibitor of muropeptide transport by SLC46s suggests that these transporters might be viable targets for small molecule anti-inflammatory agents; such SLC46 inhibitors would be predicted to retain the anti-inflammatory properties of MTX, without having antiproliferative effects, because blocking these transporters would selectively block inflammatory pathways without interfering with folate metabolism [8]. Regardless, a detailed molecular understanding of the transport mechanisms and substrate specificity of these transporters is essential for grasping their implications for immune system function, responses to bacterial infections, and potential therapeutic applications in modulating immune pathways.
Cyclic dinucleotide (CDN) transport
Like muropeptide transfer, the transport of CDNs is crucial for immune signaling pathways. CDNs are both bacterial products and host-synthesized second messengers. While bacteria use CDNs for quorum sensing and other prokaryotic communications [55, 56], animal cells produce CDNs via cGAS-like receptors to trigger a host immune response via STING signaling (Box 2). CDNs are heterocyclic structures composed of two nucleotides arranged in several configurations. Among these CDNs, 2’3’-cyclic G-A monophosphate (cGAMP) is an ‘immunotransmitter’ carrying an inflammatory signal between cells. In the classical model, cGAMP is generated when cGAS detects double-stranded DNA (dsDNA) and leads to cell-intrinsic STING activation. However, cGAMP can also be shared by producing cells and taken up by neighboring cells, or CDNs can be provided exogenously via bacterial infection or experimental manipulation (Box 2, Figure 3) [57]. Because STING is widely expressed, CDN uptake from the extracellular environment can drive responses in many cell types, although the outcomes vary between different cell types [58]. Using knockout mice and cell lines, multiple mechanisms for cGAMP movement between cells have been reported, including transport between adjacent cells through gap junctions [59] and incorporation into viral particles enabling transmission to subsequently infected cells [60, 61]. Recent studies also explored the role of extracellular CDNs in triggering immune responses when introduced exogenously [62]. Based on available reports in mice and human cells, six proteins have been linked to CDN export or import; collectively termed “cGAMP conduits,” these proteins include channels (LRRC8A:C/E), transporters (ABCC1, SLC19A1, SLC46A2), and pores (P2X7, LL-37) [47, 63–67].
Figure 3: Transport of CDNs activates the STING pathway in mammalian cells.
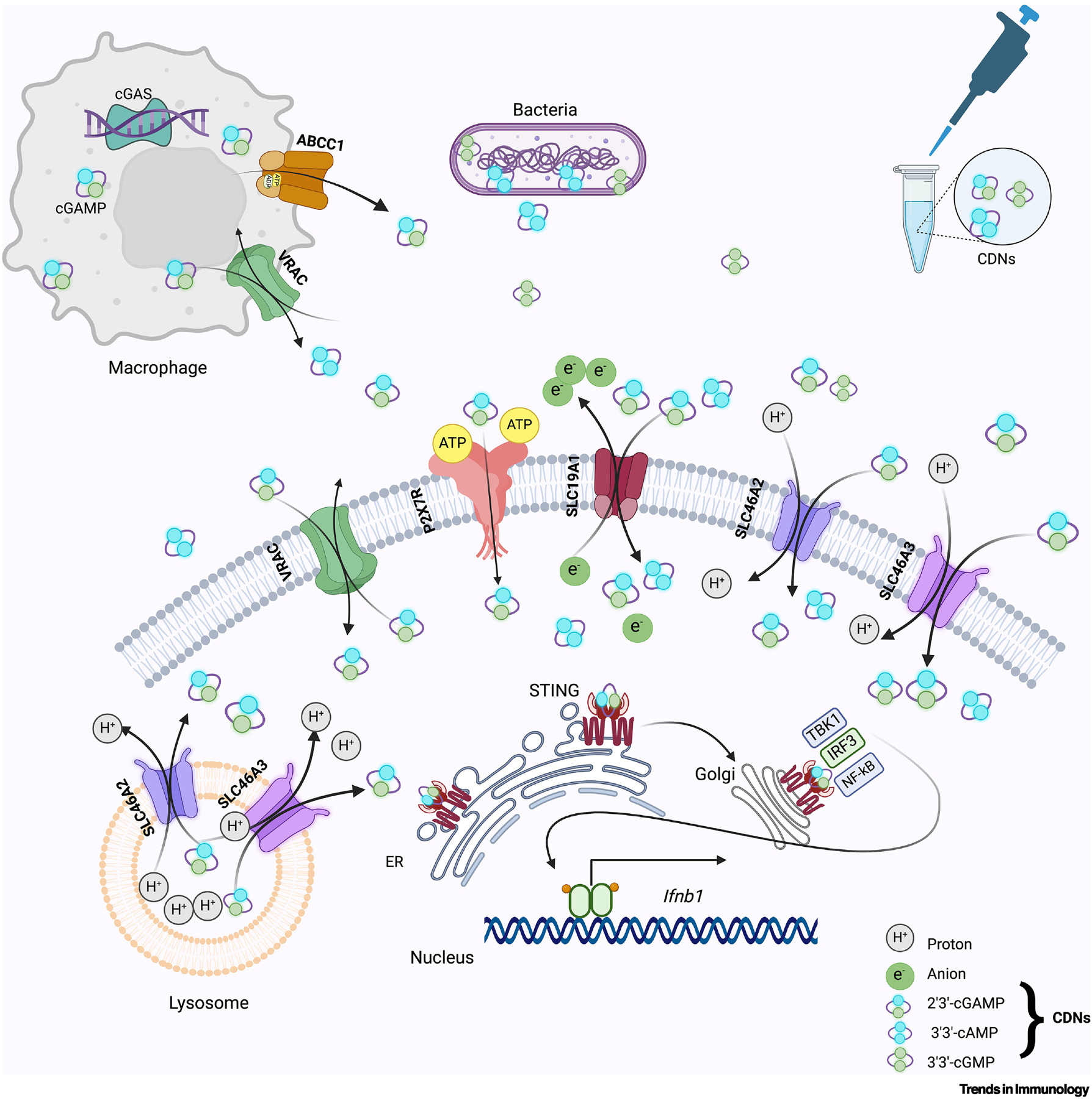
CDNs are produced and released by bacteria, released from cGLR-activated cells such as macrophages, or from permeabilized dying cells such as tumor cells or exogenously provided, and can function as a paracrine signal by entering nearby cells, binding and activating STING [109]. Reported entry mechanisms include the LRR8A:C VRAC channel, the ATP-gated P2X7R channel, and SLCs 19A1 or SLC46A2/A3, which are anion antiport or proton symport transport proteins, respectively. The ATP-dependent transporter ABCC1 is implicated in the active export of CDNs [67]. SLC19A1 imports cGAMP and other CDNs, while SLC46A2 and A3 prefer 3’3’ cAMP over 3’3’-GMP, as well as 2’3’-cGAMP [63, 83]. Once a CDN binds STING, it oligomerizes and translocates to the Golgi, where it recruits and activates TANK-binding kinase 1 (TBK1) and interferon regulatory factor 3 (IRF3); IRF3 is crucial for the transcriptional induction of type I interferons such as Ifnb1 [109].
