Abstract
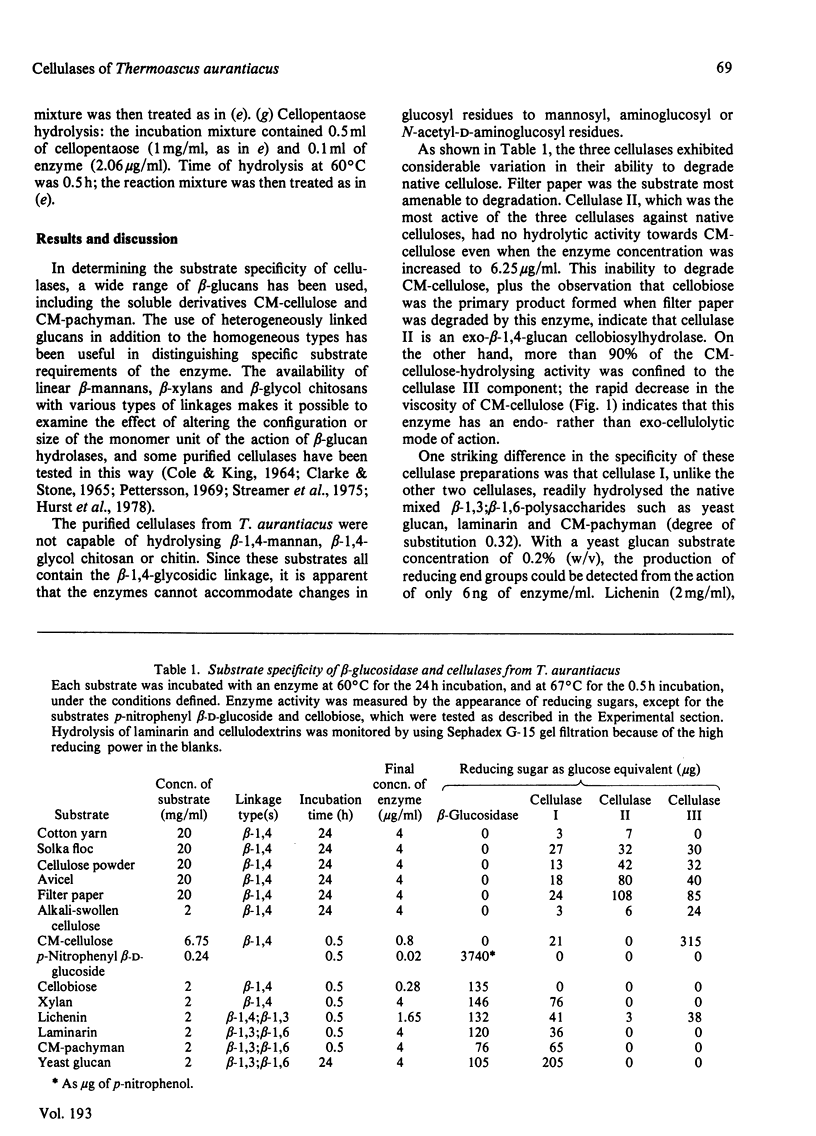
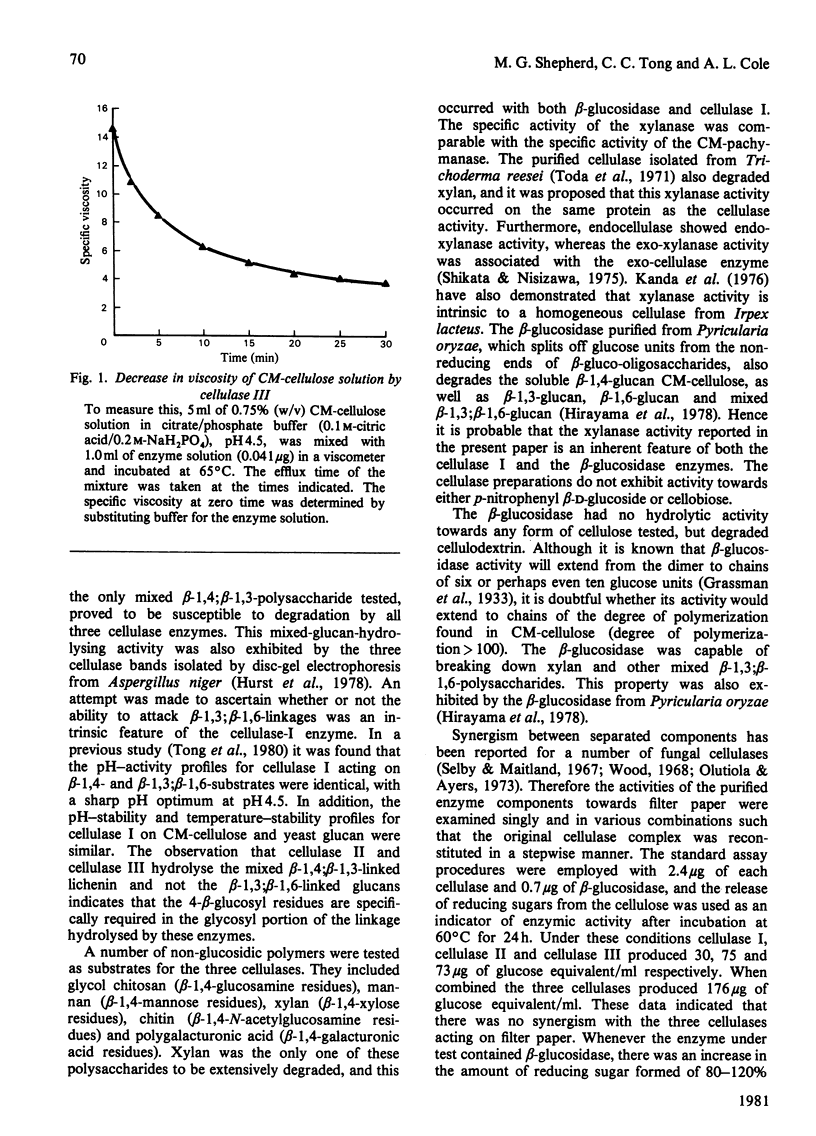
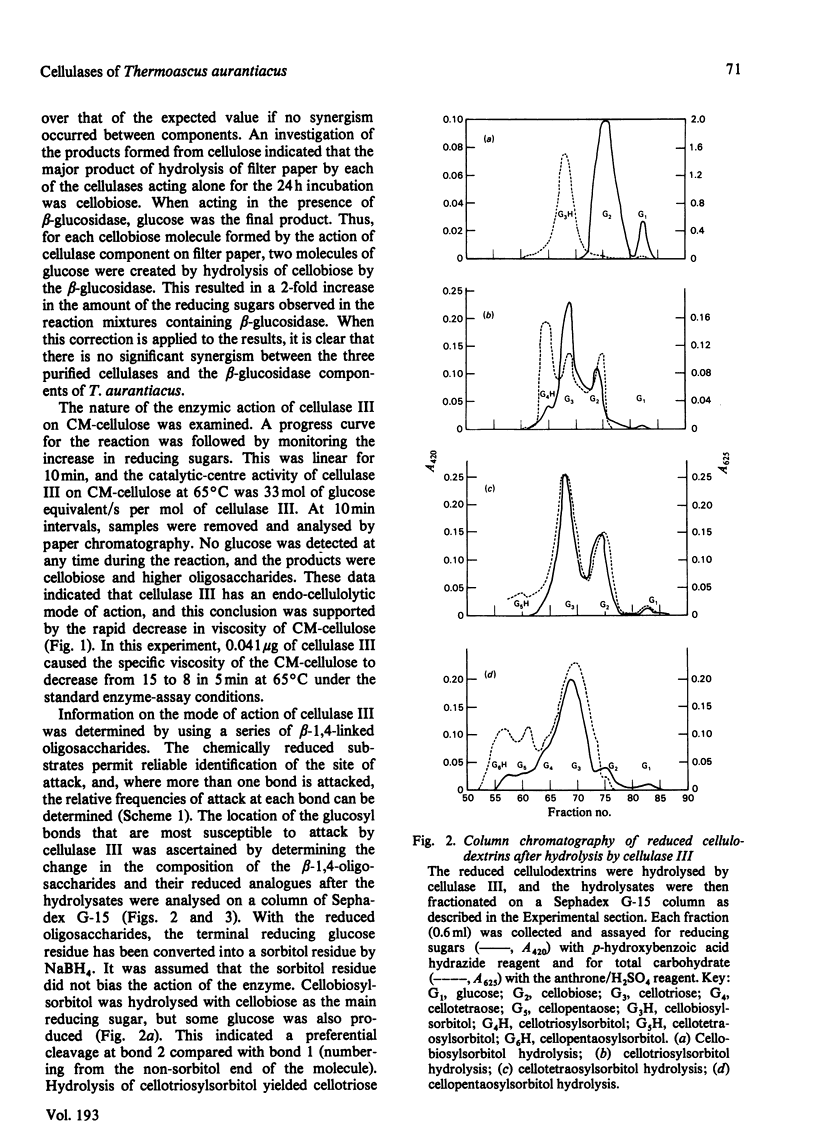
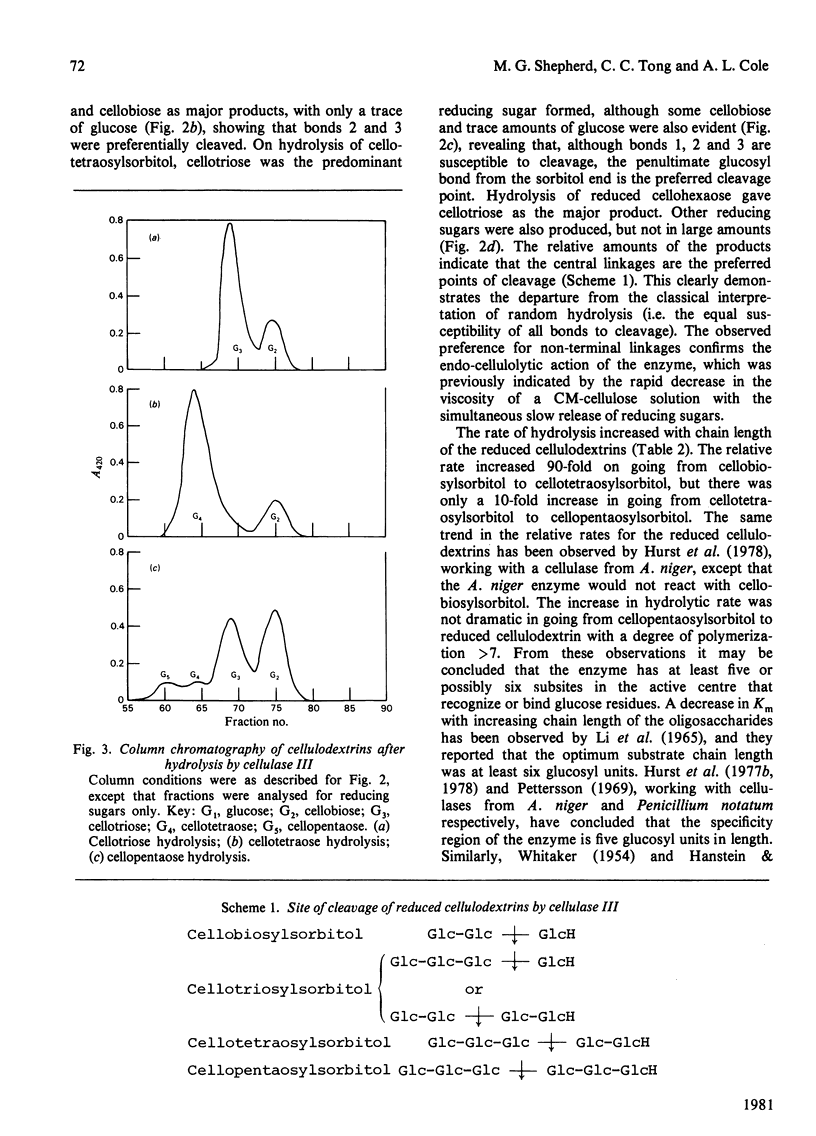
The substrate specificities of three cellulases and a beta-glucosidase purified from Thermoascus aurantiacus were examined. All three cellulases partially degraded native cellulose. Cellulase I, but not cellulase II and cellulase III, readily hydrolyzed the mixed beta-1,3; beta-1,6-polysaccharides such as carboxymethyl-pachyman, yeast glucan and laminarin. Both cellulase I and the beta-glucosidase degraded xylan, and it is proposed that the xylanase activity is an inherent feature of these two enzymes. Lichenin (beta-1,4; beta-1,3) was degraded by all three cellulases. Cellulase II cannot degrade carboxymethyl-cellulose, and with filter paper as substrate the end product was cellobiose, which indicates that cellulase II is an exo-beta-1,4-glucan cellobiosylhydrolase. Degradation of cellulose (filter paper) can be catalysed independently by each of the three cellulases; there was no synergistic effect between any of the cellulases, and cellobiose was the principal product of degradation. The mode of action of one cellulase (cellulase III) was examined by using reduced cellulodextrins. The central linkages of the cellulodextrins were