Abstract
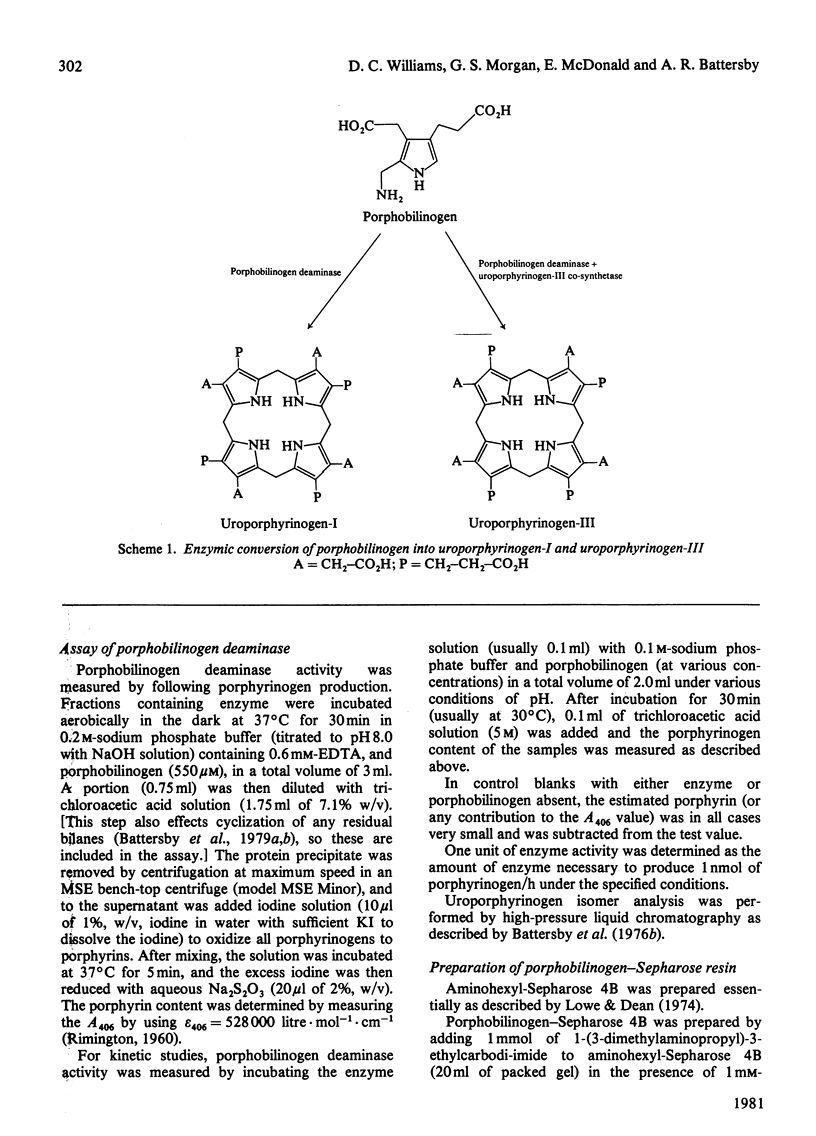
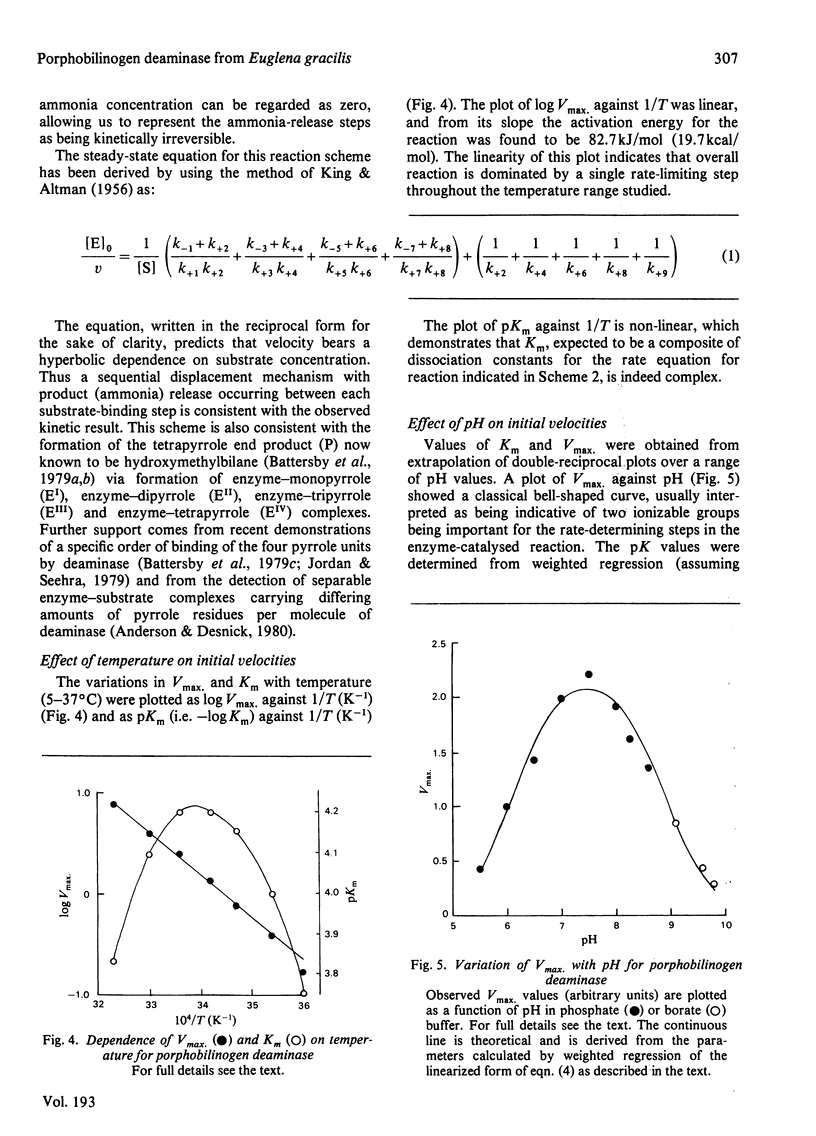
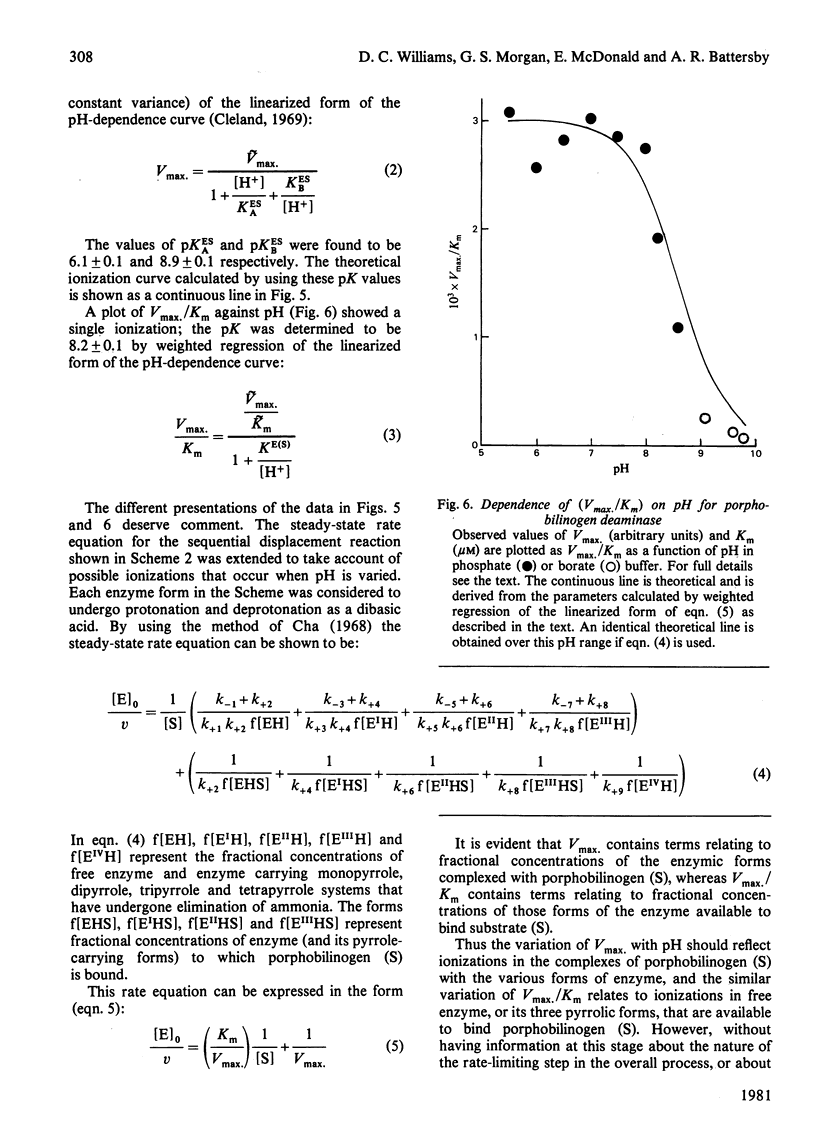
1. Porphobilinogen deaminase [porphobilinogen ammonia-lyase (polymerizing), EC 4.3.1.8] from Euglena gracilis was purified more than 200-fold. 2. The enzyme has a molecular weight of 41 000 +/- 2000, does not contain a chromophoric prosthetic group, and appears not to require metal ions for activity. 3. The stoicheiometry of the overall reaction at pH 7.4 was shown to be: 4 Porphobilinogen leads to uroporphyrinogen-I + 4 NH4+. This stoicheiometry for porphobilinogen and uroporphyrinogen was also observed over a wide range of pH values. 4. Initial-velocity studies showed a hyperbolic dependence of velocity on substrate concentration, demonstrating the existence of a displacement-type mechanism. 5. Vmax. varied with pH as a typical bell-shaped curve, indicating that two ionizable groups with pK values of 6.1 and 8.9 are important for catalysis. A plot of Vmax./Km against pH showed a single ionization (pK 8.2) to influence binding of substrate.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson P. M., Desnick R. J. Purification and properties of uroporphyrinogen I synthase from human erythrocytes. Identification of stable enzyme-substrate intermediates. J Biol Chem. 1980 Mar 10;255(5):1993–1999. [PubMed] [Google Scholar]
