Abstract
Primary analysis of the open-label, single-arm, phase II RENMIN-215 trial (primary data cutoff date: July 10, 2023) showed promising efficacy and tolerable safety with tislelizumab plus fruquintinib and fecal microbiota transplantation (FMT) in patients with refractory microsatellite stable (MSS) metastatic colorectal cancer (mCRC). Here, we reported updated survival and safety results with a median follow-up of 34.0 months (data cut-off May 20, 2024), as well as patient-reported outcomes and laboratory analysis. Twenty patients with MSS mCRC resistant or refractory to at least second-line therapy were enrolled and received tislelizumab plus fruquintinib and FMT. The primary endpoint was progression-free survival. Secondary endpoints included overall survival (OS), objective response rate (ORR), disease control rate, safety, health-related quality of life questionnaire and exploratory laboratory tests. In addition, 94 mCRC patients who received third-line or above immunotherapy in real world were screened for propensity score matching (PSM) analysis to compare efficacy. Our results showed that the median OS was 13.7 months (95% CI, 9.3-17.7), and the ORR was 20.0% (95% CI, 5.7-43.7). After PSM, the median OS benefit of the study regimen remained statistically significant (HR = 0.26; 95% CI, 0.07-0.95; P = 0.042). Patients with primary tumor surgery had better clinical outcomes. No new safety concerns were detected. Seven (35.0%) patients had one or more grade 3 treatment-related adverse events. The majority of patients had improved or stable global health status (GHS). Median time to deterioration for GHS was 7.7 months. Peripheral blood lymphocyte analysis showed that increased gamma-delta 2 T cells were positively associated with improved response and survival. To conclude, the updated results provide further evidence of sustained antitumor activity of tislelizumab plus fruquintinib and FMT in heavily pretreated MSS mCRC patients with a consistent safety profile.
Keywords: Colorectal cancer, microsatellite stable, tislelizumab, fruquintinib, fecal microbiota transplantation
Introduction
Colorectal cancer (CRC) ranks in the top three most common cancers and in the top two in terms of mortality globally [1]. Approximately 25% of patients are diagnosed with metastatic CRC (mCRC) at their initial diagnosis and almost 50% of localized CRC patients will develop mCRC, which is a heavy burden threatening human health [2].
Patients with microsatellite-stable (MSS)/proficient mismatch repair (pMMR) mCRC showed low response to immunotherapy. Indeed, only regorafenib, fruquintinib, TAS-102 plus bevacizumab or chemotherapy reintroduction are currently available options for MSS/pMMR mCRC in third-line setting [3-5]. However, the median progression free survival (mPFS) is only about 2-3 months [6-8]. More reasonable combinations of later-line treatment strategies may be clinically meaningful. Recently, some data indicated a synergistic effect of immune checkpoint inhibitor (ICI) combined with anti-angiogenetic tyrosine kinase inhibitor (TKI) in MSS mCRC [9,10].
Fecal microbiota transplantation (FMT) based on gut microbiome perturbations, as a promising immunomodulator, has been increasing reported to improve the antitumor immunity and promote cancer immunotherapy efficacy. Two phase I trials showed that responder-derived FMT effectively reversed the immuno-resistance and obtained rapid and durable clinical benefit in metastatic melanoma [11,12].
The phase II RENMIN-215 trial assessed tislelizumab plus fruquintinib and FMT in patients with MSS mCRC in third-line or above settings. Primary end point results of the study have been published [13]. Here, we provide updated overall survival and safety data with a 34-month follow-up, as well as patient-reported outcomes (PROs) and exploratory laboratory analysis.
Methods
Study design and participants
This open-label, single-arm, phase II trial was conducted at Renmin Hospital of Wuhan University. The study design was previously published and the protocol was available online [13]. Briefly, eligible patients were aged 18 years or older, had progressive and/or metastatic colorectal adenocarcinoma histologically confirmed to be MSS or pMMR phenotype, had received at least second-line of previous systemic therapy for metastatic disease, had at least one measurable/evaluable lesion by Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1, had an Eastern Cooperative Oncology Group performance status (ECOG PS) of 0 to 2, had adequate organ function, and had life expectancy of 3 months or longer.
The study was approved by an independent institutional ethics committee at Renmin Hospital of Wuhan University (WDRY2021-K049), and registered on Chictr.org.cn (ChiCTR2100046768). The study was conducted in compliance with the Declaration of Helsinki, the Guidelines for Good Clinical Practice, and applicable local laws and regulations. All patients provided written informed consent before participation.