ABCC1, also known as multidrug-resistance-associated protein 1, is an ATP-dependent cGAMP exporter in human and mouse cell lines [67]. Using chemical inhibitors, such as Verapamil and MK-571, and knockout mice, ABCC1 was shown to negatively regulate STING-mediated IFN induction. While ABCC1 overexpression increased cGAMP export in human cell lines, genetic disruption of ABCC1 did not ablate cGAMP export completely [67]. This supports the idea that multiple cGAMP exporters exist, as suggested by other studies [47, 63–66, 68].
P2X7, a purinergic receptor, is an ATP-gated ion channel expressed in all immune cells, that drives inflammation and cell death in mammals [67, 69–71]. In the presence of a high concentration of extracellular ATP, the P2X7 channel opens, allowing the efflux of potassium ions, which in turn triggers the assembly and activation of the NLRP3 inflammasome; this drives the production of pro-inflammatory cytokines, such as IL-1β [72]. In a triple-negative breast cancer murine model, blocking myeloid-epithelial-reproductive tyrosine kinase MerTK (a receptor on macrophages for apoptotic cells) prevented efferocytosis, resulting in the accumulation of necrotic tumor cells. These dying cells induced innate immune sensors and type I IFN responses in tumor-associated macrophages (TAM), driven by tumor-cell expressed cGAS, implicating a cGAMP signaling from the tumor cells to the TAMs. Extracellular ATP released by dying tumor cells opened P2X7 receptor channels on these TAMs, allowing cGAMP to enter, given that P2x7r was required in TAMs for this response [73]. Thus, these studies generated key insights into the role of CDN transport in cell death, inflammation, and cancer.
Leucine-rich repeat containing 8 (LRRC8) plays a crucial role in cGAMP transport
LRRC8s, components of VRACs also known as the SWELL channel, are also implicated in CDN transport. These channels support the passage of anions across cell membranes in response to cell swelling, for example, in hypotonic conditions. VRACs are formed by a hexameric complex of LRRC8 proteins, with LRRC8A being an essential component that partners with another LRRC8 protein (LRRC8B-E) to form a channel. LRRC8A/E is characterized by 17 leucine-rich repeat (LRR) motifs that play a role in cytosolic signaling [74]. The exact composition of these proteins within the VRAC complex influences the channel’s properties and specificity [75–78]. A study using a genome-wide CRISPR-Cas9 screen demonstrated that human LRRC8 channels were required for cGAMP transport into U937 monocytes [68]. In U937 cells, LRRC8A:C or :E complexes promoted cGAMP transport, while LRRC8D alone inhibited it [68]. Based on the electrochemical gradient, cGAMP can efflux or influx through LRRC8 channels. On activation/opening of LRRC8, HEK cells over-expressing cGAS efflux cGAMP out of cells [68]. Using LRRC8A/B/C/D/E knockouts in U937 cells, the authors showed that LRRC8A, likely in complex with LRRC8C, facilitated the transport of extracellular cGAMP across the plasma membrane [68]. Another study reported that genetic ablation or chemical inhibition of LRRC8A similarly prevented the cGAMP-triggered type I IFN response in murine cells [65]. A subsequent report argued that LRRC8C in CD4+ T cells was crucial for regulating CDN influx and activating STING and p53 signaling, because Lrrc8c−/− T cells exhibited defective CDN influx relative to wildtype cells [79]. Of note, cGAMP delivered to T cells activated STING to drive a p53-mediated cell death response, while myeloid cells were activated [80]. In Lrrc8c−/− CD4+ T cells, however, STING signaling was still observed at higher CDN concentrations, suggesting an alternative CDN transport mechanism that might involve some of the other CDN transporters discussed herein [79]. Lastly, an in vivo study demonstrated that Lrrc8e-deficient mice infected with HSV-1 failed to induce a strong type I IFN response, implicating LRRC8E transport of cGAMP in antiviral immunity [65].