- BOGORAD L. Intermediates in the biosynthesis of porphyrins from porphobilinogen. Science. 1955 Jun 17;121(3155):878–879. doi: 10.1126/science.121.3155.878. [DOI] [PubMed] [Google Scholar]
- BOGORAD L. The enzymatic synthesis of porphyrins from porphobilinogen. I. Uroporphyrin I. J Biol Chem. 1958 Aug;233(2):501–509. [PubMed] [Google Scholar]
- BOGORAD L. The enzymatic synthesis of porphyrins from porphobilinogen. II. Uroporphyrin III. J Biol Chem. 1958 Aug;233(2):510–515. [PubMed] [Google Scholar]
- Brocklehurst K., Dixon H. B. The pH-dependence of second-order rate constants of enzyme modification may provide free-reactant pKa values. Biochem J. 1977 Dec 1;167(3):859–862. doi: 10.1042/bj1670859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cha S. A simple method for derivation of rate equations for enzyme-catalyzed reactions under the rapid equilibrium assumption or combined assumptions of equilibrium and steady state. J Biol Chem. 1968 Feb 25;243(4):820–825. [PubMed] [Google Scholar]
- Cleland W. W. The statistical analysis of enzyme kinetic data. Adv Enzymol Relat Areas Mol Biol. 1967;29:1–32. doi: 10.1002/9780470122747.ch1. [DOI] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Davies R. C., Neuberger A. Polypyrroles formed from porphobilinogen and amines by uroporphyrinogen synthetase of Rhodopseudomonas spheroides. Biochem J. 1973 Jul;133(3):471–492. doi: 10.1042/bj1330471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott K. R., Tipton K. F. A kinetic analysis