Treatment
Patients received custom-made fecal microbiota capsule (10 capsules, orally taking, 3 times per day on day 1 to day 3) and tislelizumab (a PD-1 antibody, 200 mg, intravenous infusion) and fruquintinib (5 mg, oral administration, once per day, 2 weeks on/1 week off) on a 3-week cycle. Before receiving FMT, patients underwent an initial “native microbiota depletion” with ingested antibiotics. Then, oral polyethylene glycol (PEG)-based diarrhea solution was used for intestinal cleansing preparation and flora colonization. Study treatment continued until disease progression, unacceptable toxicities, patient refusal, investigator’s decision to withdraw, or completion of 18 cycles of therapy.
Outcomes
In this report, we described the updated median overall survival (mOS) and treatment-related adverse events (TRAEs). To compare the efficacy of the study regimen with that of the real-world situation, we screened a cohort of similar mCRC patients with MSS phenotype who received at least third-line immunotherapy-based treatment at our institution from May 2021 to May 2023, as an exploratory post-hoc analysis. Propensity score matching (PSM) was performed based on the following baseline variables to balance potential confounding factors: sex, age, ECOG PS, primary tumor location, liver metastasis, RAS/BRAF mutation, prior surgery of primary tumor, prior radiotherapy, and prior anti-VEGF(R) agents. A 1:2 matching ratio was used with a caliper of 0.02.
In addition, PROs and exploratory peripheral blood immune cells analysis were conducted. Two proven and frequently used health-related quality of life (HRQoL) questionnaires were administered by well-trained investigators [14-16]. Immunophenotype of peripheral blood T lymphocytes was detected by flow cytometry on FACSCalibur and FACS CANTO II in Jinan University Joint Laboratory of Immunology and Microecology. The entire γδ T cell subsets were characterized using anti-CD3 peridinin chlorophyll protein (PerCp)-, anti-Vδ1 fluorescein isothiocyanate (FITC)-, anti-Vδ2 phycoerythrin (PE)-, anti-CD45RA PE-Cy7- and anti-CD27 allophycocyanin (APC)-conjugated monoclonal antibodies (mAbs) (BD Biosciences, Mountain View, CA, USA).
Statistical analysis
Statistical methods for the efficacy and safety analyses have been reported previously [13]. Briefly, the study had 80% power to detect an increase in the mPFS from 3.7 months to 7.6 months with an one-sided type I error of 0.025 in favor of the combination of ICI plus TKI and FMT. The Kaplan-Meier method was used to estimate mOS and mPFS. TRAEs (assessed in patients who received any amount of study drug) were graded using the Common Terminology Criteria for Adverse Events version 5.0. The log-rank test was used to compare Kaplan-Meier curves, and Cox proportional hazards model was adopted to determine the hazard ratio (HR) and its associated bilateral 95% confidence interval (CI) and P value. The objective response rate (ORR), disease control rate (DCR) and 95% CI were calculated using the Clopper-Pearson method. The demographic characteristics and safety data were summarized descriptively.
The HRQoL full analysis population comprised all enrolled patients who received at least one dose of study treatment and completed at least one post-baseline questionnaire assessment. For each European Organization for Research and Treatment of Cancer Quality of Life Questionnaire Core 30 (EORTC QLQ-C30) scale or item, a linear transformation was applied to standardize the raw scores between 0 and 100. For EuroQoL 5 Dimensions 3 Levels (EQ-5D-3L) health utility score, the health status level of each dimension was encoded by numbers 1, 2 and 3, and converted into utility values according to local Chinese scoring algorithms [17]. Least squares mean (LSM) score changes from baseline to post-therapy at different point were assessed with a constrained longitudinal data analysis model, with item scores as the response variable and treatment by study visit as a covariate. LSM score change, 95% CIs, and nominal two-sided P values were calculated. Post-baseline scores of each EORTC QLQ-C30 scale were classified as “improved”, “stable”, or “deteriorated” according to a change in score of 10 points or greater. Time to deterioration was defined as the time from baseline to the first onset of a 10-point or greater decrease or increase for functional or symptom scales. Time to deterioration was estimated with the Kaplan-Meier method.
All laboratory specimens were divided into relatively effective group (responders; PFS ≥ 6 months) and inferior group (non-responders; PFS < 6 months) according to the clinical response of patients. Receiver operation characteristics (ROCs) and areas under the curves (AUCs) were analyzed. The association of lymphocyte subsets with tumor response between groups was compared using exact Mann-Whitney test. All statistical analyses were performed using SPSS v26.0.
