Abstract
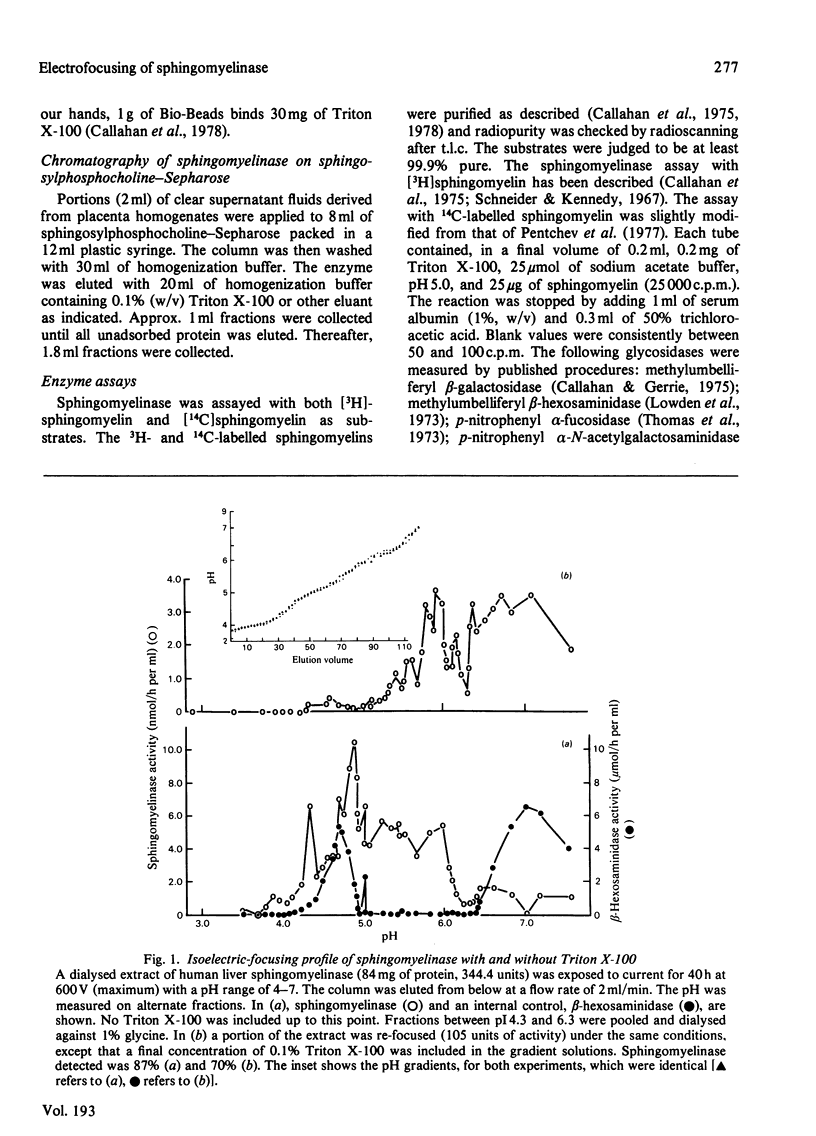
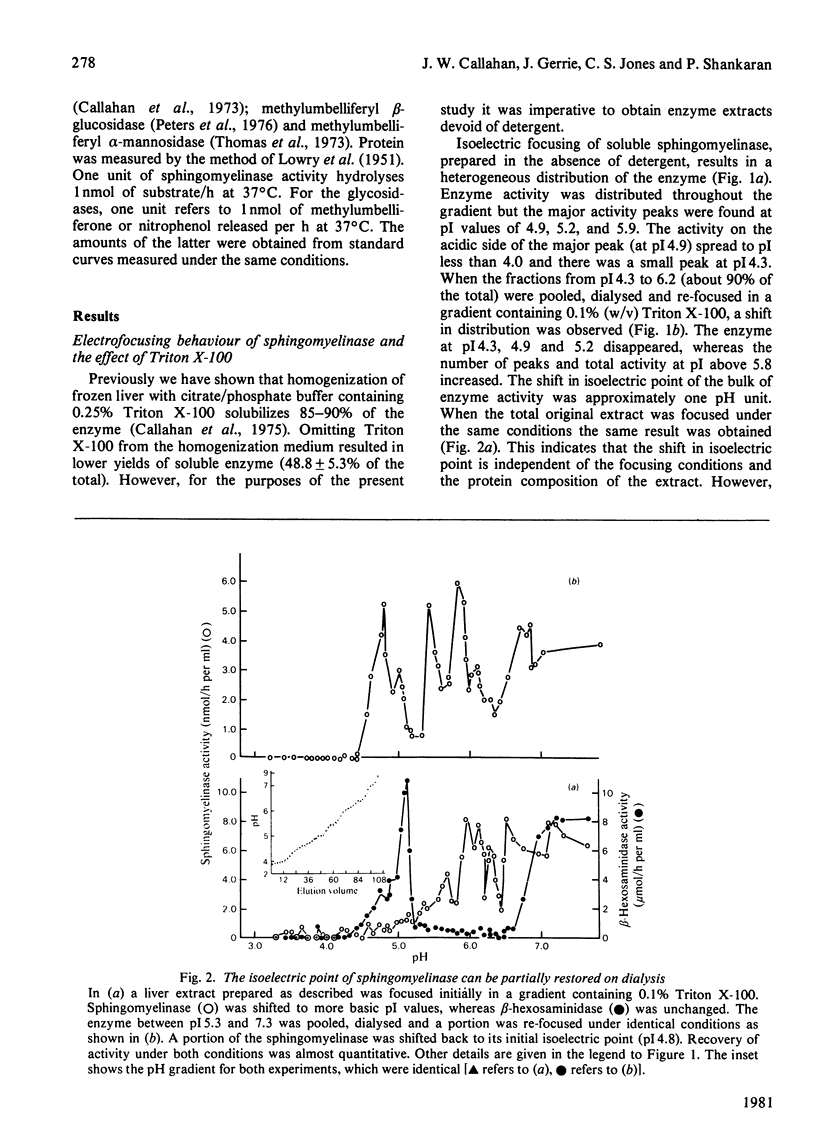
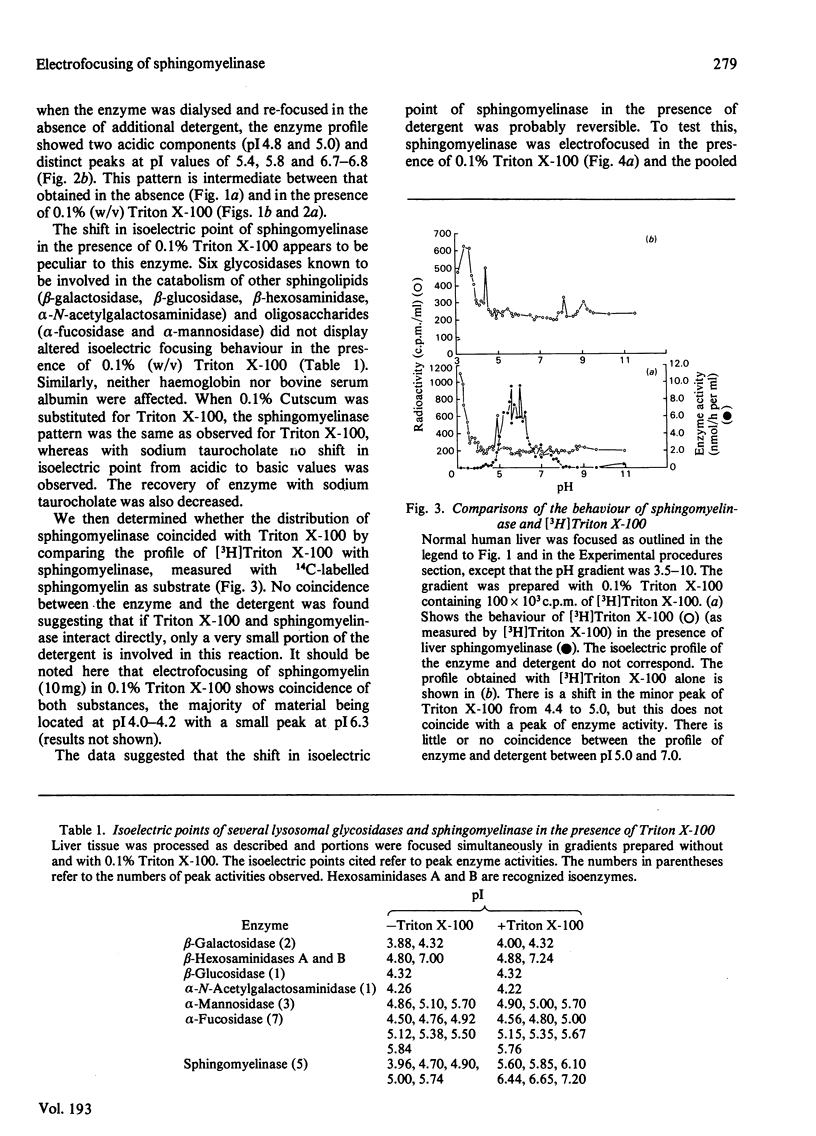
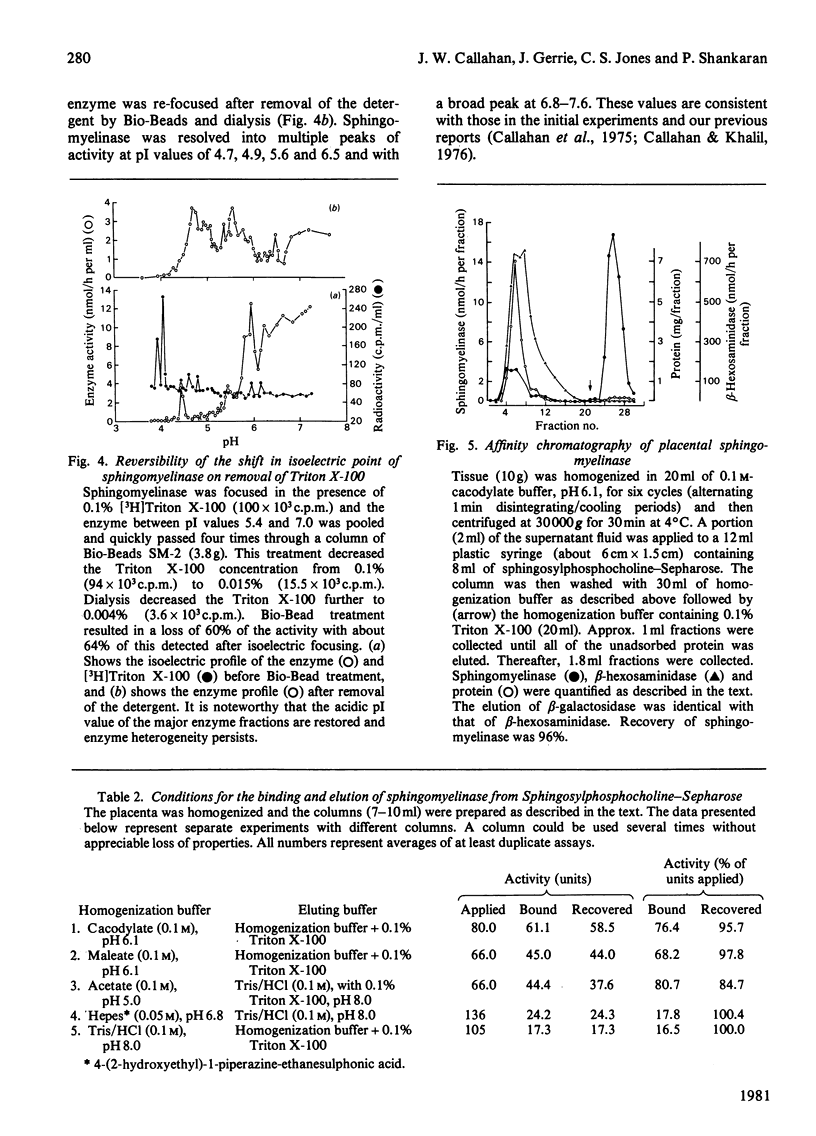
Crude liver lysosomal sphingomyelinase (EC 3.1.4.12) displays a heterogeneous electrofocusing profile. The majority of the enzyme resolves into two major components with acidic pI values near pH 4.6 and 4.8. Several additional minor peaks of activity are seen at more basic pH values (up to pH 8.0). In the presence of 0.1% Triton X-100 (or Cutscum), the location of sphingomyelinase is shifted by about 1 pH unit to more basic pH values. Triton X-100 also increases the apparent heterogeneity of sphingomyelinase. Removal of detergent by treatment with Bio Beads SM-2 restores the acidic pI profile. This behaviour appears to be specific, since it was not shared by six glycosidases several of which hydrolyse sphingolipids. The electrofocusing profile of 3H-labelled Triton X-100 was distinct and separate from sphingomyelinase, suggesting that only a small fraction of detergent interacted directly with the enzyme. To study this behaviour in more detail we examined the effect of detergents on elution of sphingomyelinase from sphingosylphosphocholine-Sepharose. Sphingosylphosphocholine is a competitive inhibitor of sphingomyelinase (Ki 0.5 mM). Binding of enzyme was pH-dependent. Triton