Abstract
Erythrocyte suspensions in buffer made with 2H2O catalyse the exchange of pyruvate protons. This process can be easly observed by spin-echo proton magnetic resonance. The dominant exchange process is shown to be due to the formation of Schiff-base links between pyruvate and amino groups of haemoglobin. Other proteins with free alpha-amino groups also catalyse the exchange. The pH*-dependence of the exchange rate due to hen-egg-white-lysozyme reflects the dissociation of the alpha-amino group.
Full text
PDF
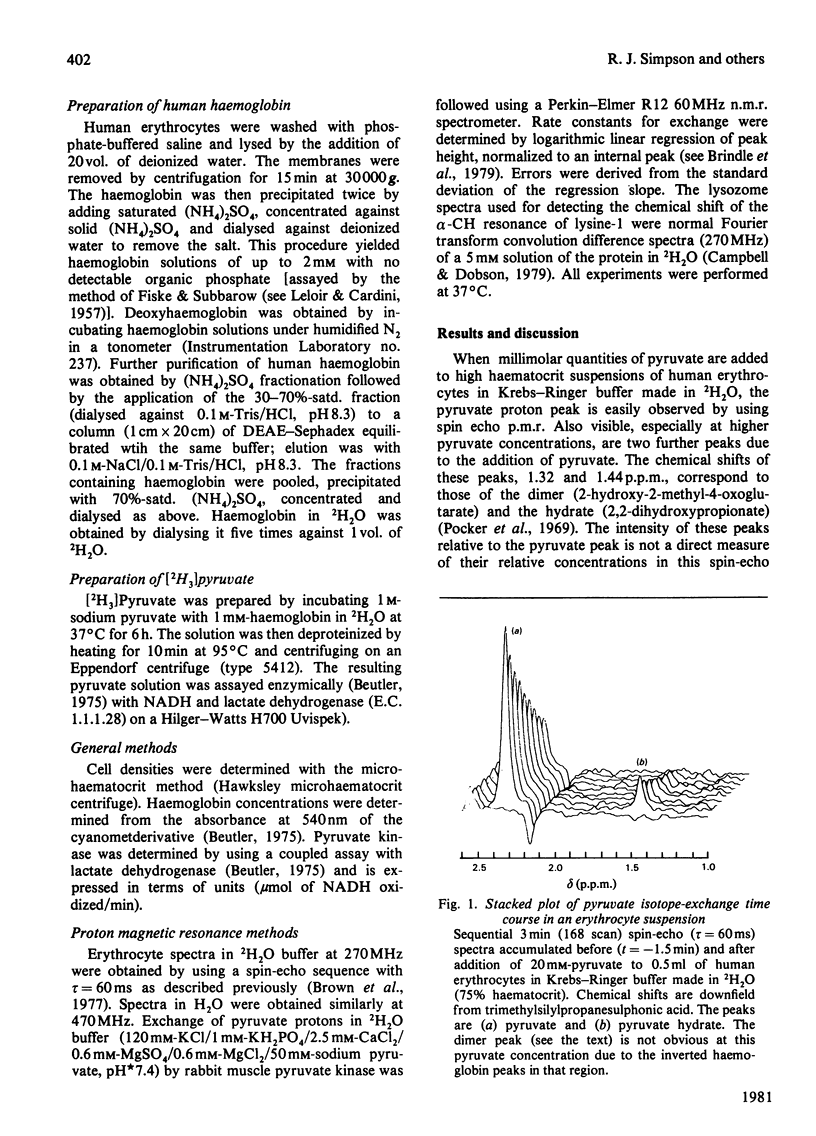
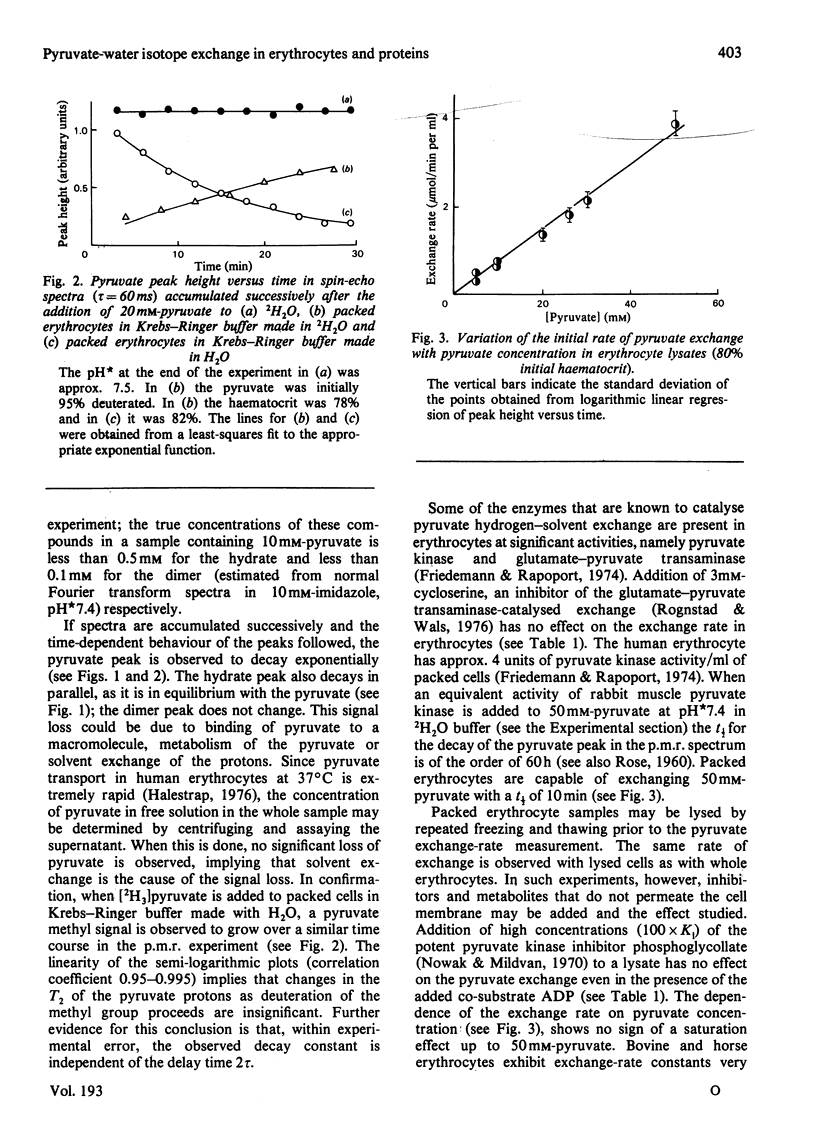
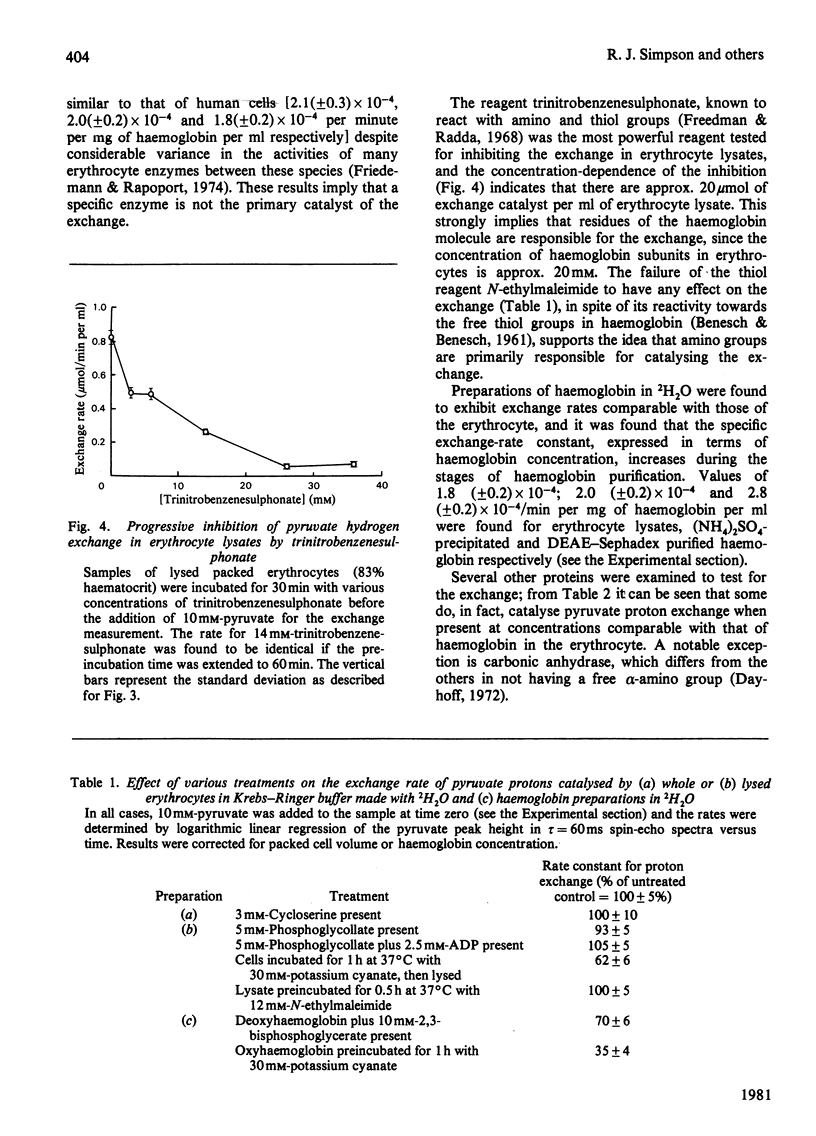


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Acharya A. S., Manning J. M. Reactivity of the amino groups of carbonmonoxyhemoglobin S with glyceraldehyde. J Biol Chem. 1980 Feb 25;255(4):1406–1412. [PubMed] [Google Scholar]
- Arnone A. X-ray diffraction study of binding of 2,3-diphosphoglycerate to human deoxyhaemoglobin. Nature. 1972 May 19;237(5351):146–149. doi: 10.1038/237146a0. [DOI] [PubMed] [Google Scholar]
- Brindle K. M., Brown F. F., Campbell I. D., Grathwohl C., Kuchel P. W. Application of spin-echo nuclear magnetic resonance to whole-cell systems. Membrane transport. Biochem J. 1979 Apr 15;180(1):37–44. doi: 10.1042/bj1800037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown F. F., Campbell I. D., Kuchel P. W., Rabenstein D. C. Human erythrocyte metabolism studies by 1H spin echo NMR. FEBS Lett. 1977 Oct 1;82(1):12–16. doi: 10.1016/0014-5793(77)80875-2. [DOI] [PubMed] [Google Scholar]