Results
Patients
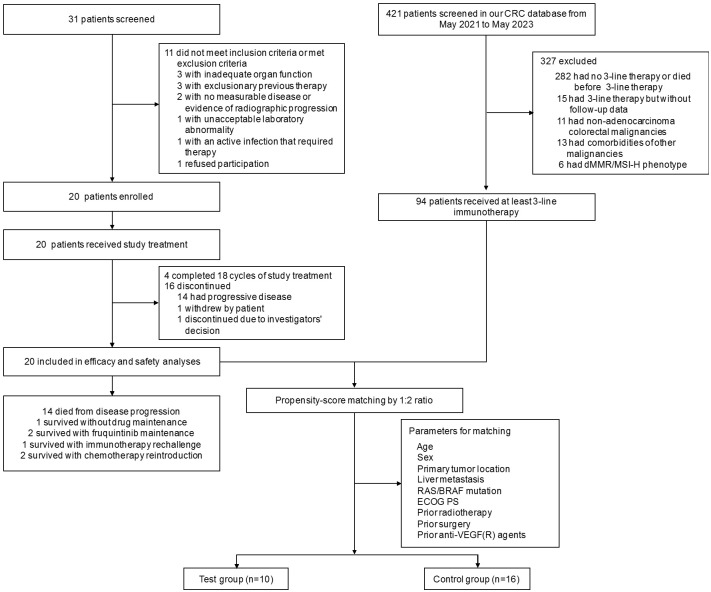
The flowchart of the study is shown in Figure 1. Baseline characteristics have been previously described (Table 1). From May 2021 to January 2022, 20 patients were enrolled. All patients received 3 to 18 cycles of study treatment. From study initiation to May 2023, a total of 286 questionnaires and 144 peripheral blood samples were collected.
Figure 1.
Study design and trial profile. CRC, colorectal cancer; dMMR, mismatch repair deficiency; ECOG PS, Eastern Cooperative Oncology Group performance status; MSI-H, high microsatellite instability.
Table 1.
Patient demographics and baseline characteristics (n = 20)
| Characteristics | Patients, No. (%) |
|---|---|
| Age, median (IQR), years | 62 (51-69) |
| > 60 | 11 (55.0) |
| ≤ 60 | 9 (45.0) |
| Sex | |
| Male | 18 (90.0) |
| Female | 2 (10.0) |
| ECOG PS | |
| 0-1 | 16 (80.0) |
| 2 | 4 (20.0) |
| Primary tumor site | |
| Left-sided | 14 (70.0) |
| Right-sided | 6 (30.0) |
| Type of metastasis | |
| With liver metastasis | 14 (70.0) |
| With lung metastasis | 11 (55.0) |
| Previous treatment | |
| Surgery | 16 (80.0) |
| Radiotherapy | 9 (45.0) |
| Chemotherapy | 20 (100.0) |
| Anti-VEGF(R) therapy | 14 (70.0) |
| Anti-EGFR therapy | 4 (20.0) |
| Lines of prior treatment | |
| 2 | 9 (45.0) |
| ≥ 3 | 11 (55.0) |
| RAS/BRAF gene status | |
| WT | 9 (45.0) |
| MT | 7 (35.0) |
| Unknown | 4 (20.0) |
| MSI status | |
| MSS/pMMR | 20 (100.0) |
Abbreviations: ECOG PS, Eastern Cooperative Oncology Group performance status; EGFR, epidermal growth factor receptor; IQR, interquartile range; MSI, microsatellite instability; MSS, microsatellite stable; MT, mutated type; pMMR, mismatch repair proficient; VEGF(R), vascular endothelial growth factor (receptor); WT, wild type.
Updated efficacy
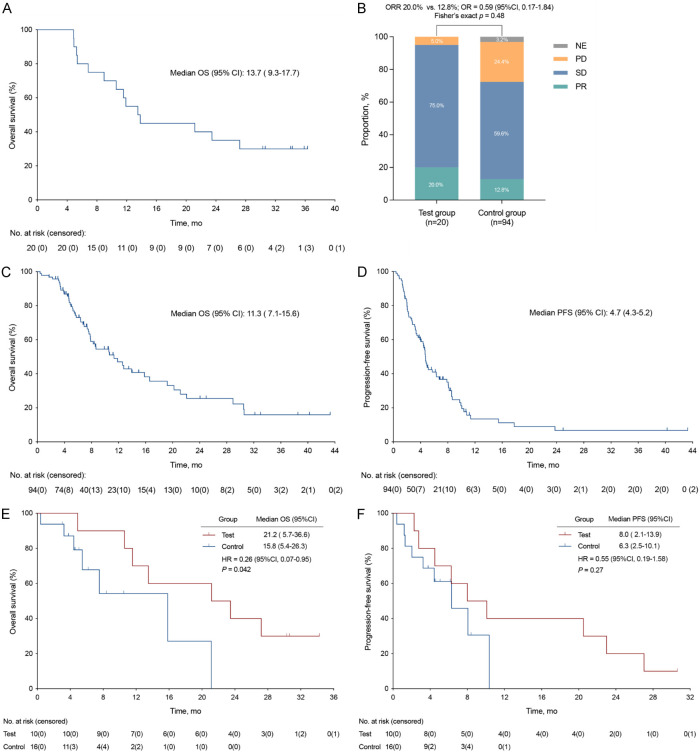
As of data cutoff on May 20, 2024, with a median follow-up of 34.0 months (95% CI, 29.7-38.4), the mOS was 13.7 months (95% CI, 9.3-7.7), the 1-year survival rate was 55.0% (95% CI, 32.1-76.2) and the 2-year survival rate was 35.0% (95% CI, 16.3-59.1) (Figure 2A). In the intention-to-treat (ITT) population, partial response was observed in 4 (20.0%) patients. The ORR was 20.0% (95% CI, 5.7-43.7), and the DCR was 95.0% (95% CI, 75.1-99.9) (Figure 2B).