X-100, Cutscum and Tween 20 eluted significant amounts of enzyme at 0.01-0.02%. Total elution was achieved with up to 0.1% detergent. These data suggest that sphingomyelinase binds to neutral detergent monomers with a high degree of affinity. In excess detergent (5-7 times the critical micellar concentration) the surface charge on the protein is changed, leading to a pI shift. This behaviour probably does not occur at the active site of the enzyme, since there is no appreciable effect on substrate hydrolysis and substrate analogues were ineffective in eluting the enzyme.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Besley G. T. Effect of Triton X-100 on the isoelectric focusing profile of fibroblast sphingomyelinase. FEBS Lett. 1976 Dec 15;72(1):101–104. doi: 10.1016/0014-5793(76)80822-8. [DOI] [PubMed] [Google Scholar]
- Besley G. T. Sphingomyelinase defect in Niemann-Pick disease, type C, fibroblasts. FEBS Lett. 1977 Aug 1;80(1):71–74. doi: 10.1016/0014-5793(77)80409-2. [DOI] [PubMed] [Google Scholar]
- Callahan J. W., Gerrie J. Purification of G-M-1-ganglioside and ceramide lactoside beta-galactosidase from rabbit brain. Biochim Biophys Acta. 1975 May 23;391(1):141–153. doi: 10.1016/0005-2744(75)90160-6. [DOI] [PubMed] [Google Scholar]