the preferred points of cleavage, which, with the rapid decrease in viscosity of carboxymethyl-cellulose, confirmed that cellulase III was an endocellulase. The rate of hydrolysis increased with chain length of the reduced cellulodextrins, and these kinetic data indicated that the specificity region of cellulase III was five or six glucose units in length.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berghem L. E., Pettersson L. G., Axiö-Fredriksson U. B. The mechanism of enzymatic cellulose degradation. Purification and some properties of two different 1,4beta-glucan glucanohydrolases from Trichoderma viride. Eur J Biochem. 1976 Jan 15;61(2):621–630. doi: 10.1111/j.1432-1033.1976.tb10058.x. [DOI] [PubMed] [Google Scholar]
- Clarke A. E., Stone B. A. beta-glucan hydrolases from Aspergillus niger. Isolation of a beta-(1-4)-glucan hydrolase and some properties of the beta-(1-3)-glucan-hydrolase components. Biochem J. 1965 Sep;96(3):793–801. doi: 10.1042/bj0960793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HANSTEIN E. G., WHITAKER D. R. Improved procedures for preparation and characterization of myrothecium cellulase. 4. Characterization of activity toward beta-methyl glycosides of 1--4-beta-d-oligoglucosides. Can J Biochem Physiol. 1963 Mar;41:707–718. [PubMed] [Google Scholar]