Figure 2.
Kaplan-Meier curve and tumor response. A. Overall survival of study patients. B. Tumor response in test and control group patients. C, D. Overall survival and progression-free survival of control patients in real world. E, F. Overall survival and progression-free survival of matched patients after propensity score matching. CI, confidence interval; HR, hazard ratio; NE, not evaluable; OR, odds ratio; ORR, objective response rate; OS, overall survival; PD, progressive disease; PFS, progression-free survival; PR, partial response; SD, stable disease.
A total of 94 MSS mCRC patients were screened for PSM analysis (Table S1). The ORR was 12.8% (95% CI, 7.1-21.6). An increase in ORR (20.0% vs 12.8%, OR = 0.59; 95% CI, 0.17-1.84; P = 0.48) was observed here (Figure 2B). The mOS and mPFS of the real-world cohort was 11.3 months (95% CI, 7.1-15.6) and 4.6 months (95% CI, 4.3-5.2), respectively (Figure 2C, 2D). After PSM, 16 of 94 patients (control group) were matched with 10 of 20 patients (test group). Significantly improved mOS (21.2 months vs 15.8 months, HR = 0.26; 95% CI, 0.07-0.95; P = 0.042) was found in the test group (Figure 2E). The study treatment also showed a statistically non-significant increase in mPFS (8.0 months vs 6.3 months, HR = 0.55; 95% CI, 0.19-1.58; P = 0.27) (Figure 2F).
Multivariate analysis indicated that primary tumor surgical resection was an independent prognostic factor for OS (HR = 0.16; 95% CI, 0.029-0.90; P = 0.037) (Table S2). Patients who had primary tumor surgery exhibited a mOS of 21.2 months (95% CI, 7.8-34.6), while that for patients without primary tumor surgery was 6.8 months (95% CI, 4.3-9.3) (Figure S1C). Significant mOS differences were also observed in patients with better ECOG PS score (22.3 months vs 7.2 months, HR = 0.23; 95% CI, 0.036-1.52; P = 0.0053), and in patients with or without liver metastasis (10.6 months vs 27.2 months, HR = 2.97; 95% CI, 1.04-8.52; P = 0.037), but they were not independent prognostic factors for OS here (Figure S1A, S1B and Table S2). Besides, we observed directional improvements in mOS in patients with prior RT (Figure S1E) and patients without prior anti-VEGF(R) or regorafenib therapy (Figure S1D, S1F).
Updated safety
As of May 20, 2024, investigator-reported drug-related TRAEs had occurred in 100.0% of patients and the majority were grade 1 or 2 (Table 2). Most common TRAEs were generally decreased appetite, albuminuria and fecal occult blood. Grade 3/4 TRAEs were observed in 7 patients (35.0%), mainly including albuminuria, urine or fecal occult blood, hypertension, hyperglycemia, liver dysfunction, hand-foot skin reaction and hypothyroidism.
Table 2.