SLC19A1 is a cGAMP importer
SLC19A1 (Reduced Folate Carrier 1) is another transporter linked to cGAMP delivery. Using a CRISPR-Cas9 knockout screen, SLC19A1 was identified as a cGAMP importer in monocytic U937 cells [64]. SLC19A1 selectively transported 2’3’-cGAMP analogs in U937 cells, as evidenced by an IRF3 phosphorylation assay to monitor IFN signaling [64]. Another study also identified SLC19A1 as a cGAMP importer, using a CRISPRi screen in human THP-1 cells, while overexpression of SLC19A1 increased CDN responsiveness in THP-1 and other human cell lines [63]. However, Slc19a1 depletion in mouse bone marrow cells did not block CDN-induced Ifn gene expression, and blocking Slc19a1 in mouse splenocytes using MTX or other antifolates did not inhibit cGAMP-induced gene expression either [63]. However, both of these studies suggested that using (anti)folates such as MTX or folic acid almost completely blocked CDN uptake in human THP-1 cells, based on IRF3 activation, while the Slc19a1 knockout resulted in only a partial loss of CDN stimulation, suggesting that other transporters sensitive to these drugs may also play a role in CDN uptake [63, 64]. Together, these studies argue that different cell types, both primary and transformed cell lines, use distinct and not-fully identified transporters to import cGAMP and other CDNs[81].
cGAMP entry has also been linked to LL-37, an antimicrobial peptide that binds cGAMP and facilitates its entry directly across cell membranes via endocytosis in THP-1 cells [82]. Specifically, using LL-37CRISPR−/− and Slc19a1 knockout in human and murine monocyte/macrophage cell lines, cGAMP transport by LL37 was monitored by IFNβ reporter, as well as STING and IRF3 phosphorylation assays and found to be SLC19A1-independent.
SLC46As as cGAMP transporters
Early reports of CDN import via SLC19A1 showed that this transporter was not required in cGAMP transport in many cell types or cell lines, particularly monocytes [83]. However, in human CD14+ monocytes, the response to exogenous cGAMP was sensitive to sulfasalazine (SSZ), an inhibitor of SLC19A1 and other proteins, suggesting that another target of this drug might be involved. Specifically, using the U937 monocytic cell line, the expression of human SLC46A2 was sufficient to support responsiveness to exogenous cGAMP and was sensitive to SSZ, as measured by IRF3 phosphorylation. In these U937 cell assays, expression of the closely related transporter SLC46A3 also showed a strong enhancement in the response to extracellular cGAMP response, while SLC46A1 (PFCT) was less effective [83]. In primary human monocytes, examined using a lentivirus-delivery CRISPR-Cas9 targeting strategy, SLC46A2, but not SLC46A3, was partially required for the response to exogenous cGAMP, suggesting that SLC46A2 might play distinct roles in different cell types [83]. Altogether, the SLC46 family of transporters are potentially essential for CDN delivery, in a cell type-specific manner.
Concluding Remarks
Here, we highlighted the emerging role of SLCs and other channels and pores in modulating cytosolic innate immune responses. The complex interplay between cytosolic sensors and their agonists is currently a major research focus, and this review aimed to clarify some of the contradictory claims in the literature, regarding the role of different SLCs in the delivery of innate immune agonists (muropeptides and CDNs) from the extracellular space into the cell cytosol. Surprisingly, recent reports suggest some connectivity between the transport mechanism for these two agonists; SLC46s are implicated in the delivery of muropeptides as well as CDNs even though they are chemically dissimilar [8, 83]. It is unclear how these distinct cargos can be recognized by the same transport proteins and whether they utilize the same binding pocket and biochemical mechanism (see Outstanding Questions). Recent work also uncovered a surprising connection between the delivery of these innate immune elicitors and folate metabolism [8, 10, 83–85]. Two different folate/antifolate transporters, SLC19A1 and the SLC46 family, have been implicated in CDN and muropeptide delivery, as detailed above; it has been argued that this connection provides novel mechanistic insight as to why anti-folates, originally developed as anti-proliferative cancer therapies, are such potent anti-inflammatory agents [8, 49, 86]. These findings and hypotheses underscore the potential of SLCs as candidate therapeutic targets for autoimmune diseases and related conditions. The role of SLCs in IBD is more complex. Mouse intestinal epithelial cells with a hyperactive Sting allele display an IBD-like phenotype [87]. Perhaps the transporters discussed here might be targeted to block this pathological STING response in IBD. However, an intact NOD2 pathway is protective against IBD [88], and blocking this pathway by inhibiting SLC46A3 could exacerbate IBD. Although the identity of the key CDN transporter in the gut epithelium is unclear, this potential functional overlap of SLC46s in IBD-causing or -protective pathways might limit the use of these transporters as putative IBD drug targets. The intriguing relationship between SLCs, especially SLC46s and SLC19A1, and their transport of various cargo molecules — including glutamic acid-containing small molecules such as folate derivatives and muropeptides as well as unrelated compounds such as CDNs — raises important questions about the specificity and selectivity of these transporters. The differential effects observed with MTX treatment – an agent that perturbs SLC46-mediated muropeptide delivery but not CDN delivery – suggest that these transporters use distinct binding pockets or mechanisms for different cargoes. Understanding this selectivity might pave the way for the development of more targeted anti-inflammatory therapies that exploit these distinct mechanisms. SLCs of the MFS class, with their large cargo-binding pockets, offer many opportunities for developing small molecule binders and inhibitors, as seen with SLC6 family proteins – the targets of many neuroactive drugs [1].
Outstanding Questions.
What are the biochemical binding properties of the SLCs implicated in muropeptide and/or CDN transport? While genetic and cell biological assays provide insights into function, the observed phenotypes implicating transporters in cytosolic innate immune responses could be indirect. Therefore, binding studies of purported innate immune cargos for SLC15, SLC19, and SLC46 transporters, along with structural determinations, are essential to conclusively demonstrate the claimed transporting activities.
