Abstract
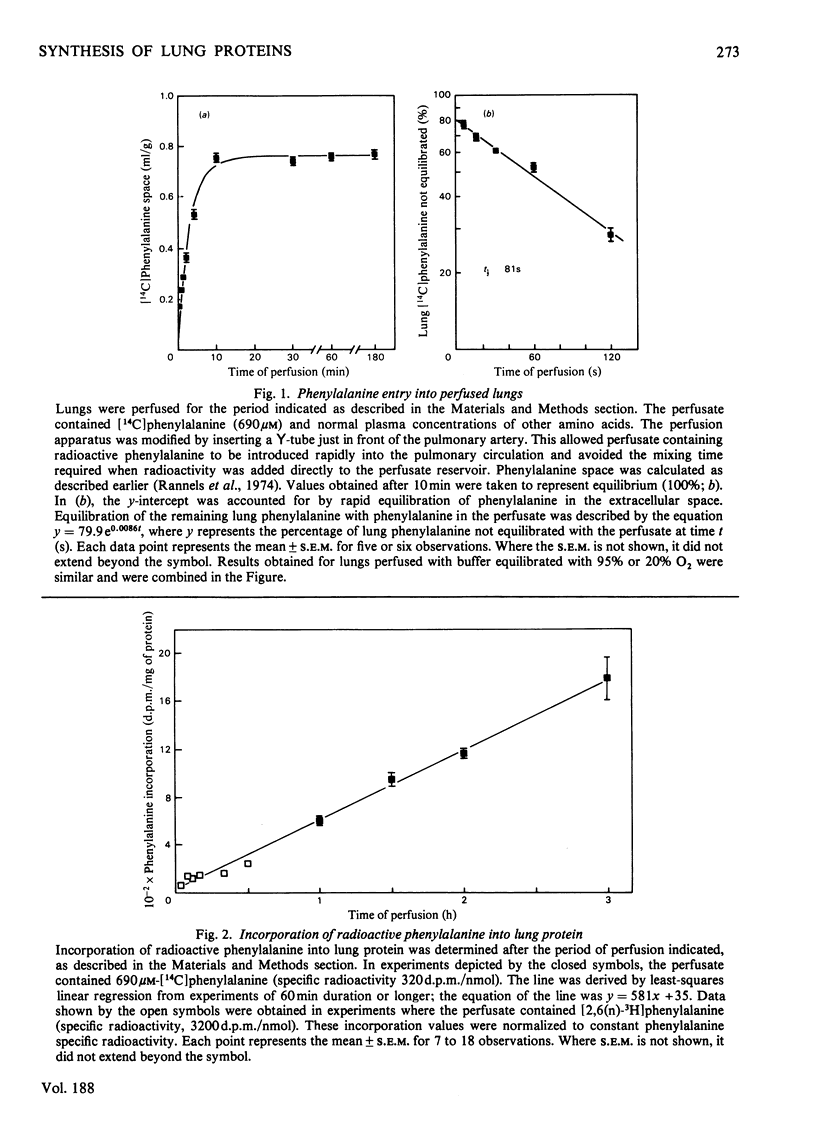
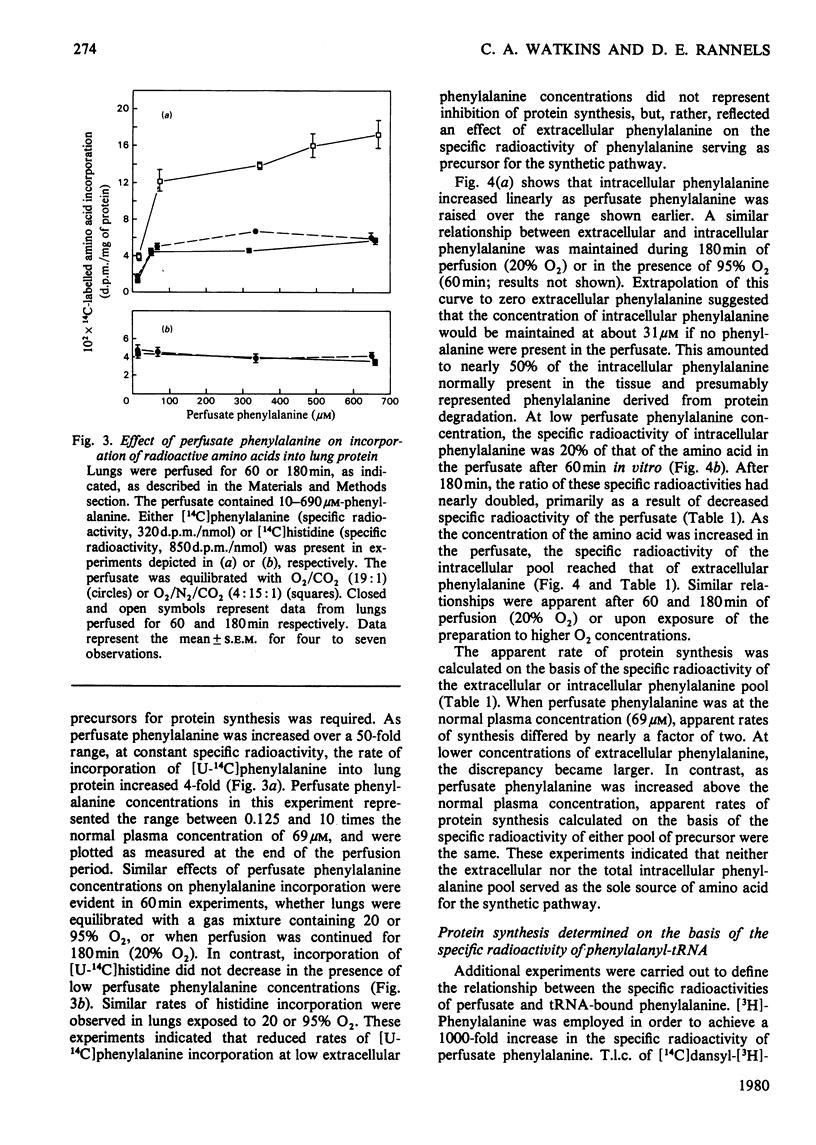
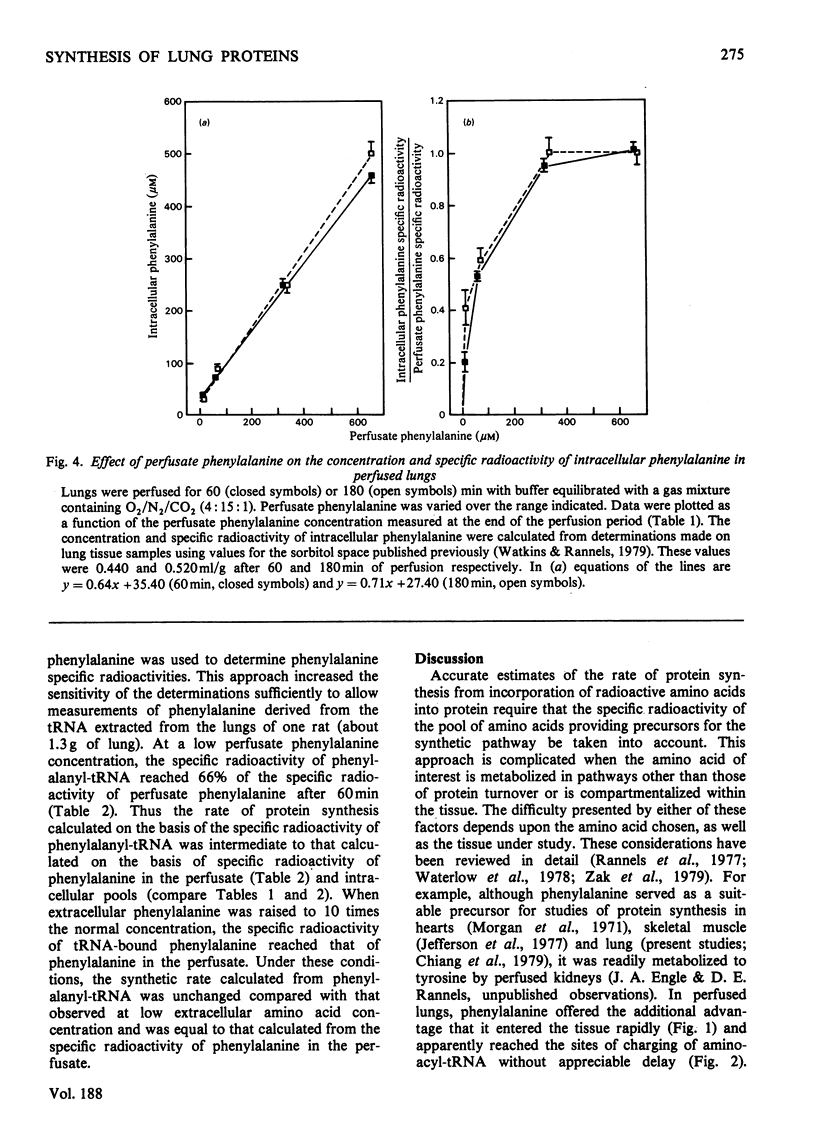
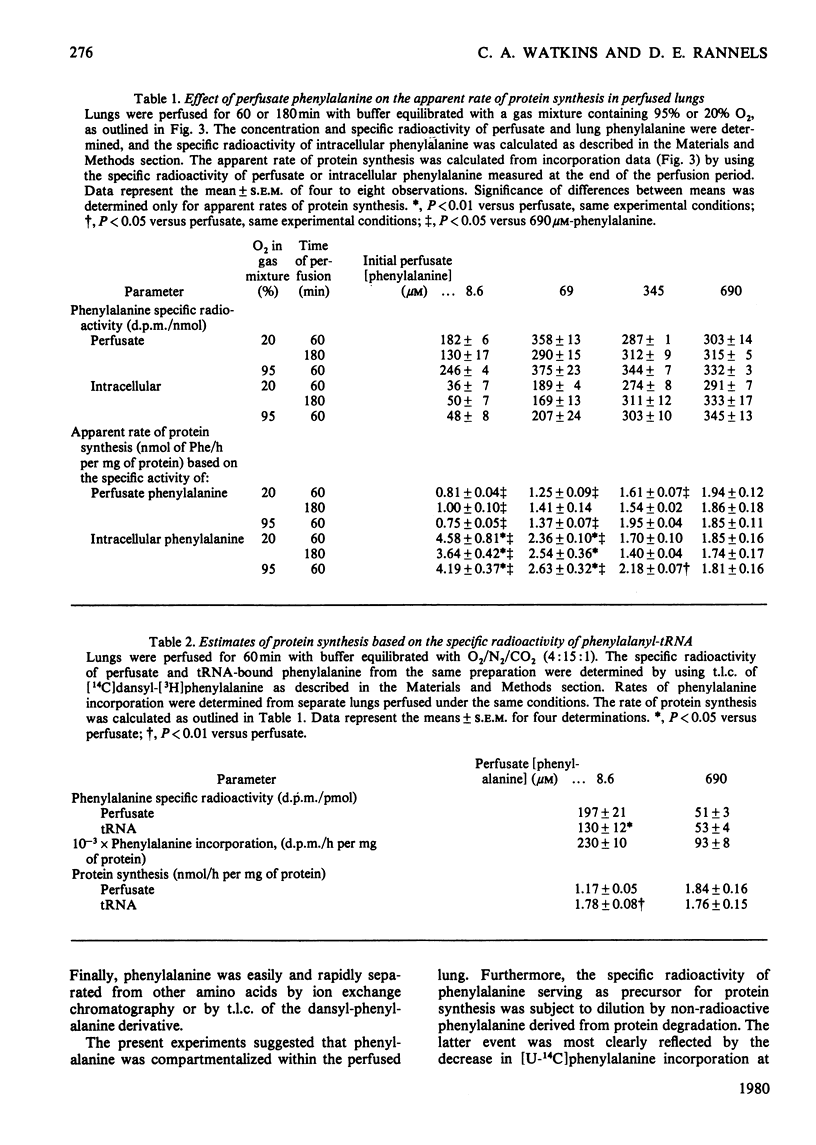
Compartmentalization of amino acid was investigated to define conditions required for accurate measurements of rates of protein synthesis in rat lungs perfused in situ. Lungs were perfused with Krebs–Henseleit bicarbonate buffer containing 4.5% (w/v) bovine serum albumin, 5.6mm-glucose, normal plasma concentrations of 19 amino acids, and 8.6–690μm-[U-14C]phenylalanine. The perfusate was equilibrated with the same humidified gas mixture used to ventilate the lungs [O2/CO2 (19:1) or O2/N2/CO2 (4:15:1)]. [U-14C]Phenylalanine was shown to be a suitable precursor for studies of protein synthesis in perfused lungs: it entered the tissue rapidly (t½, 81s) and was not converted to other compounds. As perfusate phenylalanine was decreased below 5 times the normal plasma concentration, the specific radioactivity of the pool of phenylalanine serving as precursor for protein synthesis, and thus [14C]phenylalanine incorporation into protein, declined. In contrast, incorporation of [14C]histidine into lung protein was unaffected. At low perfusate phenylalanine concentrations, rates of protein synthesis that were based on the specific radioactivity of phenylalanyl-tRNA were between rates calculated from the specific radioactivity of phenylalanine in the extracellular or intracellular pools. Rates based on the specific radioactivities of these three pools of phenylalanine were the same when extracellular phenylalanine was increased. These observations suggested that: (1) phenylalanine was compartmentalized in