- Bunn H. F., Gabbay K. H., Gallop P. M. The glycosylation of hemoglobin: relevance to diabetes mellitus. Science. 1978 Apr 7;200(4337):21–27. doi: 10.1126/science.635569. [DOI] [PubMed] [Google Scholar]
- Campbell I. D., Dobson C. M. The application of high resolution nuclear magnetic resonance to biological systems. Methods Biochem Anal. 1979;25:1–133. doi: 10.1002/9780470110454.ch1. [DOI] [PubMed] [Google Scholar]
- Cerami A., Manning J. M. Potassium cyanate as an inhibitor of the sickling of erythrocytes in vitro. Proc Natl Acad Sci U S A. 1971 Jun;68(6):1180–1183. doi: 10.1073/pnas.68.6.1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman R. B., Radda G. K. The reaction of 2,4,6-trinitrobenzenesulphonic acid with amino acids, Peptides and proteins. Biochem J. 1968 Jul;108(3):383–391. doi: 10.1042/bj1080383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garner M. H., Bogardt R. A., Jr, Gurd F. R. Determination of the pK values for the alpha-amino groups of human hemoglobin. J Biol Chem. 1975 Jun 25;250(12):4398–4404. [PubMed] [Google Scholar]
- Halestrap A. P. Transport of pyruvate nad lactate into human erythrocytes. Evidence for the involvement of the chloride carrier and a chloride-independent carrier. Biochem J. 1976 May 15;156(2):193–207. doi: 10.1042/bj1560193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz J., Rognstad R. Futile cycles in the metabolism of glucose. Curr Top Cell Regul. 1976;10:237–289. doi: 10.1016/b978-0-12-152810-2.50013-9. [DOI] [PubMed] [Google Scholar]
