Abstract
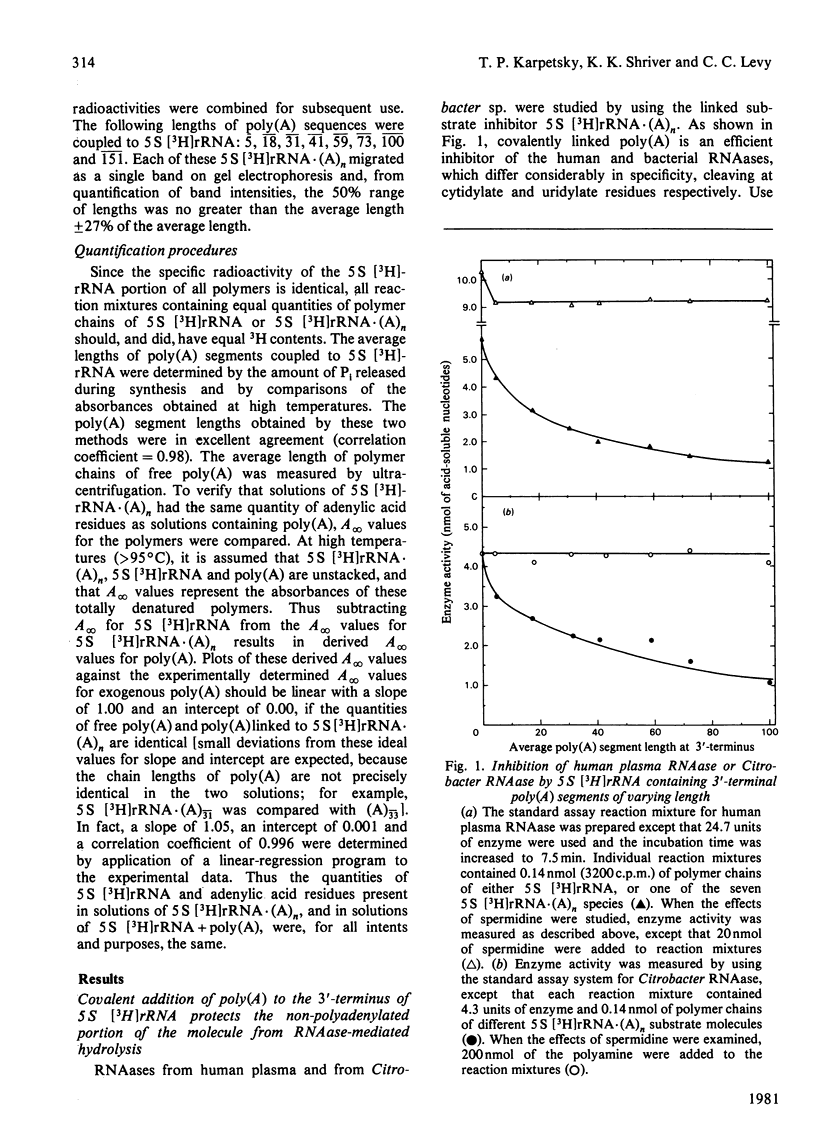
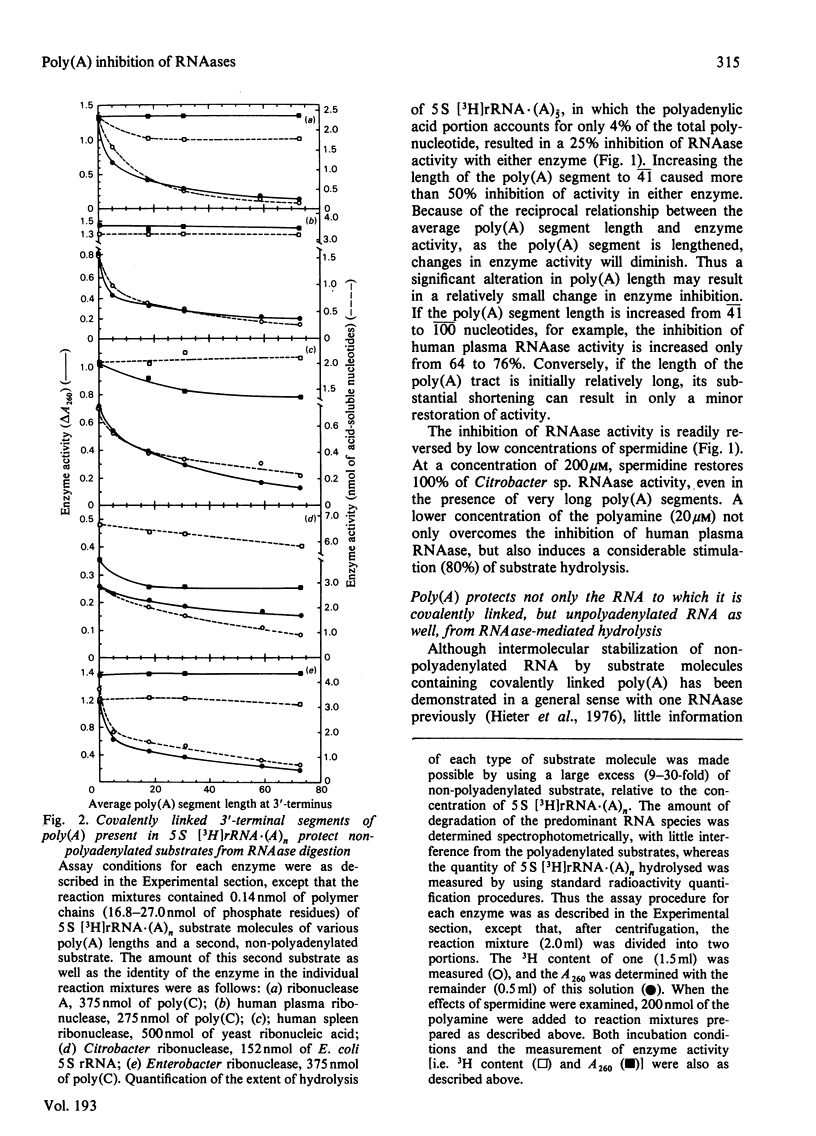
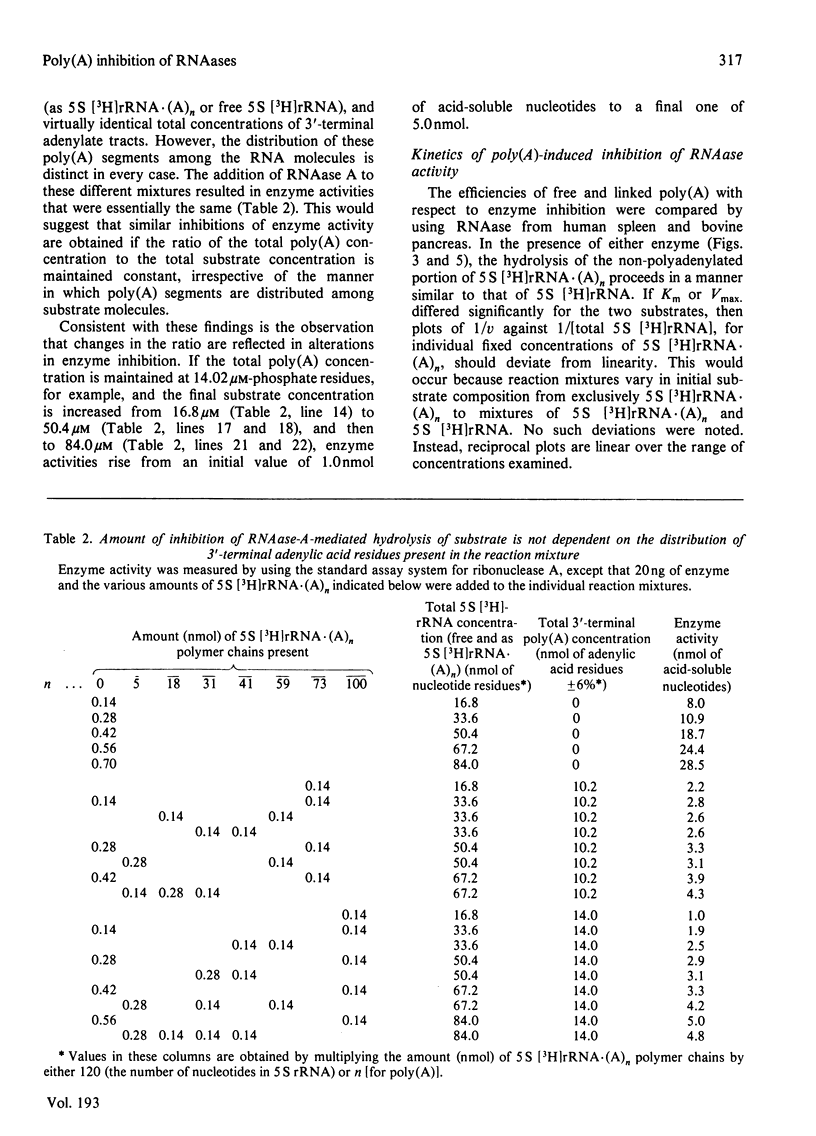
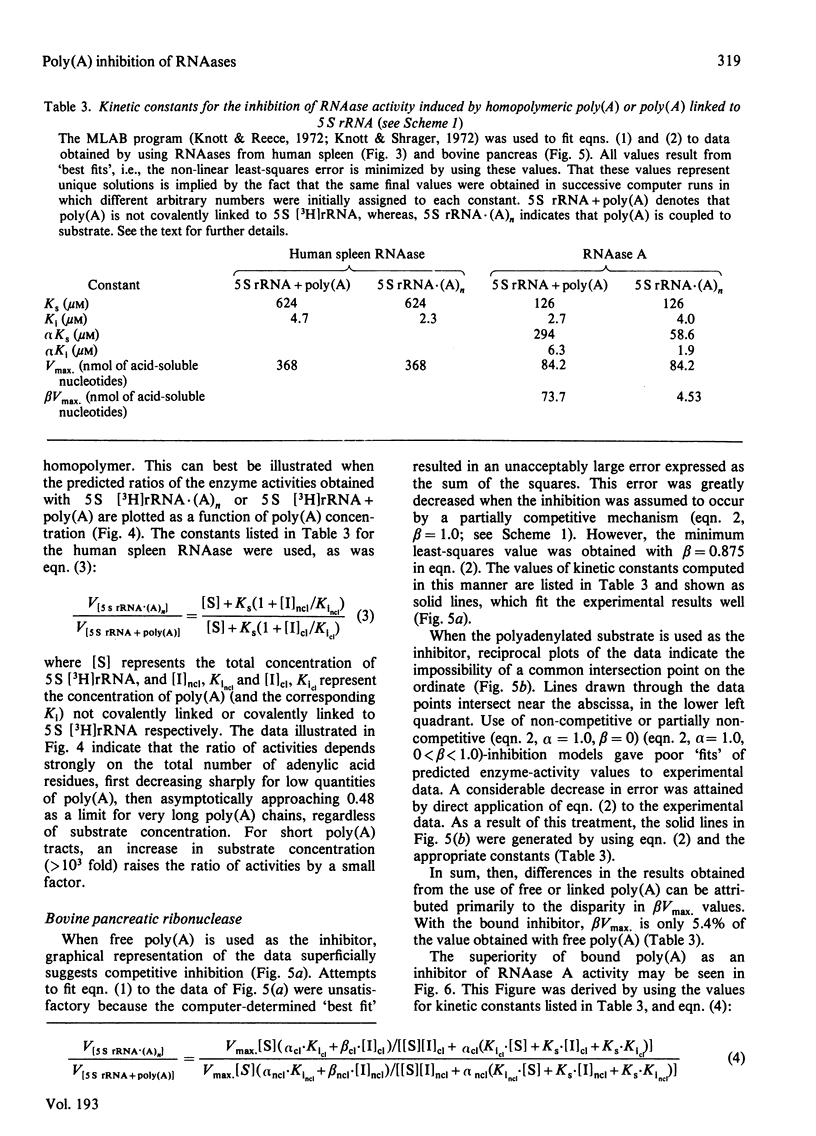
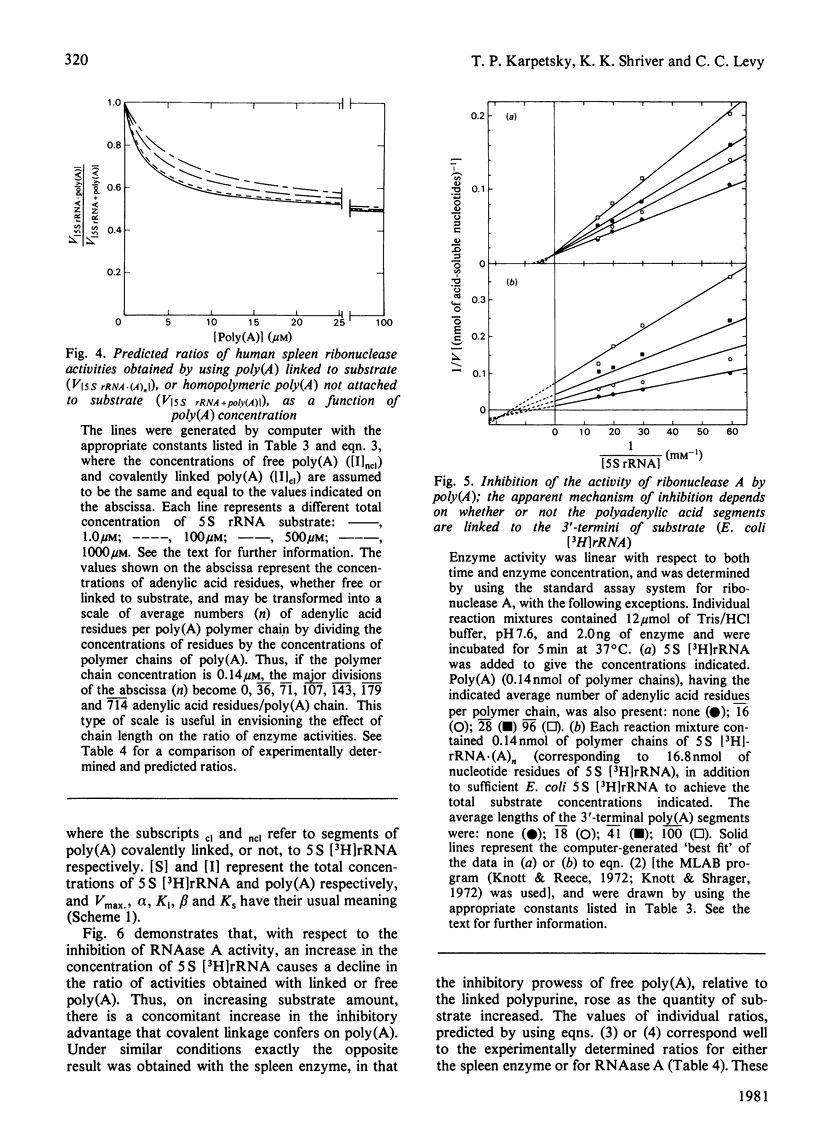
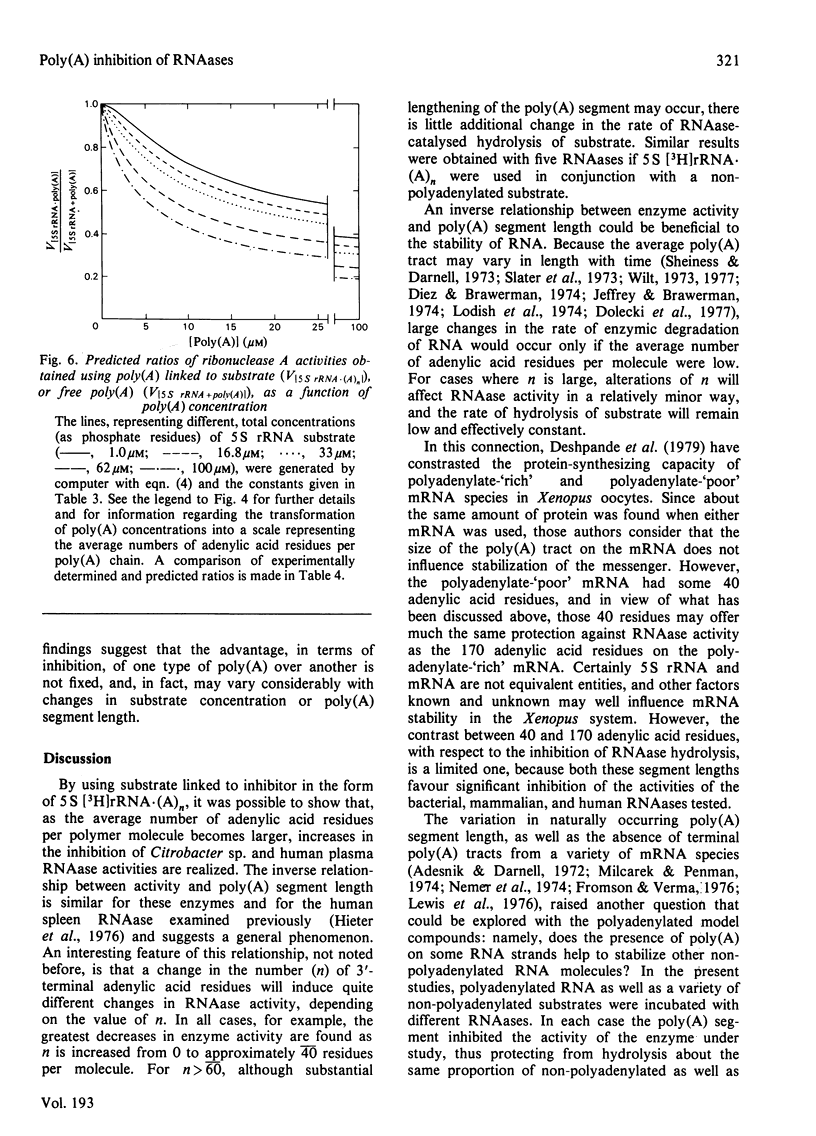
Short lengths (18 residues) of poly(A), covalently linked to the 3'-termini of Escherichia coli 5 S rRNA, induce powerful inhibitions (38-87%) of the activities of RNAases (ribonucleases) from Citrobacter sp., Enterobacter sp., bovine pancreas, human spleen and human plasma. As the polypurine chain length is extended, enzyme activity declines. Furthermore, poly(A) sequences, present only on a small subpopulation of RNA, and accounting for less than 1% of total RNA, serve to protect all RNA, polyadenylated or not, from enzyme-catalysed degradation. The quantity of 3'-terminal adenylic acid residues, relative to the amount of substrate, determines enzyme activity. The exact distribution of a fixed amount of poly(A) residues on the 3'-termini of substrate molecules is unimportant in this respect. Comparison of the efficacies of inhibition of RNAase activity, by using linked poly(A) and similar quantities of free poly(A), revealed that although the free polypurine inhibits RNAase activity, covalent linkage of poly(A) to RNA is more advantageous to the stability of an RNA substrate. However, the ratio of inhibited activities obtained by using linked or free poly(A) may change considerably with alterations in either substrate concentration or polyadenylic acid segment length.