lung tissue; (2) neither the extracellular nor the total intracellular pool of phenylalanine served as the sole source of precursor for protein; (3) at low extracellular phenylalanine concentrations, rates of protein synthesis were in error if calculated from the specific radioactivity of the free amino acid; (4) at high extracellular phenylalanine concentrations, the effects of compartmentalization were negligible and protein synthesis could be calculated accurately from the specific radioactivity of the free or tRNA-bound phenylalanine pool.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Airhart J., Kelley J., Brayden J. E., Low R. B., Stirewalt W. S. An ultramicro method of amino acid analysis: application to studies of protein metabolism in cultured cells. Anal Biochem. 1979 Jul 1;96(1):45–55. doi: 10.1016/0003-2697(79)90552-9. [DOI] [PubMed] [Google Scholar]
- Bassett D. J., Fisher A. B. Glucose metabolism in rat lung during exposure to hyperbaric O2. J Appl Physiol Respir Environ Exerc Physiol. 1979 May;46(5):943–949. doi: 10.1152/jappl.1979.46.5.943. [DOI] [PubMed] [Google Scholar]
- Block E. R., Fisher A. B. Depression of serotonin clearance by rat lungs during oxygen exposure. J Appl Physiol Respir Environ Exerc Physiol. 1977 Jan;42(1):33–38. doi: 10.1152/jappl.1977.42.1.33. [DOI] [PubMed] [Google Scholar]
- Chiang M. J., Whitney P., Jr, Massaro D. Protein metabolism in lung: use of isolated perfused lung to study protein degradation. J Appl Physiol Respir Environ Exerc Physiol. 1979 Jul;47(1):72–78. doi: 10.1152/jappl.1979.47.1.72. [DOI] [PubMed] [Google Scholar]