TRAEs
| Patients (n = 20) | ||
|
| ||
| Any Grade TRAEs, No. (%) | 20 (100.0) | |
| Grade ≥ 3 | 7 (35.0) | |
| Treatment suspension | 4 (20.0) | |
| Dose reduction | 3 (15.0) | |
| Death | 0 | |
|
| ||
| Incidence rate | Any Grade | Grade ≥ 3 |
|
| ||
| HFSR | 5 (25.0) | 1 (5.0) |
| Rash | 4 (20.0) | 0 |
| Pruritus | 3 (15.0) | 0 |
| Diarrhea | 6 (30.0) | 0 |
| Bloating | 4 (20.0) | 0 |
| Constipation | 2 (10.0) | 0 |
| Ileus | 3 (15.0) | 0 |
| Hypothyroidism | 8 (40.0) | 1 (5.0) |
| Hyperthyroidism | 1 (5.0) | 0 |
| Liver dysfunction | 5 (25.0) | 1 (5.0) |
| Hypertension | 7 (35.0) | 1 (5.0) |
| Hyperglycemia | 4 (20.0) | 1 (5.0) |
| Weight loss | 5 (25.0) | 0 |
| Fatigue | 11 (55.0) | 0 |
| Decreased appetite | 13 (65.0) | 0 |
| Hoarseness | 9 (45.0) | 0 |
| Dry mouth | 5 (25.0) | 0 |
| Mucositis oral | 3 (15.0) | 0 |
| Periodontal disease | 3 (15.0) | 0 |
| Epistaxis | 8 (40.0) | 0 |
| FOBT positive | 12 (60.0) | 2 (10.0) |
| Albuminuria | 13 (65.0) | 3 (15.0) |
| Urine occult blood | 7 (35.0) | 2 (10.0) |
| Fever | 3 (15.0) | 0 |
| Leukopenia | 3 (15.0) | 0 |
| Thrombocytopenia | 2 (10.0) | 0 |
| Hypoalbuminemia | 2 (10.0) | 0 |
| Anemia | 3 (15.0) | 0 |
| Hypokalemia | 3 (15.0) | 0 |
| Hyponatremia | 1 (5.0) | 0 |
| Headache | 4 (20.0) | 0 |
| Musculoskeletal pain | 2 (10.0) | 0 |
| Visual fatigue | 1 (5.0) | 0 |
| Insomnia | 3 (15.0) | 0 |
| Hypomnesis | 1 (5.0) | 0 |
| Nausea | 2 (10.0) | 0 |
| Vomiting | 1 (5.0) | 0 |
| Cough | 2 (10.0) | 0 |
Abbreviations: TRAEs, treatment-related adverse events; HFSR, hand-foot skin reaction; FOBT, fecal occult blood test.
Immune related AEs (irAEs) of any grade occurred in 9 (45.0%) patients, and most irAEs were grade 1-2. Two patients (10.0%) experienced grade 3 irAEs (one with grade 3 elevated aminotransferases, and one with grade 3 hypertension and hyperglycemia). Cumulative drug-related TRAEs associated with dose reduction, drug discontinuation, or dose interruption were reported in 3 (15.0%), 2 (10.0%), and 2 (10.0%) patients, respectively. Drug-related TRAEs associated with death were not reported.
By May 2024, 4 (20.0%) patients completed 18 cycles of study therapy as scheduled, of which two were continuing fruquintinib maintenance and one was in drug-free survival. Fourteen (70.0%) patients developed progression and died, including one who had completed all study treatments. The remaining three living patients are currently receiving chemotherapy reintroduction and/or immunotherapy rechallenge, respectively (Figure 1).
Patient-reported outcomes
EORTC QLQ-C30
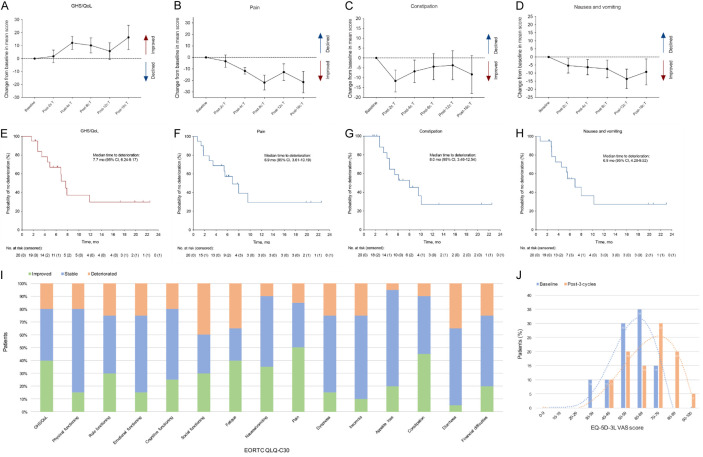
Compared with baseline, a significant improvement was showed in global health status/quality of life (GHS/QoL) (LSM change +12.13; 95% CI, 2.09-22.18; P = 0.019) and pain (LSM change -11.76; 95% CI, -22.80 to -0.72; P = 0.037) scores after 4 cycles of treatment (Figure 3A, 3B). Significant improvements were also observed in constipation (LSM change -11.67; 95% CI, -22.71 to -0.62; P = 0.039 at post 2 cycles of treatment) and nausea and vomiting (LSM change -13.59; 95% CI, -25.68 to -1.50; P = 0.028 at post 12 cycles of treatment) (Figure 3C, 3D). Patients’ scores in most other HRQoL domains were also generally stable or improved, except for diarrhoea (Figure S2 and Table S3).
Figure 3.