Do SLC46s use the same or different binding pockets for muropeptides versus CDNs? Given the dual reported roles of SLC46s as muropeptide and CDN transporters, it is essential to learn if these chemically distinct cargoes bind the same or distinct cargo-binding pockets within these transporters.
Do any of the SLC15 transporters, individually or in combination, play an essential role in NOD1 or NOD2 pathways? Studies are needed to determine the role, if any, of SLC15s in NOD1 or NOD2 pathways with rigorous loss-of-function analyses in multiple cell types in vitro and in vivo.
What are the roles of the transporters and channels discussed here in different cell types from both humans and mice? Given the role of both the NOD1/2 and STING pathways in immune cells, e.g. macrophages and dendritic cells and also at epithelial surfaces such as the gut and skin, it is important to rigorously determine the role of these different transporters in a variety of cell types, myeloid and epithelial alike. Studies in human systems, as well as mice, are also essential.
Are SLC46s or SLC19s important anti-inflammatory targets of drugs such as methotrexate and sulfasalazine? The role of these innate immune agonist transporters in the mechanism of action of commonly used but complex and poorly understood anti-inflammatory drugs, such as methotrexate and sulfasalazine, requires more in-depth investigation and the development of more specific inhibitors.
How does NOD1 activation trigger Caspase-1-dependent pyroptosis in keratinocytes? In primary mouse keratinocytes, the SLC46A2/NOD1 pathway was linked to pyroptotic-like cell permeabilization and IL-1α release, but it is unknown if a similar pathway functions in other cell types. The molecular mechanisms linking the NOD1-osome to Caspase 1, Gasdermin D, and cell permeabilization are also unclear.
Do SLC46A2 or SLC46A3 transport folates or anti-folates? Folate and methotrexate compete with the transport of DAP-muropeptides through SLC46A2. However, it remains to be determined whether SLC46A2 functions as a folate transporter in skin keratinocytes and if folate transport through SLC46A2 has any impact on psoriasis in mouse models or patients.
Do other members of the SLC15 family interact with TASL or similar scaffolding proteins? TASL interacts with the cargo-binding pocket of SLC15A4 in the endosomal membrane and prevents the transport of specific cargo by SLC15A4. It is unclear if TASL also binds other SLC15 family members or if these /transporter proteins interact with additional yet-to-be-identified TASL-like adaptors. It will also be important to probe how the TASL/SLC15A4 interaction is modulated during immune responses and if disrupting this interaction is a viable therapeutic approach, as suggested by recent in vitro studies.
Highlights:
Cytosolic innate immune sensors, such as NOD1/2 or STING, are activated by exogenously provided agonists in many cell types
Transporters and channels are essential for delivering agonists to these cytosolic receptors
SLC15s and SLC46As are linked to the transport of bacterial muropeptides that activate the NOD1/2 signaling pathway, although the connectivity and selectivity of these disinfect families of transporters are unclear
SLC19A1, SLC46A2, and SLC46A3 are all linked to the transport of cGAMP and activation of the STING pathway
Along with SLC transporters LRRC8, P2X7, and ABCC1 are implicated in cGAMP transport
SLC and channels represent druggable targets and the effect of classic anti-inflammatory drugs such as methotrexate and sulfasalazine may function by inhibiting cytosolic delivery of innate immune agonists
Significance:
While cytosolic innate immune receptors are critical for defending against pathogens that invade the intracellular space, it is well established that these receptors sense numerous innate triggers even when provided exogenously by neighboring cells or experimental manipulation. The molecular mechanisms by which these innate agonists access essential cytosolic sensors have been the focus of much recent discovery and constitute promising targets for perturbing the propagation of inflammation. In cancer immunotherapy, selectively targeting these pathways in the tumor microenvironment might also harbor therapeutic potential worth exploring.
Acknowledgments
This work was supported by a grant from the NIH/NIAID (AI060025) to N.S. All figures are created in BioRender.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Interests
A provisional patent on targeting SLC46s to inhibit inflammation in psoriasis and other auto-inflammatory diseases has been filed by some of the authors (N.S., R.B.)