- Chua B., Kao R. L., Rannels D. E., Morgan H. E. Inhibition of protein degradation by anoxia and ischemia in perfused rat hearts. J Biol Chem. 1979 Jul 25;254(14):6617–6623. [PubMed] [Google Scholar]
- Exton J. H. The perfused rat liver. Methods Enzymol. 1975;39:25–36. doi: 10.1016/s0076-6879(75)39006-x. [DOI] [PubMed] [Google Scholar]
- Garlick P. J., Burk T. L., Swick R. W. Protein synthesis and RNA in tissues of the pig. Am J Physiol. 1976 Apr;230(4):1108–1112. doi: 10.1152/ajplegacy.1976.230.4.1108. [DOI] [PubMed] [Google Scholar]
- Hod Y., Hershko A. Relationship of the pool of intracellular valine to protein synthesis and degradation in cultured cells. J Biol Chem. 1976 Jul 25;251(14):4458–4457. [PubMed] [Google Scholar]
- Jefferson L. S., Li J. B., Rannels S. R. Regulation by insulin of amino acid release and protein turnover in the perfused rat hemicorpus. J Biol Chem. 1977 Feb 25;252(4):1476–1483. [PubMed] [Google Scholar]
- Khairallah E. A., Mortimore G. E. Assessment of protein turnover in perfused rat liver. Evidence for amino acid compartmentation from differential labeling of free and tRNA-gound valine. J Biol Chem. 1976 Mar 10;251(5):1375–1384. [PubMed] [Google Scholar]
- Martin A. F., Rabinowitz M., Blough R., Prior G., Zak R. Measurements of half-life of rat cardiac myosin heavy chain with leucyl-tRNA used as precursor pool. J Biol Chem. 1977 May 25;252(10):3422–3429. [PubMed] [Google Scholar]
- McKee E. E., Cheung J. Y., Rannels D. E., Morgan H. E. Measurement of the rate of protein synthesis and compartmentation of heart phenylalanine. J Biol Chem. 1978 Feb 25;253(4):1030–1040. [PubMed] [Google Scholar]