HRQoL questionnaire assessment. A-D. LSM change of GHS/QoL, pain, constipation and nausea/vomiting item scores in EORTC QLQ-C30. Error bars indicate 95% CIs around the mean. E-H. Kaplan-Meier curves of time to deterioration in EORTC QLQ-C30 item scores. Time to deterioration was defined as first onset of a 10-point or greater change in relevant items score from baseline with confirmation. I. Proportion of patients with improved, stable, and deteriorated EORTC QLQ-C30 scale and item scores after 3 cycles of study treatment. J. Changes of patients’ proportion in EQ-5D-3L VAS scores from baseline to post 3 cycles of study treatment. CI, confidence interval; HRQoL, health-related quality of life; LSM, least-squares mean; GHS, global health status; EORTC QLQ-C30, European Organization for the Research and Treatment of Cancer Quality of Life Questionnaire Core 30; EQ-5D-3L, EuroQoL 5 Dimensions 3 Levels; VAS, visual analogue scale.
After 3 cycles of treatment (week 9 to week 12), more than 60% of patients had improved or stable scores in GHS/QoL and most functioning and symptom domains (Figure 3I). GHS/QoL, pain and constipation were domains with improved percentage more than 30%. Specifically, median time to deterioration was 7.7 months (95% CI, 6.24-9.17) in GHS/QoL (Figure 3E). Median time to deterioration was 6.9 months for pain (95% CI, 3.61-10.19; 6 events) and nausea/vomiting (95% CI, 4.28-9.52; 2 events) and 8.0 months for constipation (95% CI, 3.46-12.54; 2 events) (Figure 3F-H). Median deterioration time of other items were reported in Figure S3.
EQ-5D-3L
The EQ-5D-3L visual analogue scale (VAS) scores showed small and sustained increments in the patient’s health status during the course of study treatment (Table S4). It is, and in particular, the VAS score achieved a statistically significant improvement (LSM change +10.09; 95% CI, 1.22-18.96; P = 0.026) after 8 cycles of therapy. The patients’ VAS score presented a non-normal distribution, so median and interquartile range (IQR) were used to describe it. Data showed that after 3 cycles of treatment, the median VAS score was 70 (IQR, 57.25-76.25), and half of the patients’ scores were in 70-89 range (Figure 3J). In comparison, the median VAS score at baseline was 59 (IQR, 50-65.25), and more than 60% of the patients scored in the 50-69 range.
Similarly, in health utility values of EQ-5D-3L, a steady increase has also been witnessed. Statistically significant changes were observed after 8 and 12 cycles of treatment (LSM change +0.10; 95% CI, 0.02-0.19; P = 0.017 and LSM change +0.10; 95% CI, 0.00-0.19; P = 0.047) (Table S4).
Flow cytometry (FCM) analysis
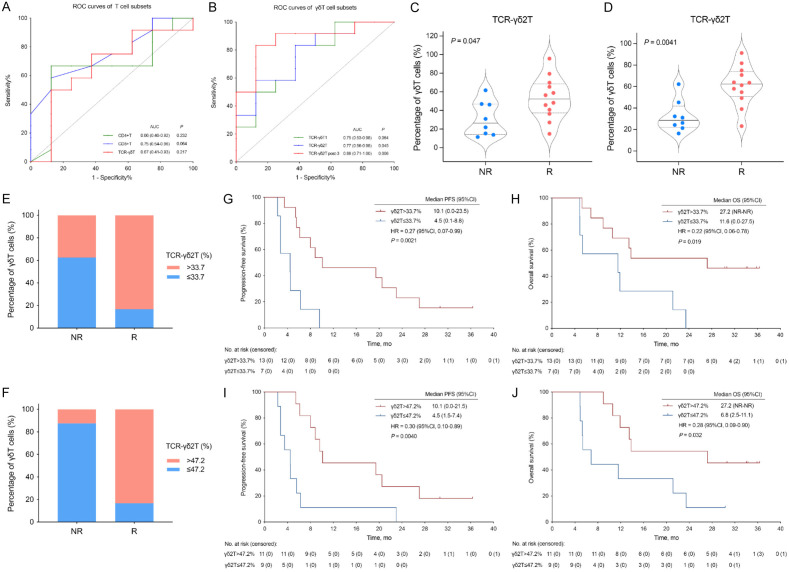
T cell subsets were measured by FCM. The ROC curves were plotted to find the optimal index and cutoff value between responders and non-responders, and the results showed that the AUC of γδ2 T cell at baseline and post-3 courses of treatment reached 0.77 (95% CI, 0.56-0.98; P = 0.045) and 0.88 (95% CI, 0.71-1.00; P = 0.006), respectively (Figure 4A, 4B).
Figure 4.
Changes of peripheral blood T lymphocytes during the study treatment were related to the therapeutic response in responders. A, B. ROC curves of T cell and γδT cell subsets. C, D. Percentage of γδ2 T cells in total peripheral blood T cells in responders and non-responders before and after 3 cycles of study treatment. E, F. Percentage of γδ2 T cells above and below the threshold in responders and non-responders. G-J. Kaplan-Meier survival curves in two groups of patients with γδ2 T cells above and below the threshold at baseline and after 3 cycles of treatment. AUC, area under the curve; CI, confidence interval; HR, hazard ratio; NR, not reached; OS, overall survival; γδ T cell, gamma-delta T cells; R, responders (defined as patients with PFS ≥ 6 months); NR, non-responders (defined as patients with PFS < 6 months); PFS, progression-free survival; ROC, receiver operator characteristic.