- HASH J. H., KING K. W. On the nature of the beta-glucosidases of Myrothecium verrucaria. J Biol Chem. 1958 May;232(1):381–393. [PubMed] [Google Scholar]
- Hirayama T., Horie S., Nagayama H., Matsuda K. Studies on cellulases of a phytopathogenic fungus, Pyricularia oryzae cavara. II. Purification and properties of a beta-glucosidase. J Biochem. 1978 Jul;84(1):27–37. doi: 10.1093/oxfordjournals.jbchem.a132116. [DOI] [PubMed] [Google Scholar]
- Hurst P. L., Nielsen J., Sullivan P. A., Shepherd M. G. Purification and properties of a cellulase from Aspergillus niger. Biochem J. 1977 Jul 1;165(1):33–41. doi: 10.1042/bj1650033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurst P. L., Sullivan P. A., Shepherd M. G. Chemical modification of cellulase from Aspergillus niger. Biochem J. 1977 Dec 1;167(3):549–556. doi: 10.1042/bj1670549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurst P. L., Sullivan P. A., Shepherd M. G. Substrate specificity and mode of action of a cellulase from Aspergillus niger. Biochem J. 1978 Feb 1;169(2):389–395. doi: 10.1042/bj1690389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanda T., Wakabayashi K., Nisizawa K. Xylanase activity of an endo-cellulase of carboxymethyl-cellulase type from Irpex lacteus (Polyporus tulipiferae). J Biochem. 1976 May;79(5):989–995. doi: 10.1093/oxfordjournals.jbchem.a131166. [DOI] [PubMed] [Google Scholar]
