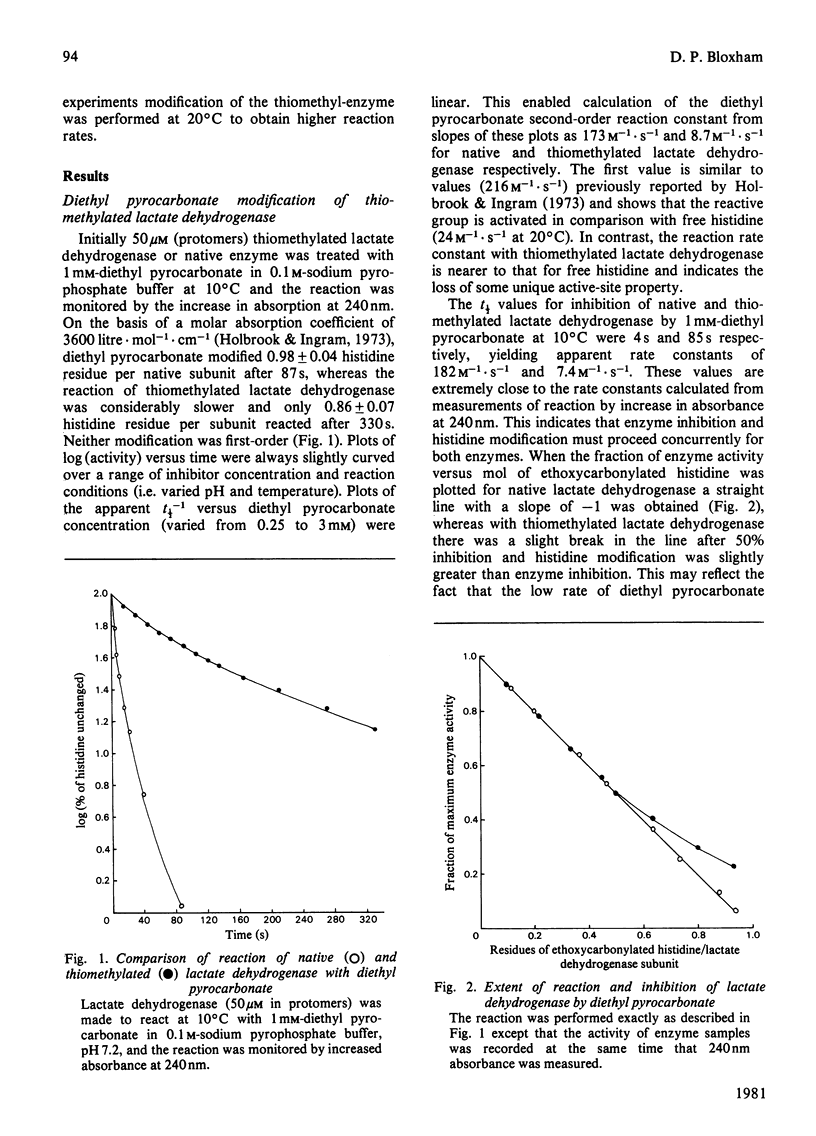
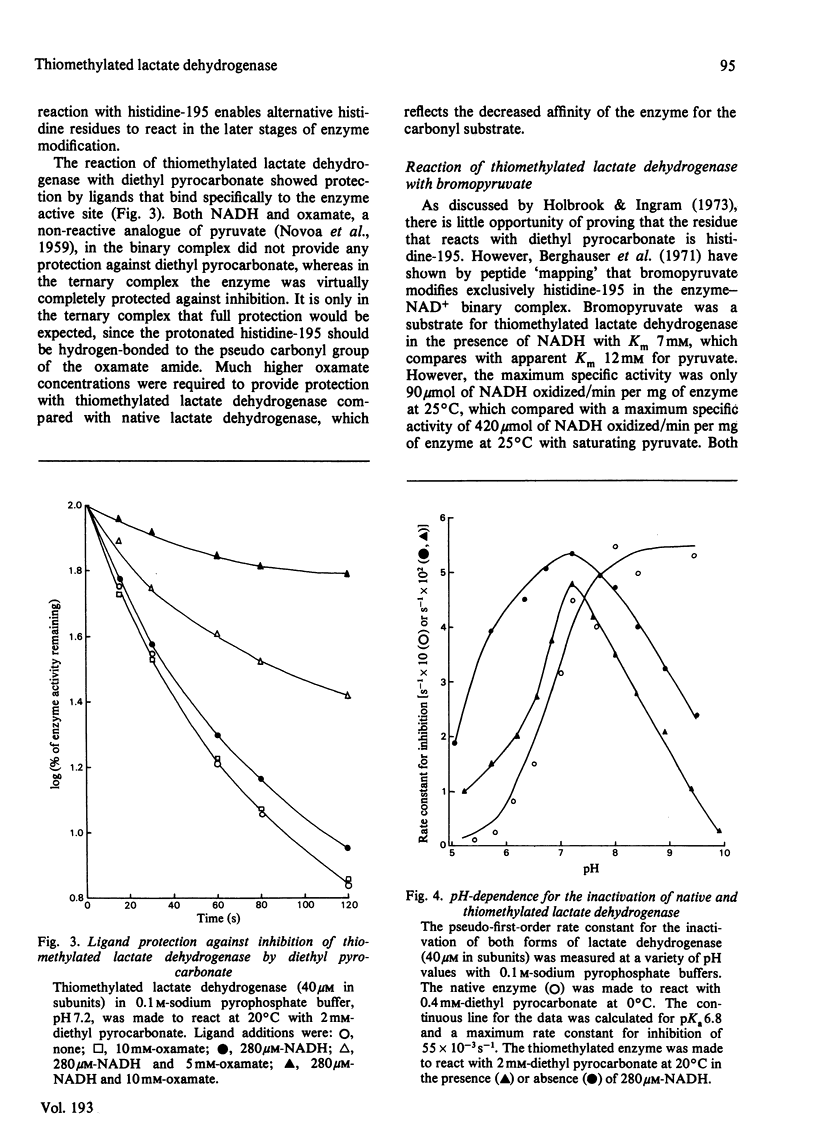
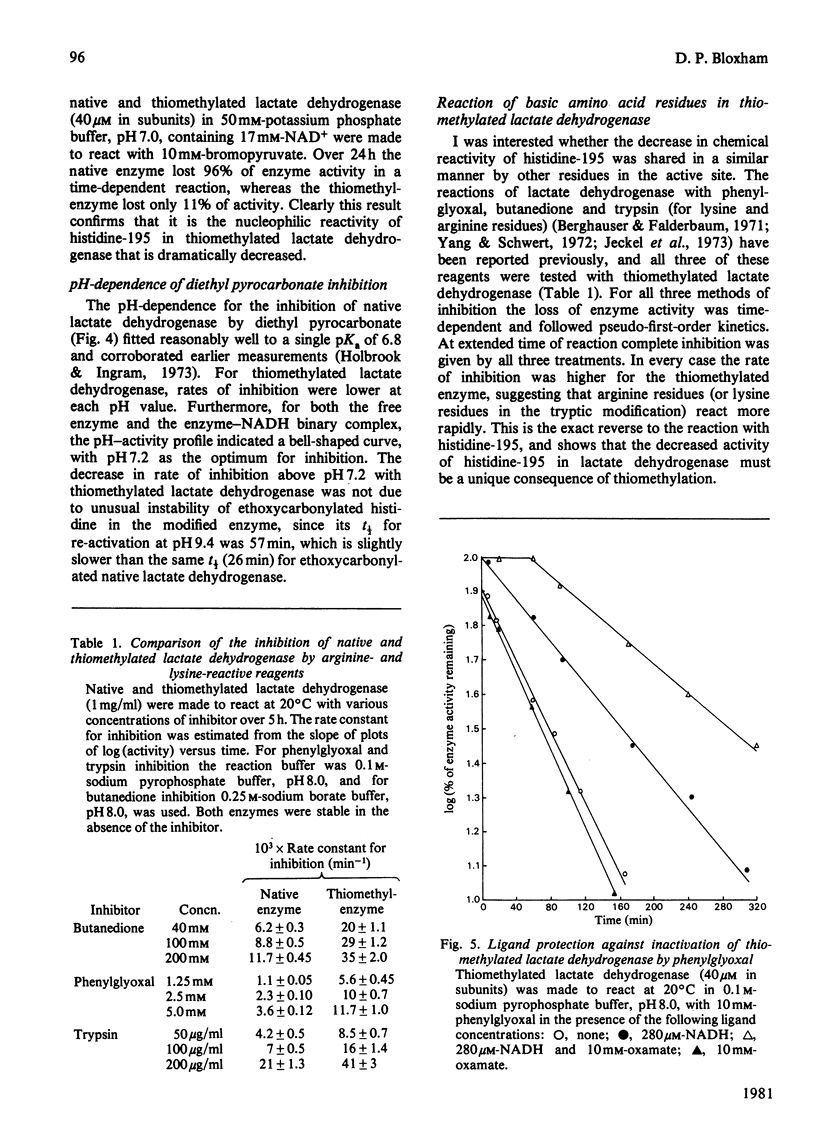
Abstract
The specific thiomethylation of cysteine-165 (insertion of a methylthio group, CH3-S-) in pig heart lactate dehydrogenase results in a decreased affinity for carbonyl ligands that is accompanied by a decreased nucleophilic reaction of histidine-195 with diethyl pyrocarbonate. The rate constants at 10 degrees C for the modification of native and thiomethylated lactate dehydrogenase by diethyl pyrocarbonate were 173 M-1 . s-1 and 8.7 M-1 . s-1 respectively. It was found that 0.86 +/- 0.07 histidine residue per subunit reacted with diethyl pyrocarbonate in thiomethylated lactate dehydrogenase. This reaction was not affected in the enzyme-NADH binary complex, but was diminished in the enzyme-NADH-oxamate ternary complex. In the enzyme-NADH complex the reaction of diethyl pyrocarbonate was controlled by two groups with pKa 6.8 and 7.9. The decreased reactivity of histidine-195 was selective in thiomethylated lactate dehydrogenase, since the reactivity of arginine and/or lysine residues was enhanced.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berghäuser J., Falderbaum I. Modifizierung eines essentiellen Argininrestes in Lactat-Dehydrogenase mit Phenylglyoxal. Hoppe Seylers Z Physiol Chem. 1971 Sep;352(9):1189–1194. [PubMed] [Google Scholar]
- Berghäuser J., Falderbaum I., Woenckhaus C. Zuordnung eines essentiellen Histidinrestes der Lactat-Dehydrogenase zur Substratbindungsstelle. Hoppe Seylers Z Physiol Chem. 1971 Jan;352(1):52–58. doi: 10.1515/bchm2.1971.352.1.52. [DOI] [PubMed] [Google Scholar]