References
- 1.Schlessinger A, et al. , Targeting SLC transporters: small molecules as modulators and therapeutic opportunities. Trends Biochem Sci, 2023. 48(9): p. 801–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Colas C and Laine E, Targeting Solute Carrier Transporters through Functional Mapping. Trends Pharmacol Sci, 2021. 42(1): p. 3–6. [DOI] [PubMed] [Google Scholar]
- 3.Hediger MA, et al. , The ABCs of membrane transporters in health and disease (SLC series): introduction. Mol Aspects Med, 2013. 34(2–3): p. 95–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bharadwaj R, et al. , Targeting Solute Carrier Transporters (SLCs) as a Therapeutic Target in Different Cancers. Diseases, 2024. 12(3): p. 63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Song W, et al. , Solute carrier transporters: the metabolic gatekeepers of immune cells. Acta Pharm Sin B, 2020. 10(1): p. 61–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weiss HJ and Angiari S, Metabolite Transporters as Regulators of Immunity. Metabolites, 2020. 10(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ren W, et al. , Amino-acid transporters in T-cell activation and differentiation. Cell Death Dis, 2017. 8(5): p. e2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bharadwaj R, et al. , Methotrexate suppresses psoriatic skin inflammation by inhibiting muropeptide transporter SLC46A2 activity. Immunity, 2023. 56(5): p. 998–1012 e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kaneko T, et al. , PGRP-LC and PGRP-LE have essential yet distinct functions in the drosophila immune response to monomeric DAP-type peptidoglycan. Nat Immunol, 2006. 7(7): p. 715–23. [DOI] [PubMed] [Google Scholar]
- 10.Paik D, et al. , SLC46 Family Transporters Facilitate Cytosolic Innate Immune Recognition of Monomeric Peptidoglycans. J Immunol, 2017. 199(1): p. 263–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Skopelja-Gardner S, An J, and Elkon KB, Role of the cGAS-STING pathway in systemic and organ-specific diseases. Nat Rev Nephrol, 2022. 18(9): p. 558–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Samson N and Ablasser A, The cGAS–STING pathway and cancer. Nature cancer, 2022. 3(12): p. 1452–1463. [DOI] [PubMed] [Google Scholar]
- 13.Rosenzweig HL, et al. , Activation of NOD2 in vivo induces IL-1beta production in the eye via caspase-1 but results in ocular inflammation independently of IL-1 signaling. J Leukoc Biol, 2008. 84(2): p. 529–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smith DE, Clemencon B, and Hediger MA, Proton-coupled oligopeptide transporter family SLC15: physiological, pharmacological and pathological implications. Mol Aspects Med, 2013. 34(2–3): p. 323–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vavricka SR, et al. , hPepT1 transports muramyl dipeptide, activating NF-kappaB and stimulating IL-8 secretion in human colonic Caco2/bbe cells. Gastroenterology, 2004. 127(5): p. 1401–9. [DOI] [PubMed] [Google Scholar]
- 16.Charrier L and Merlin D, The oligopeptide transporter hPepT1: gateway to the innate immune response. Lab Invest, 2006. 86(6): p. 538–46. [DOI] [PubMed] [Google Scholar]
- 17.Wojtal KA, et al. , Changes in mRNA expression levels of solute carrier transporters in inflammatory bowel disease patients. Drug Metab Dispos, 2009. 37(9): p. 1871–7. [DOI] [PubMed] [Google Scholar]
- 18.Dalmasso G, et al. , The PepT1-NOD2 signaling pathway aggravates induced colitis in mice. Gastroenterology, 2011. 141(4): p. 1334–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wuensch T, et al. , Colonic expression of the peptide transporter PEPT1 is downregulated during intestinal inflammation and is not required for NOD2-dependent immune activation. Inflamm Bowel Dis, 2014. 20(4): p. 671–84. [DOI] [PubMed] [Google Scholar]
- 20.Buyse M, et al. , PepT1-mediated fMLP transport induces intestinal inflammation in vivo. Am J Physiol Cell Physiol, 2002. 283(6): p. C1795–800. [DOI] [PubMed] [Google Scholar]
- 21.Zucchelli M, et al. , PepT1 oligopeptide transporter (SLC15A1) gene polymorphism in inflammatory bowel disease. Inflamm Bowel Dis, 2009. 15(10): p. 1562–9. [DOI] [PubMed] [Google Scholar]
- 22.Marina-Garcia N, et al. , Clathrin- and dynamin-dependent endocytic pathway regulates muramyl dipeptide internalization and NOD2 activation. J Immunol, 2009. 182(7): p. 4321–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Daniel H and Kottra G, The proton oligopeptide cotransporter family SLC15 in physiology and pharmacology. Pflügers Archiv, 2004. 447: p. 610–618. [DOI] [PubMed] [Google Scholar]
- 24.Sun D, et al. , Functional and molecular expression of the proton-coupled oligopeptide transporters in spleen and macrophages from mouse and human. Mol Pharm, 2013. 10(4): p. 1409–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Charriere GM, et al. , Identification of Drosophila Yin and PEPT2 as evolutionarily conserved phagosome-associated muramyl dipeptide transporters. J Biol Chem, 2010. 285(26): p. 20147–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kudo M, et al. , H(+)/peptide transporter (PEPT2) is expressed in human epidermal keratinocytes and is involved in skin oligopeptide transport. Biochem Biophys Res Commun, 2016. 475(4): p. 335–41. [DOI] [PubMed] [Google Scholar]
- 27.Kudo M, et al. , Alternate expression of PEPT1 and PEPT2 in epidermal differentiation is required for NOD2 immune responses by bacteria-derived muramyl dipeptide. Biochem Biophys Res Commun, 2020. 522(1): p. 151–156. [DOI] [PubMed] [Google Scholar]