- Li L. H., Flora R. M., King K. W. Individual roles of cellulase components derived from Trichoderma viride. Arch Biochem Biophys. 1965 Aug;111(2):439–447. doi: 10.1016/0003-9861(65)90207-9. [DOI] [PubMed] [Google Scholar]
- Lloyd J. B., Whelan W. J. An improved method for enzymic determination of glucose in the presence of maltose. Anal Biochem. 1969 Sep;30(3):467–470. doi: 10.1016/0003-2697(69)90143-2. [DOI] [PubMed] [Google Scholar]
- Pettersson G. Studies on cellulolytic enzymes. VI. Specificity and mode of action on different substrates of a cellulase from Penicillium notatum. Arch Biochem Biophys. 1969 Mar;130(1):286–294. doi: 10.1016/0003-9861(69)90035-6. [DOI] [PubMed] [Google Scholar]
- Rautela G. S., Cowling E. B. Simple cultural test for relative cellulolytic activity of fungi. Appl Microbiol. 1966 Nov;14(6):892–898. doi: 10.1128/am.14.6.892-898.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SMOGYI M. Notes on sugar determination. J Biol Chem. 1952 Mar;195(1):19–23. [PubMed] [Google Scholar]
- Selby K., Maitland C. C. The cellulase of Trichoderma viride. Separation of the components involved in the solubilization of cotton. Biochem J. 1967 Sep;104(3):716–724. doi: 10.1042/bj1040716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shikata S., Nsizawa K. Purification and properties of an exo-cellulase component of novel type from Trichoderma miride. J Biochem. 