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adesnik M., Darnell J. E. Biogenesis and characterization of histone messenger RNA in HeLa cells. J Mol Biol. 1972 Jun 28;67(3):397–406. doi: 10.1016/0022-2836(72)90458-5. [DOI] [PubMed] [Google Scholar]
- Blackburn P., Wilson G., Moore S. Ribonuclease inhibitor from human placenta. Purification and properties. J Biol Chem. 1977 Aug 25;252(16):5904–5910. [PubMed] [Google Scholar]
- CRESTFIELD A. M., SMITH K. C., ALLEN F. W. The preparation and characterization of ribonucleic acids from yeast. J Biol Chem. 1955 Sep;216(1):185–193. [PubMed] [Google Scholar]
- Chou J. Y., Singer M. F. Deoxyadenosine diphosphate as a substrate and inhibitor of polynucleotide phosphorylase of Micrococcus luteus. II. Inhibition of the initiation of adenosine diphosphate polymerization by deoxyadenosine diphosphate. J Biol Chem. 1971 Dec 25;246(24):7497–7504. [PubMed] [Google Scholar]
- Deshpande A. K., Chatterjee B., Roy A. K. Translation and stability of rat liver messenger RNA for alpha 2 mu-globulin in Xenopus oocyte. The role of terminal poly(A). J Biol Chem. 1979 Sep 25;254(18):8937–8942. [PubMed] [Google Scholar]