- Morgan H. E., Earl D. C., Broadus A., Wolpert E. B., Giger K. E., Jefferson L. S. Regulation of protein synthesis in heart muscle. I. Effect of amino acid levels on protein synthesis. J Biol Chem. 1971 Apr 10;246(7):2152–2162. [PubMed] [Google Scholar]
- Mortimore G. E., Woodside K. H., Henry J. E. Compartmentation of free valine and its relation to protein turnover in perfused rat liver. J Biol Chem. 1972 May 10;247(9):2776–2784. [PubMed] [Google Scholar]
- Rannels D. E., Hjalmarson A. C., Morgan H. E. Effects of noncarbohydrate substrates on protein synthesis in muscle. Am J Physiol. 1974 Mar;226(3):528–539. doi: 10.1152/ajplegacy.1974.226.3.528. [DOI] [PubMed] [Google Scholar]
- Rannels D. E., Kao R., Morgan H. E. Effect of insulin on protein turnover in heart muscle. J Biol Chem. 1975 Mar 10;250(5):1694–1701. [PubMed] [Google Scholar]
- Rannels D. E., Sahms R. H., Watkins C. A. Effects of starvation and diabetes on protein synthesis in lung. Am J Physiol. 1979 Apr;236(4):E421–E428. doi: 10.1152/ajpendo.1979.236.4.E421. [DOI] [PubMed] [Google Scholar]
- Rannels D. E., White D. M., Watkins C. A. Rapidity of compensatory lung growth following pneumonectomy in adult rats. J Appl Physiol Respir Environ Exerc Physiol. 1979 Feb;46(2):326–333. doi: 10.1152/jappl.1979.46.2.326. [DOI] [PubMed] [Google Scholar]
- Simon L. M., Raffin T. A., Douglas W. H., Theodore J., Robin E. D. Effects of high oxygen exposure on bioenergetics in isolated type II pneumocytes. J Appl Physiol Respir Environ Exerc Physiol. 1979 Jul;47(1):98–103. doi: 10.1152/jappl.1979.47.1.98. [DOI] [PubMed] [Google Scholar]
- Thet L. A., Delaney M. D., Gregorio C. A., Massaro D. Protein metabolism by rat lung: influence of fasting, glucose, and insulin. J Appl Physiol Respir Environ Exerc Physiol. 1977 Sep;43(3):463–467. doi: 10.1152/jappl.1977.43.3.463. [DOI] [PubMed] [Google Scholar]