- Kosicki G. W. Oxaloacetate decarboxylase from cod. Catalysis of hydrogen-deuterium exchange in pyruvate. Biochemistry. 1968 Dec;7(12):4310–4314. doi: 10.1021/bi00852a024. [DOI] [PubMed] [Google Scholar]
- Meloche H. P., Monti C. T., Cleland W. W. Magnitude of the equilibrium isotope effect on carbon-tritium bond synthesis. Biochim Biophys Acta. 1977 Feb 9;480(2):517–519. doi: 10.1016/0005-2744(77)90047-x. [DOI] [PubMed] [Google Scholar]
- Nowak T., Mildvan A. S. Stereoselective interactions of phosphoenolpyruvate analogues with phosphoenolpyruvate-utilizing enzymes. J Biol Chem. 1970 Nov 25;245(22):6057–6064. [PubMed] [Google Scholar]
- ROSE I. A. Studies on the enolization of pyruvate by pyruvate kinase. J Biol Chem. 1960 Apr;235:1170–1177. [PubMed] [Google Scholar]
- Rognstad R. Pyruvate cycling involving possible oxaloacetate decarboxylase activity. Biochim Biophys Acta. 1979 Aug 22;586(2):242–249. doi: 10.1016/0304-4165(79)90096-5. [DOI] [PubMed] [Google Scholar]
- Rognstad R., Wals P. The metabolism of l-[3-3h]lactate by isolated hamster liver cells. Biochim Biophys Acta. 1976 Jun 23;437(1):16–21. doi: 10.1016/0304-4165(76)90343-3. [DOI] [PubMed] [Google Scholar]
- SHINODA T. THE APPARENT HIGH REACTIVITY OF SOME AMINO GROUPS OF NATIVE HEMOGLOBIN. Biochim Biophys Acta. 1965 Feb 15;97:382–384. doi: 10.1016/0304-4165(65)90117-0. [DOI] [PubMed] [Google Scholar]