The responders had relatively higher baseline level of γδ2 T cells in peripheral blood and the higher baseline level of γδ2 T cells was associated with better mPFS (10.1 months vs 4.5 months, HR = 0.27; 95% CI, 0.07-0.99; P = 0.0021) and mOS (27.2 months vs 11.6 months, HR = 0.22; 95% CI, 0.06-0.78; P = 0.019) (Figure 4C, 4E, 4G, 4H). In addition, the induced increase of γδ2 T cells after 3 courses of study treatment was associated with high response and improved mPFS (10.1 months vs 4.5 months, HR = 0.30; 95% CI, 0.10-0.89; P = 0.0040) and mOS (27.2 months vs 6.8 months, HR = 0.28; 95% CI, 0.09-0.90; P = 0.032) (Figure 4D, 4F, 4I, 4J).
Discussion
In this clinical trial, which is, to our knowledge, the first prospective study to evaluate the antitumor activity and safety of tislelizumab (anti-PD-1 ICI) plus fruquintinib (anti-VEGFR TKI) and FMT in MSS mCRC patients as a third-line or above therapy. The combination continued to exhibit compelling ORR and OS benefits in comparison with what would be expected on the basis of the real-world data at this treatment setting. The regimen also reported a consistent and controllable safety profile, which is generally comparable to the established profile of the monotherapy per agent. What’s more, there have been limited reports focusing on mCRC patients’ HRQoL at later-line setting. Our study, therefore, included assessing the impact of study treatment on patients’ HRQoL. And the updated PROs further verified the improvement in survival did not negatively affect the health status in these heavily pre-treated mCRC patients.
It is well-known that fluorouracil plus irinotecan and/or oxaliplatin are the standard first- and second-line chemotherapeutic regimens for mCRC patients, with satisfactory survival benefit [3,4,18]. However, in the third-line or above setting, rechallenging chemotherapy in those heavily pre-treated mCRC patients yielded a mOS of only 6 to 8 months, with more than half of patients discontinuing treatment due to worsening functioning and symptoms, including myelosuppression, nausea and vomiting, appetite loss, malnutrition, fatigue, constipation and diarrhoea [19,20]. Immunotherapy, represented by anti-PD (L)-1 inhibitors, has significantly changed the tumor treatment landscape [21-24]. For mCRC, not only patients with MSI-H phenotype but also MSS patients have been consistently reported to benefit from ICI-based therapies [9,10,25,26]. Increasing researches have explored ICI in combination with TKI and/or radiotherapy or other cytotoxic agents in MSS mCRC patients and reported mixed results. In view of the existing evidence that gut microbiota can promote the activation of immune cells and participate in the regulation of anti-tumor immune response, the ICI, TKI and FMT in triple combinations was designed in this study.
After a median follow-up of 34.0 months, patients treated with tislelizumab plus fruquintinib and FMT achieved a mOS of 13.7 months versus 11.3 months with ICI-based therapy in the real world at third-line or above setting. After PSM analysis, the difference in mOS was still significant. It is worth noting that 38.2% (36/94) of patients in the real-world cohort received a triple combination of immunotherapy plus targeted therapy and chemotherapy. A recent randomized phase II study of a triple combination of a PD-1 antibody (sintilimab), a histone deacetylase inhibitor (chidamide) and a VEGF antibody (bevacizumab) for patients with unresectable chemotherapy-refractory locally advanced or metastatic MSS/pMMR CRC reported an ORR of 44.0% and mPFS of 7.3 months [27]. The survival outcomes shown in the above CAPability-01 trial further reflects the promising therapeutic efficacy of immunotherapy-based combinations compared to a targeted agent plus a cytotoxic drug in MSS/pMMR mCRC population.
In the updated safety analysis, the regimen was well tolerated and patients had controllable TRAEs. Most TRAEs were grade 1 to 2, and no unexpected safety signals were observed. One additional grade 3 TRAE, two TRAEs leading to dose reductions and one leading to dose interruption were reported since 10 July 2023, with no new reports of drug discontinuation or death due to TRAEs.
Consistent with these safety profiles, stability or improvement was also generally observed in the corresponding EORTC QLQ-C30 general health, functioning and symptom scores, especially pain, constipation, nausea and vomiting, as well as EQ-5D-3L VAS and health utility scores. In contrast to the significant improvement in above items, symptoms of diarrhoea persisted throughout the study, which may be related to the fluctuations of gut microecology caused by FMT. But all of the diarrhoea AEs were grade 1 to 2, and no patients dropped out of the study due to diarrhoea. Time to deterioration in GHS and most QoL measures (4.8 months to 8.0 months) was as long as expected, which was superior to the reported mPFS data (1.9 months to 5.6 months) of the recommended third-line treatment options in current clinical guidelines.