1975 Sep;78(3):499–512. doi: 10.1093/oxfordjournals.jbchem.a130934. [DOI] [PubMed] [Google Scholar]
- Streamer M., Eriksson K. E., Pettersson B. Extracellular enzyme system utilized by the fungus Sporotrichum pulverulentum (Chrysosporium lignorum) for the breakdown of cullulose. Functional characterization of five endo-1,4-beta-glucanases and one exo-1,4-beta-glucanase. Eur J Biochem. 1975 Nov 15;59(2):607–613. doi: 10.1111/j.1432-1033.1975.tb02489.x. [DOI] [PubMed] [Google Scholar]
- Tong C. C., Cole A. L., Shepherd M. G. Purification and properties of the cellulases from the thermophilic fungus Thermoascus aurantiacus. Biochem J. 1980 Oct 1;191(1):83–94. doi: 10.1042/bj1910083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHITAKER D. R. Hydrolysis of a series of beta-1,4'-oligoglucosides by Myrothecium verrucaria cellulase. Arch Biochem Biophys. 1954 Dec;53(2):439–449. doi: 10.1016/0003-9861(54)90425-7. [DOI] [PubMed] [Google Scholar]
- Wood T. M. Cellulolytic enzyme system of Trichoderma koningii. Separation of components attacking native cotton. Biochem J. 1968 Sep;109(2):217–227. doi: 10.1042/bj1090217. [DOI] [PMC free article] [PubMed] [Google Scholar]