- Diez J., Brawerman G. Elongation of the polyadenylate segment of messenger RNA in the cytoplasm of mammalian cells. Proc Natl Acad Sci U S A. 1974 Oct;71(10):4091–4095. doi: 10.1073/pnas.71.10.4091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolecki G. J., Duncan R. F., Humphreys T. Complete turnover of poly(A) on maternal mRNA of sea urchin embryos. Cell. 1977 Jun;11(2):339–344. doi: 10.1016/0092-8674(77)90050-2. [DOI] [PubMed] [Google Scholar]
- Fromson D., Verma D. P. Translation of nonpolyadenylylated messenger RNA of sea urchin embryos. Proc Natl Acad Sci U S A. 1976 Jan;73(1):148–151. doi: 10.1073/pnas.73.1.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GAREN A., LEVINTHAL C. A fine-structure genetic and chemical study of the enzyme alkaline phosphatase of E. coli. I. Purification and characterization of alkaline phosphatase. Biochim Biophys Acta. 1960 Mar 11;38:470–483. doi: 10.1016/0006-3002(60)91282-8. [DOI] [PubMed] [Google Scholar]
- Hieter P. A., LeGendre S. M., Levy C. C. Stabilization of an RNA molecule by 3'-terminal poly (A)-induced inhibition of RNase activity. J Biol Chem. 1976 Jun 10;251(11):3287–3293. [PubMed] [Google Scholar]
- Hindley J. Fractionation of 32P-labelled ribonucleic acids on polyacrylamide gels and their characterization by fingerprinting. J Mol Biol. 1967 Nov 28;30(1):125–136. doi: 10.1016/0022-2836(67)90248-3. [DOI] [PubMed] [Google Scholar]