- Bloxham D. P., Giles I. G., Wilton D. C., Akhtar M. The mechanism of the bond forming events in pyridine nucleotide linked oxidoreductases. Studies with epoxide inhibitors of lactic dehydrogenase and beta-hydroxybutyrate dehydrogenase. Biochemistry. 1975 May 20;14(10):2235–2241. doi: 10.1021/bi00681a030. [DOI] [PubMed] [Google Scholar]
- Bloxham D. P., Sharma R. P., Wilton D. C. A detailed investigation of the properties of lactate dehydrogenase in which the 'Essential' cysteine-165 is modified by thioalkylation. Biochem J. 1979 Mar 1;177(3):769–780. doi: 10.1042/bj1770769a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloxham D. P., Wilton D. C. Modification of pig heart lactate dehydrogenase with methyl methanethiosulphonate to produce an enzyme with altered catalytic activity. Biochem J. 1977 Mar 1;161(3):643–651. doi: 10.1042/bj1610643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fondy T. P., Everse J., Driscoll G. A., Castillo F., Stolzenbach F. E., Kaplan N. O. The comparative enzymology of lactic dehydrogenases. IV. Function of sulfhydryl groups in lactic dehydrogenases and the sequence around the essential group. J Biol Chem. 1965 Nov;240(11):4219–4234. [PubMed] [Google Scholar]
- Holbrook J. J., Gutfreund H. Approaches to the study of enzyme mechanisms lactate dehydrogenase. FEBS Lett. 1973 Apr 15;31(2):157–169. doi: 10.1016/0014-5793(73)80095-x. [DOI] [PubMed] [Google Scholar]
- Holbrook J. J., Ingram V. A. Ionic properties of an essential histidine residue in pig heart lactate dehydrogenase. Biochem J. 1973 Apr;131(4):729–738. doi: 10.1042/bj1310729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holbrook J. J., Pfleiderer G. Bedeutung von SH-Gruppen für die enzymatische Aktivität. 3. Eine Methode, um die essentiellen Cysteinreste in nativer Schweineherz-Lactatdehydrogenase (Isozym I) radioaktiv zu markieren. Biochem Z. 1965 Jun 3;342(1):111–114. [PubMed] [Google Scholar]