of enzyme systems involving four substrates. Biochem J. 1974 Sep;141(3):789–805. doi: 10.1042/bj1410789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frydman R. B., Feinstein G. Studies on porphobilinogen deaminase and uroporphyrinogen 3 cosynthase from human erythrocytes. Biochim Biophys Acta. 1974 Jun 18;350(2):358–373. doi: 10.1016/0005-2744(74)90510-5. [DOI] [PubMed] [Google Scholar]
- Frydman R. B., Frydman B. Purification and properties of porphobilinogen deaminase from wheat germ. Arch Biochem Biophys. 1970 Jan;136(1):193–202. doi: 10.1016/0003-9861(70)90341-3. [DOI] [PubMed] [Google Scholar]
- Hanes C. S. Studies on plant amylases: The effect of starch concentration upon the velocity of hydrolysis by the amylase of germinated barley. Biochem J. 1932;26(5):1406–1421. doi: 10.1042/bj0261406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higuchi M., Bogorad L. The purification and properties of uroporphyrinogen I synthases and uroporphyrinogen III cosynthase. Interactions between the enzymes. Ann N Y Acad Sci. 1975 Apr 15;244:401–418. doi: 10.1111/j.1749-6632.1975.tb41545.x. [DOI] [PubMed] [Google Scholar]
- Jordan P. M., Seehra J. S. The biosynthesis of uroporphyrinogen III: order of assembly of the four porphobilinogen molecules in the formation of the tetrapyrrole ring. FEBS Lett. 1979 Aug 15;104(2):364–366. doi: 10.1016/0014-5793(79)80853-4. [DOI] [PubMed] [Google Scholar]