- 28.Hu Y, et al. , SLC15A2 and SLC15A4 Mediate the Transport of Bacterially Derived Di/Tripeptides To Enhance the Nucleotide-Binding Oligomerization Domain-Dependent Immune Response in Mouse Bone Marrow-Derived Macrophages. J Immunol, 2018. 201(2): p. 652–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sakata K, et al. , Cloning of a lymphatic peptide/histidine transporter. Biochem J, 2001. 356(Pt 1): p. 53–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Capo F, et al. , Oligopeptide Transporters of the SLC15 Family Are Dispensable for Peptidoglycan Sensing and Transport in Drosophila. J Innate Immun, 2017. 9(5): p. 483–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nakamura N, et al. , Endosomes are specialized platforms for bacterial sensing and NOD2 signalling. Nature, 2014. 509(7499): p. 240–4. [DOI] [PubMed] [Google Scholar]
- 32.Wang Y, et al. , Expression and regulation of the proton-coupled oligopeptide transporter PhT2 by LPS in macrophages and mouse spleen. Mol Pharm, 2014. 11(6): p. 1880–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Song F, et al. , Regulation and biological role of the peptide/histidine transporter SLC15A3 in Toll-like receptor-mediated inflammatory responses in macrophage. Cell Death Dis, 2018. 9(7): p. 770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang Y, et al. , Expression and regulation of proton-coupled oligopeptide transporters in colonic tissue and immune cells of mice. Biochem Pharmacol, 2018. 148: p. 163–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.He L, et al. , The Solute Carrier Transporter SLC15A3 Participates in Antiviral Innate Immune Responses against Herpes Simplex Virus-1. J Immunol Res, 2018. 2018: p. 5214187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vanaki N, et al. , Increased inflammatory responsiveness of peripheral blood mononuclear cells (PBMCs) to in vitro NOD2 ligand stimulation in patients with ankylosing spondylitis. Immunopharmacol Immunotoxicol, 2018. 40(5): p. 393–400. [DOI] [PubMed] [Google Scholar]
- 37.Kobayashi T, et al. , Human SLC15A4 is crucial for TLR-mediated type I interferon production and mitochondrial integrity. Int Immunol, 2021. 33(7): p. 399–406. [DOI] [PubMed] [Google Scholar]
- 38.Lee J, et al. , pH-dependent internalization of muramyl peptides from early endosomes enables Nod1 and Nod2 signaling. J Biol Chem, 2009. 284(35): p. 23818–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li S, et al. , miR-31–5p Regulates Type I Interferon by Targeting SLC15A4 in Plasmacytoid Dendritic Cells of Systemic Lupus Erythematosus. J Inflamm Res, 2022. 15: p. 6607–6616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sasawatari S, et al. , The solute carrier family 15A4 regulates TLR9 and NOD1 functions in the innate immune system and promotes colitis in mice. Gastroenterology, 2011. 140(5): p. 1513–25. [DOI] [PubMed] [Google Scholar]
- 41.Han JW, et al. , Genome-wide association study in a Chinese Han population identifies nine new susceptibility loci for systemic lupus erythematosus. Nat Genet, 2009. 41(11): p. 1234–7. [DOI] [PubMed] [Google Scholar]
- 42.Boeszoermenyi A, et al. , A conformation-locking inhibitor of SLC15A4 with TASL proteostatic anti-inflammatory activity. Nat Commun, 2023. 14(1): p. 6626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Heinz LX, et al. , TASL is the SLC15A4-associated adaptor for IRF5 activation by TLR7–9. Nature, 2020. 581(7808): p. 316–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang H, et al. , SLC15A4 controls endolysosomal TLR7–9 responses by recruiting the innate immune adaptor TASL. Cell Rep, 2023. 42(8): p. 112916. [DOI] [PubMed] [Google Scholar]
- 45.Chen X, et al. , Structural basis for recruitment of TASL by SLC15A4 in human endolysosomal TLR signaling. Nat Commun, 2023. 14(1): p. 6627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhao R, Aluri S, and Goldman ID, The proton-coupled folate transporter (PCFT-SLC46A1) and the syndrome of systemic and cerebral folate deficiency of infancy: Hereditary folate malabsorption. Mol Aspects Med, 2017. 53: p. 57–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cordova AF, et al. , Human SLC46A2 Is the Dominant cGAMP Importer in Extracellular cGAMP-Sensing Macrophages and Monocytes. ACS Cent Sci, 2021. 7(6): p. 1073–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fagerberg L, et al. , Analysis of the human tissue-specific expression by genome-wide integration of transcriptomics and antibody-based proteomics. Mol Cell Proteomics, 2014. 13(2): p. 397–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bharadwaj R, et al. , Synthesis and validation of click-modified NOD1/2 agonists. Innate Immun, 2023. 29(8): p. 186–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kim KY, et al. , Expression Analyses Revealed Thymic Stromal Co-Transporter/Slc46A2 Is in Stem Cell Populations and Is a Putative Tumor Suppressor. Mol Cells, 2015. 38(6): p. 548–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kim JH, et al. , Lysosomal SLC46A3 modulates hepatic cytosolic copper homeostasis. Nat Commun, 2021. 12(1): p. 290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Boehnke N, et al. , Massively parallel pooled screening reveals genomic determinants of nanoparticle delivery. Science, 2022. 377(6604): p. eabm5551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tomabechi R, et al. , SLC46A3 is a lysosomal proton-coupled steroid conjugate and bile acid transporter involved in transport of active catabolites of T-DM1. PNAS Nexus, 2022. 1(3): p. pgac063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tomabechi R, et al. , Identification of 5-Carboxyfluorescein as a Probe Substrate of SLC46A3 and Its Application in a Fluorescence-Based In Vitro Assay Evaluating the Interaction with SLC46A3. Mol Pharm, 2023. 20(1): p. 491–499. [DOI] [PubMed] [Google Scholar]