- Callahan J. W., Khalil M., Philippart M. Sphingomyelinases in human tissues. II. Absence of a specific enzyme from liver and brain of Niemann-Pick disease, type C. Pediatr Res. 1975 Dec;9(12):908–913. doi: 10.1203/00006450-197512000-00009. [DOI] [PubMed] [Google Scholar]
- Callahan J. W., Khalil M. Sphingomyelinases in human tissues. III. Expression of Niemann-Pick disease in cultured skin fibroblasts. Pediatr Res. 1975 Dec;9(12):914–918. doi: 10.1203/00006450-197512000-00010. [DOI] [PubMed] [Google Scholar]
- Callahan J. W., Lassila E. L., Den Tandt W., Philippart M. Alpha-N-acetylgalactosaminidase: isolation, properties and distribution of the human enzyme. Biochem Med. 1973 Jun;7(3):424–431. doi: 10.1016/0006-2944(73)90063-x. [DOI] [PubMed] [Google Scholar]
- Callahan J. W., Shankaran P., Khalil M., Gerrie J. Sphingomyelinases in human tissues. IV. Purification of sphingomyelinase from human placenta and effect of Triton X-100. Can J Biochem. 1978 Sep;56(9):885–891. doi: 10.1139/o78-137. [DOI] [PubMed] [Google Scholar]
- Clarke S. The interaction of Triton X-100 with soluble proteins: possible implications for the transport of proteins across membranes. Biochem Biophys Res Commun. 1977 Nov 7;79(1):46–52. doi: 10.1016/0006-291x(77)90058-4. [DOI] [PubMed] [Google Scholar]
- Clarke S. The size and detergent binding of membrane proteins. J Biol Chem. 1975 Jul 25;250(14):5459–5469. [PubMed] [Google Scholar]