- Iqbal Z. M. The kinetics of pancreatic ribonuclease reaction with alkaline and acidic forms of poly A. Mol Cell Biochem. 1975 Oct 31;9(1):17–20. doi: 10.1007/BF01731729. [DOI] [PubMed] [Google Scholar]
- Jeffery W. R., Brawerman G. Characterization of the steady-state population of messenger RNA and its poly(adenylic acid) segment in mammalian cells. Biochemistry. 1974 Oct 22;13(22):4633–4637. doi: 10.1021/bi00719a026. [DOI] [PubMed] [Google Scholar]
- Karpetsky T. P., Shriver K. K., Levy C. C. Covalent linkage of poly(A) to RNA produces a molecule altered in both structure and susceptibility to ribonuclease-mediated hydrolysis. J Biol Chem. 1980 Apr 10;255(7):2713–2721. [PubMed] [Google Scholar]
- Karpetsky T. P., Shriver K. K., Levy C. C. The effect of polyamines on the poly(adenylic acid)-induced inhibition of ribonuclease activity. Biochem J. 1981 Jan 1;193(1):325–337. doi: 10.1042/bj1930325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy C. C., Goldman P. Residue specificity of a ribonuclease which hydrolyzes polycytidylic acid. J Biol Chem. 1970 Jun;245(12):3257–3262. [PubMed] [Google Scholar]
- Levy C. C., Hieter P. A., LeGendre S. M. Evidence for the direct binding of polyamines to a ribonuclease that hydrolyzes ribonucleic acid at uridylic acid residues. J Biol Chem. 1974 Nov 10;249(21):6762–6769. [PubMed] [Google Scholar]