- Jordan P. M., Shemin D. Purification and properties of uroporphyrinogen I synthetase from Rhodopseudomonas spheroides. J Biol Chem. 1973 Feb 10;248(3):1019–1024. [PubMed] [Google Scholar]
- Levin E. Y. Enzymatic properties of uroporphyrinogen 3 cosynthetase. Biochemistry. 1971 Dec 7;10(25):4669–4675. doi: 10.1021/bi00801a012. [DOI] [PubMed] [Google Scholar]
- Levin E. Y. Uroporphyrinogen 3 cosynthetase from mouse spleen. Biochemistry. 1968 Nov;7(11):3781–3788. doi: 10.1021/bi00851a001. [DOI] [PubMed] [Google Scholar]
- MAUZERALL D., GRANICK S. The occurrence and determination of delta-amino-levulinic acid and porphobilinogen in urine. J Biol Chem. 1956 Mar;219(1):435–446. [PubMed] [Google Scholar]
- Rimington C. Spectral-absorption coefficients of some porphyrins in the Soret-band region. Biochem J. 1960 Jun;75(3):620–623. doi: 10.1042/bj0750620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sancovich H. A., Battle A. M., Grinstein M. Porphyrin biosynthesis. VI. Separation and purification of porphobilinogen deaminase and uroporphyrinogen isomerase from cow liver. Porphobilinogenase an allosteric enzyme. Biochim Biophys Acta. 1969 Sep 30;191(1):130–143. doi: 10.1016/0005-2744(69)90322-2. [DOI] [PubMed] [Google Scholar]
- Stevens E., Frydman B. Isolation and properties of wheat germ uroporphyrinogen 3 cosynthetase. Biochim Biophys Acta. 1968 Feb 5;151(2):429–437. doi: 10.1016/0005-2744(68)90111-3. [DOI] [PubMed] [Google Scholar]