- Cuatrecasas P. Protein purification by affinity chromatography. Derivatizations of agarose and polyacrylamide beads. J Biol Chem. 1970 Jun;245(12):3059–3065. [PubMed] [Google Scholar]
- Harzer K., Anzil A. P., Schuster I. Resolution of tissue sphingomyelinase isoelectric profile in multiple components is extraction-dependent: evidence for a component defect in Niemann-Pick disease type C is spurious. J Neurochem. 1977 Dec;29(6):1155–1157. doi: 10.1111/j.1471-4159.1977.tb06525.x. [DOI] [PubMed] [Google Scholar]
- Helenius A., Simons K. The binding of detergents to lipophilic and hydrophilic proteins. J Biol Chem. 1972 Jun 10;247(11):3656–3661. [PubMed] [Google Scholar]
- KALLER H. [Preparative production of sphingosinephosphorvlcholine]. Biochem Z. 1961;334:451–456. [PubMed] [Google Scholar]
- Lowden J. A., Skomorowski M. A., Henderson F., Kaback M. Automated assay of hexosaminidases in serum. Clin Chem. 1973 Dec;19(12):1345–1349. [PubMed] [Google Scholar]
- Makino S., Reynolds J. A., Tanford C. The binding of deoxycholate and Triton X-100 to proteins. J Biol Chem. 1973 Jul 25;248(14):4926–4932. [PubMed] [Google Scholar]
- Nakajin S., Ishii Y., Shinoda M., Shikita M. Binding of Triton X-100 to purified cytochrome P-450scc and enhancement of the cholesterol side chain cleavage activity. Biochem Biophys Res Commun. 1979 Mar 30;87(2):524–531. doi: 10.1016/0006-291x(79)91827-8. [DOI] [PubMed] [Google Scholar]