- Levy C. C., Mitch W. E., Schmukler M. Effect of polyamines on a ribonuclease which hydrolyzes ribonucleic acid at uridylic acid residues. J Biol Chem. 1973 Aug 25;248(16):5712–5719. [PubMed] [Google Scholar]
- Levy C. C., Schmukler M., Frank J. J., Karpetsky T. P., Jewett P. B., Hieter P. A., LeGendre S. M., Dorr R. G. Possible role for poly(A) as an inhibitor of endonuclease activity in eukaryotic cells. Nature. 1975 Jul 24;256(5515):340–342. doi: 10.1038/256340a0. [DOI] [PubMed] [Google Scholar]
- Lewin B. Units of transcription and translation: the relationship between heterogeneous nuclear RNA and messenger RNA. Cell. 1975 Jan;4(1):11–20. doi: 10.1016/0092-8674(75)90128-2. [DOI] [PubMed] [Google Scholar]
- Lewis F. S., Rutman R. J., Avadhani N. G. Messenger ribonucleic acid metabolism in mammalian mitochondria. Discrete poly(adenylic acid) lacking messenger ribonucleic acid species associated with mitochondrial polysomes. Biochemistry. 1976 Jul 27;15(15):3367–3372. doi: 10.1021/bi00660a031. [DOI] [PubMed] [Google Scholar]
- Lodish H. F., Jacobson A., Firtel R., Alton T., Tuchman J. Synthesis of messenger RNA and chromosome structure in the cellular slime mold. Proc Natl Acad Sci U S A. 1974 Dec;71(12):5103–5108. doi: 10.1073/pnas.71.12.5103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milcarek C., Penman S. Membrane-bound polyribosomes in HeLa cells: association of polyadenylic acid with membranes. J Mol Biol. 1974 Oct 25;89(2):327–338. doi: 10.1016/0022-2836(74)90522-1. [DOI] [PubMed] [Google Scholar]