- 55.Srivastava D and Waters CM, A tangled web: regulatory connections between quorum sensing and cyclic Di-GMP. J Bacteriol, 2012. 194(17): p. 4485–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Aline Dias da P, et al. , The World of Cyclic Dinucleotides in Bacterial Behavior. Molecules, 2020. 25(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chen Q, Sun L, and Chen ZJ, Regulation and function of the cGAS-STING pathway of cytosolic DNA sensing. Nat Immunol, 2016. 17(10): p. 1142–9. [DOI] [PubMed] [Google Scholar]
- 58.Decout A, et al. , The cGAS-STING pathway as a therapeutic target in inflammatory diseases. Nat Rev Immunol, 2021. 21(9): p. 548–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ablasser A, et al. , Cell intrinsic immunity spreads to bystander cells via the intercellular transfer of cGAMP. Nature, 2013. 503(7477): p. 530–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bridgeman A, et al. , Viruses transfer the antiviral second messenger cGAMP between cells. Science, 2015. 349(6253): p. 1228–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gentili M, et al. , Transmission of innate immune signaling by packaging of cGAMP in viral particles. Science, 2015. 349(6253): p. 1232–6. [DOI] [PubMed] [Google Scholar]
- 62.Blest HTW and Chauveau L, cGAMP the travelling messenger. Front Immunol, 2023. 14: p. 1150705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Luteijn RD, et al. , SLC19A1 transports immunoreactive cyclic dinucleotides. Nature, 2019. 573(7774): p. 434–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ritchie C, et al. , SLC19A1 Is an Importer of the Immunotransmitter cGAMP. Mol Cell, 2019. 75(2): p. 372–381 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhou C, et al. , Transfer of cGAMP into Bystander Cells via LRRC8 Volume-Regulated Anion Channels Augments STING-Mediated Interferon Responses and Anti-viral Immunity. Immunity, 2020. 52(5): p. 767–781 e6. [DOI] [PubMed] [Google Scholar]
- 66.Zhou Y, et al. , Blockade of the Phagocytic Receptor MerTK on Tumor-Associated Macrophages Enhances P2X7R-Dependent STING Activation by Tumor-Derived cGAMP. Immunity, 2020. 52(2): p. 357–373 e9. [DOI] [PubMed] [Google Scholar]
- 67.Maltbaek JH, et al. , ABCC1 transporter exports the immunostimulatory cyclic dinucleotide cGAMP. Immunity, 2022. 55(10): p. 1799–1812 e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lahey LJ, et al. , LRRC8A:C/E Heteromeric Channels Are Ubiquitous Transporters of cGAMP. Mol Cell, 2020. 80(4): p. 578–591 e5. [DOI] [PubMed] [Google Scholar]
- 69.Shokoples BG, Paradis P, and Schiffrin EL, P2X7 Receptors: An Untapped Target for the Management of Cardiovascular Disease. Arterioscler Thromb Vasc Biol, 2021. 41(1): p. 186–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Liu J. p., et al. , ATP ion channel P2X purinergic receptors in inflammation response. Biomedicine & Pharmacotherapy, 2023. 158: p. 114205. [DOI] [PubMed] [Google Scholar]
- 71.Di Virgilio F, et al. , The P2X7 Receptor in Infection and Inflammation. Immunity, 2017. 47(1): p. 15–31. [DOI] [PubMed] [Google Scholar]
- 72.Kong H, et al. , Targeted P2X7/NLRP3 signaling pathway against inflammation, apoptosis, and pyroptosis of retinal endothelial cells in diabetic retinopathy. Cell Death Dis, 2022. 13(4): p. 336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhou Y, et al. , Blockade of the Phagocytic Receptor MerTK on Tumor-Associated Macrophages Enhances P2X7R-Dependent STING Activation by Tumor-Derived cGAMP. Immunity, 2020. 52(2): p. 357–373.e9. [DOI] [PubMed] [Google Scholar]
- 74.Abascal F and Zardoya R, LRRC8 proteins share a common ancestor with pannexins, and may form hexameric channels involved in cell-cell communication. Bioessays, 2012. 34(7): p. 551–60. [DOI] [PubMed] [Google Scholar]
- 75.Qiu Z, et al. , SWELL1, a plasma membrane protein, is an essential component of volume-regulated anion channel. Cell, 2014. 157(2): p. 447–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Voss FK, et al. , Identification of LRRC8 heteromers as an essential component of the volume-regulated anion channel VRAC. Science, 2014. 344(6184): p. 634–8. [DOI] [PubMed] [Google Scholar]
- 77.Syeda R, et al. , LRRC8 Proteins Form Volume-Regulated Anion Channels that Sense Ionic Strength. Cell, 2016. 164(3): p. 499–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Deneka D, et al. , Structure of a volume-regulated anion channel of the LRRC8 family. Nature, 2018. 558(7709): p. 254–259. [DOI] [PubMed] [Google Scholar]
- 79.Concepcion AR, et al. , The volume-regulated anion channel LRRC8C suppresses T cell function by regulating cyclic dinucleotide transport and STING-p53 signaling. Nat Immunol, 2022. 23(2): p. 287–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gulen MF, et al. , Signalling strength determines proapoptotic functions of STING. Nat Commun, 2017. 8(1): p. 427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ritchie C, Cordova AF, and Li L, In response to Luteijn et al.: Concerns regarding cGAMP uptake assay and evidence that SLC19A1 is not the major cGAMP importer in human PBMCs. bioRxiv, 2019: p. 798397. [Google Scholar]
- 82.Wei X, et al. , LL-37 transports immunoreactive cGAMP to activate STING signaling and enhance interferon-mediated host antiviral immunity. Cell Rep, 2022. 39(9): p. 110880. [DOI] [PubMed] [Google Scholar]
- 83.Cordova AF, et al. , Human SLC46A2 Is the Dominant cGAMP Importer in Extracellular cGAMP-Sensing Macrophages and Monocytes. ACS Central Science, 2021. 7(6): p. 1073–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ritchie C, et al. , SLC19A1 Is an Importer of the Immunotransmitter cGAMP. Mol. Cell, 2019. 75(2): p. 372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Luteijn RD, et al. , SLC19A1 transports immunoreactive cyclic dinucleotides. Nature, 2019. 573(7774): p. 434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cronstein BN and Aune TM, Methotrexate and its mechanisms of action in inflammatory arthritis. Nat Rev Rheumatol, 2020. 16(3): p. 145–154. [DOI] [PubMed] [Google Scholar]