- UDENFRIEND S., COOPER J. R. The enzymatic conversion of phenylalanine to tyrosine. J Biol Chem. 1952 Feb;194(2):503–511. [PubMed] [Google Scholar]
- Vidrich A., Airhart J., Bruno M. K., Khairallah E. A. Compartmentation of free amino acids for protein biosynthesis. Influence of diurnal changes in hepatic amino acid concentrations of the composition of the precursor pool charging aminoacyl-transfer ribonucleic acid. Biochem J. 1977 Feb 15;162(2):257–266. doi: 10.1042/bj1620257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WOLLENBERGER A., RISTAU O., SCHOFFA G. [A simple technic for extremely rapid freezing of large pieces of tissue]. Pflugers Arch Gesamte Physiol Menschen Tiere. 1960;270:399–412. [PubMed] [Google Scholar]
- Wallyn C. S., Vidrich A., Airhart J., Khairallah E. A. Analysis of the specific radioactivity of valine isolated from aminoacyl-transfer ribonucleic acid of rat liver. Biochem J. 1974 Jun;140(3):545–548. doi: 10.1042/bj1400545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins C. A., Morgan H. E. Relationship between rates of methylation and synthesis of heart protein. J Biol Chem. 1979 Feb 10;254(3):693–701. [PubMed] [Google Scholar]
- Watkins C. A., Rannels D. E. In situ perfusion of rat lungs: stability and effects of oxygen tension. J Appl Physiol Respir Environ Exerc Physiol. 1979 Aug;47(2):325–329. doi: 10.1152/jappl.1979.47.2.325. [DOI] [PubMed] [Google Scholar]
- Zak R., Martin A. F., Blough R. Assessment of protein turnover by use of radioisotopic tracers. Physiol Rev. 1979 Apr;59(2):407–447. doi: 10.1152/physrev.1979.59.2.407. [DOI] [PubMed] [Google Scholar]