- Nemer M., Graham M., Dubroff L. M. Co-existence of non-histone messenger RNA species lacking and containing polyadenylic acid in sea urchin embryos. J Mol Biol. 1974 Nov 5;89(3):435–454. doi: 10.1016/0022-2836(74)90474-4. [DOI] [PubMed] [Google Scholar]
- Neuwelt E. A., Frank J. J., Levy C. C. Purification of human spleen ribonuclease by immunoabsorption. Similarity of the enzyme with human liver ribonuclease. J Biol Chem. 1976 Sep 25;251(18):5752–5758. [PubMed] [Google Scholar]
- Robins H. I., Raacke I. D. A simplified procedure for the simultaneous isolation of 4S and 5S RNA. Biochem Biophys Res Commun. 1968 Oct 24;33(2):240–246. doi: 10.1016/0006-291x(68)90775-4. [DOI] [PubMed] [Google Scholar]
- SINGER M. F., GUSS J. K. The dependence of reactions catalyzed by polynucleotide phosphorylase on oligonucleotides. J Biol Chem. 1962 Jan;237:182–189. [PubMed] [Google Scholar]
- Schmukler M., Jewett P. B., Levy C. C. The effects of polyamines on a residue-specific human plasma ribonuclease. J Biol Chem. 1975 Mar 25;250(6):2206–2212. [PubMed] [Google Scholar]
- Sheiness D., Darnell J. E. Polyadenylic acid segment in mRNA becomes shorter with age. Nat New Biol. 1973 Feb 28;241(113):265–268. doi: 10.1038/newbio241265a0. [DOI] [PubMed] [Google Scholar]