- Holbrook J. J. The importance of SH-groups for enzymic activity. V. The coenzyme-binding capacity of pig heart lactate dehydrogenase, isozyme I, after inhibition by various maleinimides. Biochem Z. 1966 Mar 28;344(2):141–152. [PubMed] [Google Scholar]
- Jeckel D., Anders R., Pfleiderer G. Proteolytische Spaltung nativer Lactat-Dehydrogenase aus Schweineherz, I. Untersuchungen über die Möglichkeit, durch Einwirkung von Trypsin enzymatisch aktive Spaltprodukte zu erhalten. Hoppe Seylers Z Physiol Chem. 1973 Jul;354(7):737–748. [PubMed] [Google Scholar]
- Lodola A., Parker D. M., Jeck R., Holbrook J. J. Malate dehydrogenase of the cytosol. Ionizations of the enzyme-reduced-coenzyme complex and a comparison with lactate dehydrogenase. Biochem J. 1978 Aug 1;173(2):597–605. doi: 10.1042/bj1730597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NOVOA W. B., WINER A. D., GLAID A. J., SCHWERT G. W. Lactic dehydrogenase. V. Inhibition by oxamate and by oxalate. J Biol Chem. 1959 May;234(5):1143–1148. [PubMed] [Google Scholar]
- Whitaker J. R., Yates D. W., Bennett N. G., Holbrook J. J., Gutfreund H. The identification of intermediates in the reaction of pig heart lactate dehydrogenase with its substrates. Biochem J. 1974 Jun;139(3):677–697. doi: 10.1042/bj1390677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woenckhaus C., Berghäuser J., Pfleiderer G. Markierung essentieller Aminosäurereste der Lactat-Dehydrogenase aus Schweineherz mit (Carbonyl-14C)3-(2-Brom-acetyl)-pyridin. Hoppe Seylers Z Physiol Chem. 1969 Apr;350(4):473–483. [PubMed] [Google Scholar]
- Yang P. C., Schwert G. W. Inactivation of lactate dehydrogenase by butanedione. Biochemistry. 1972 Jun 6;11(12):2218–2224. doi: 10.1021/bi00762a002. [DOI] [PubMed] [Google Scholar]