- Pentchev P. G., Brady R. O., Gal A. E., Hibbert S. R. The isolation and characterization of sphingomyelinase from human placental tissue. Biochim Biophys Acta. 1977 Aug 24;488(2):312–321. doi: 10.1016/0005-2760(77)90189-8. [DOI] [PubMed] [Google Scholar]
- Peters S. P., Coyle P., Glew R. H. Differentiation of beta-glucocerebrosidase from beta-glucosidase in human tissues using sodium taurocholate. Arch Biochem Biophys. 1976 Aug;175(2):569–582. doi: 10.1016/0003-9861(76)90547-6. [DOI] [PubMed] [Google Scholar]
- Rao B. G., Spence M. W. Sphingomyelinase activity at pH 7.4 in human brain and a comparison to activity at pH 5.0. J Lipid Res. 1976 Sep;17(5):506–515. [PubMed] [Google Scholar]
- Schneider P. B., Kennedy E. P. Sphingomyelinase in normal human spleens and in spleens from subjects with Niemann-Pick disease. J Lipid Res. 1967 May;8(3):202–209. [PubMed] [Google Scholar]
- Thomas G. H., Taylor H. A., Reynolds L. W., Miller C. S. Mucolipidosis 3 (Pseudo-Hurler polydystrophy): multiple lysosomal enzyme abnormalities in serum and cultured fibroblast cells. Pediatr Res. 1973 Sep;7(9):751–756. doi: 10.1203/00006450-197309000-00004. [DOI] [PubMed] [Google Scholar]
- Yamaguchi S., Suzuki K. Purification and characterization of sphingomyelinase from human brain. J Biol Chem. 1977 Jun 10;252(11):3805–3813. [PubMed] [Google Scholar]