- 87.Shmuel-Galia L, et al. , Dysbiosis exacerbates colitis by promoting ubiquitination and accumulation of the innate immune adaptor STING in myeloid cells. Immunity, 2021. 54(6): p. 1137–1153.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ashton JJ, et al. , NOD2 in Crohn’s Disease-Unfinished Business. J Crohns Colitis, 2023. 17(3): p. 450–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Egan AJF, Errington J, and Vollmer W, Regulation of peptidoglycan synthesis and remodelling. Nat Rev Microbiol, 2020. 18(8): p. 446–460. [DOI] [PubMed] [Google Scholar]
- 90.Dörr T, Moynihan PJ, and Mayer C, Editorial: Bacterial Cell Wall Structure and Dynamics. Front Microbiol, 2019. 10: p. 2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Vollmer W, Blanot D, and de Pedro MA, Peptidoglycan structure and architecture. FEMS Microbiol Rev, 2008. 32(2): p. 149–67. [DOI] [PubMed] [Google Scholar]
- 92.Schleifer KH and Kandler O, Peptidoglycan types of bacterial cell walls and their taxonomic implications. Bacteriol Rev, 1972. 36(4): p. 407–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Viswanathan VK, Muramyl dipeptide: Not just another brick in the wall. Gut Microbes, 2014. 5(3): p. 275–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bastos PAD, Wheeler R, and Boneca IG, Uptake, recognition and responses to peptidoglycan in the mammalian host. FEMS Microbiol Rev, 2021. 45(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Girardin SE, et al. , Nod1 Detects a Unique Muropeptide from Gram-Negative Bacterial Peptidoglycan. Science, 2003. 300(5625): p. 1584–1587. [DOI] [PubMed] [Google Scholar]
- 96.Inohara N, et al. , Human Nod1 Confers Responsiveness to Bacterial Lipopolysaccharides*. Journal of Biological Chemistry, 2001. 276(4): p. 2551–2554. [DOI] [PubMed] [Google Scholar]
- 97.Laroui H, et al. , L-Ala-γ-D-Glu-meso-diaminopimelic acid (DAP) interacts directly with leucine-rich region domain of nucleotide-binding oligomerization domain 1, increasing phosphorylation activity of receptor-interacting serine/threonine-protein kinase 2 and its interaction with nucleotide-binding oligomerization domain 1. J Biol Chem, 2011. 286(35): p. 31003–31013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Grimes CL, et al. , The innate immune protein Nod2 binds directly to MDP, a bacterial cell wall fragment. J Am Chem Soc, 2012. 134(33): p. 13535–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Fu J, Schroder K, and Wu H, Mechanistic insights from inflammasome structures. Nat Rev Immunol, 2024. 24(7): p. 518–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Inohara N, et al. , Nod1, an Apaf-1-like activator of caspase-9 and nuclear factor-kappaB. J Biol Chem, 1999. 274(21): p. 14560–7. [DOI] [PubMed] [Google Scholar]
- 101.Lowry RC, et al. , Production of 3’,3’-cGAMP by a Bdellovibrio bacteriovorus promiscuous GGDEF enzyme, Bd0367, regulates exit from prey by gliding motility. PLoS Genet, 2022. 18(5): p. e1010164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Tak U, Walth P, and Whiteley AT, Bacterial cGAS-like enzymes produce 2’,3’-cGAMP to activate an ion channel that restricts phage replication. bioRxiv, 2023. [Google Scholar]
- 103.Li Y, et al. , cGLRs are a diverse family of pattern recognition receptors in innate immunity. Cell, 2023. 186(15): p. 3261–3276 e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Slavik KM, et al. , cGAS-like receptors sense RNA and control 3’2’-cGAMP signalling in Drosophila. Nature, 2021. 597(7874): p. 109–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Cai H, et al. , The virus-induced cyclic dinucleotide 2’3’-c-di-GMP mediates STING-dependent antiviral immunity in Drosophila. Immunity, 2023. 56(9): p. 1991–2005 e9. [DOI] [PubMed] [Google Scholar]
- 106.Motani K, et al. , The Golgi-resident protein ACBD3 concentrates STING at ER-Golgi contact sites to drive export from the ER. Cell Rep, 2022. 41(12): p. 111868. [DOI] [PubMed] [Google Scholar]
- 107.Xie Z, et al. , Structural insights into a shared mechanism of human STING activation by a potent agonist and an autoimmune disease-associated mutation. Cell Discov, 2022. 8(1): p. 133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Decout A, et al. , The cGAS–STING pathway as a therapeutic target in inflammatory diseases. Nature Reviews Immunology, 2021. 21(9): p. 548–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Dong M and Fitzgerald KA, DNA-sensing pathways in health, autoinflammatory and autoimmune diseases. Nature Immunology, 2024. [DOI] [PubMed] [Google Scholar]
- 110.Kurata S, Peptidoglycan recognition proteins in Drosophila immunity. Dev Comp Immunol, 2014. 42(1): p. 36–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Wolf AJ and Underhill DM, Peptidoglycan recognition by the innate immune system. Nat Rev Immunol, 2018. 18(4): p. 243–254. [DOI] [PubMed] [Google Scholar]
- 112.McFarland AP, et al. , Sensing of Bacterial Cyclic Dinucleotides by the Oxidoreductase RECON Promotes NF-kappaB Activation and Shapes a Proinflammatory Antibacterial State. Immunity, 2017. 46(3): p. 433–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Mudgal S, et al. , Cyclic di-AMP: Small molecule with big roles in bacteria. Microbial Pathogenesis, 2021. 161: p. 105264. [DOI] [PubMed] [Google Scholar]
- 114.Daniel H and Kottra G, The proton oligopeptide cotransporter family SLC15 in physiology and pharmacology. Pflugers Arch, 2004. 447(5): p. 610–8. [DOI] [PubMed] [Google Scholar]
- 115.Caruso R, et al. , NOD1 and NOD2: signaling, host defense, and inflammatory disease. Immunity, 2014. 41(6): p. 898–908. [DOI] [PMC free article] [PubMed] [Google Scholar]