- Slater I., Gillespie D., Slater D. W. Cytoplasmic adenylylation and processing of maternal RNA. Proc Natl Acad Sci U S A. 1973 Feb;70(2):406–411. doi: 10.1073/pnas.70.2.406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stannard B. S., Felsenfeld G. The conformation of polyriboadenylic acid at low temperature and neutral pH. A single-stranded rodlike structure. Biopolymers. 1975 Feb;14(2):299–307. doi: 10.1002/bip.1975.360140205. [DOI] [PubMed] [Google Scholar]
- Walker E. J., Ralston G. B., Darvey I. G. An allosteric model for ribonuclease. Biochem J. 1975 Jun;147(3):425–433. doi: 10.1042/bj1470425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker E. J., Ralston G. B., Darvey I. G. Further evidence for an allosteric model for ribonuclease. Biochem J. 1976 Feb 1;153(2):329–337. doi: 10.1042/bj1530329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilt F. H. Polyadenylation of maternal RNA of sea urchin eggs after fertilization. Proc Natl Acad Sci U S A. 1973 Aug;70(8):2345–2349. doi: 10.1073/pnas.70.8.2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilt F. H. The dynamics of maternal poly(A)-containing mRNA in fertilized sea urchin eggs. Cell. 1977 Jul;11(3):673–681. doi: 10.1016/0092-8674(77)90084-8. [DOI] [PubMed] [Google Scholar]
- Yu R. S. A simple method for the preparation of 5S RNA from Escherichia coli. Hoppe Seylers Z Physiol Chem. 1973 Feb;354(2):125–130. doi: 10.1515/bchm2.1973.354.1.125. [DOI] [PubMed] [Google Scholar]