The impact of ICIs treatment on HRQoL has been increasing compared with that of chemotherapy. Statistically significant and clinically meaningful improvements in HRQoL were also observed with ICIs in non-small-cell lung cancer, mCRC, esophageal squamous cell carcinoma, urothelial cancer (KEYNOTE-010, CheckMate-142, RATIONALE 302, KEYNOTE-045), etc. [28-31]. In the ICI cohort, most patients had stabled functioning and symptom scores without further deterioration, with significant improvements in some HRQoL outcomes. The EORTC QLQ-C30 and EQ-5D-3L questionnaires were selected in the study design to maximize coverage of the impact of the study treatment on HRQoL in mCRC patients. The results of the HRQoL analysis observed in our study align with these of previous studies that showed stability or improvement in most HRQoL measures with ICI-based therapy. The observed improvements in HRQoL may also be related to the convenience of the regimen, which involves easier medication methods and shorter hospital stays compared to traditional chemotherapy. In short, rational therapeutic strategies that could improve survival as well as patients’ HRQoL have always been the direction of our exploration.
To explore potential mechanisms related to the immune response mediated by this combination treatment strategy, we collected peripheral blood lymphocyte samples from patients for FCM analysis. In the analysis, γδ2 T cells were more prevalent in responders than non-responders. In addition, there was an induced increase in γδ2 T cells in responders after treatment, which was associated with improvements in OS and PFS. Data showed that γδ2 T cells are the most common circulating γδ T lymphocytes that may play a critical role in cancer immunity [32]. Recently, increasing study data support the use of γδ2 T cells as immunotherapeutic agents in infectious diseases and tumors [33-35]. Li et al. applied antibody-cell conjugation technology to generate a rituximab-conjugated γδ2 T cells complex with superior cytotoxicity against CD20-expressing relapsed/refractory B-cell lymphoma [35]. Data on the relationship between γδ2 T cell and anti-tumor outcomes in mCRC are sorely lacking, and our finding is novel and clinically valuable, and of course further research is required.
Several limitations should be considered. Firstly, the study was conducted in a single-center with small sample size and open-label design. Patients were well aware of the study purpose, which may have certain influence on the results. A low sample size will undoubtedly reduce the statistical power and the results should, thus, be interpreted with caution. Secondly, it was a single-arm design without randomization and control groups. We only use the real-world population and relevant data reported in the literature for comparison. Certainly, we have the limitation that the control cohort might give misleading comparisons. Thirdly, the PD-L1 expression and tumor mutation burden of patients are unknown and, therefore, cannot be used to identify patients who would derive the greatest benefit from ICI-based treatment. Besides, further laboratory studies of additional biomarkers and detailed molecular mechanisms are still needed. With all its defects, the current result is encouraging and a dedicated, randomized further study in larger populations is warranted.
In summary, this study of ICI plus TKI and FMT demonstrated promising efficacy and controllable safety profile in MSS mCRC patients. The induced increase of γδ2 T cells was associated with better response and improved survival. On the basis of the present analysis and previous RENMIN-215 results, tislelizumab combined with fruquintinib and FMT, is a worthwhile therapeutic option for heavily pre-treated mCRC patients at later-line setting.
Acknowledgements
This work was funded by National Natural Science Foundation of China (No. 82102954) and the Special Project of Central Government for Local Science and Technology Development of Hubei Province (No. ZYYD2020000169). We thank all patients and their families, and the investigators and staff involved with the study.
Written informed consent was provided from the participants.
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72:7–33. doi: 10.3322/caac.21708. [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Goding Sauer A, Fedewa SA, Butterly LF, Anderson JC, Cercek A, Smith RA, Jemal A. Colorectal cancer statistics, 2020. CA Cancer J Clin. 2020;70:145–164. doi: 10.3322/caac.21601. [DOI] [PubMed] [Google Scholar]
- 3.Benson AB, Venook AP, Al-Hawary MM, Arain MA, Chen YJ, Ciombor KK, Cohen S, Cooper HS, Deming D, Farkas L, Garrido-Laguna I, Grem JL, Gunn A, Hecht JR, Hoffe S, Hubbard J, Hunt S, Johung KL, Kirilcuk N, Krishnamurthi S, Messersmith WA, Meyerhardt J, Miller ED, Mulcahy MF, Nurkin S, Overman MJ, Parikh A, Patel H, Pedersen K, Saltz L, Schneider C, Shibata D, Skibber JM, Sofocleous CT, Stoffel EM, Stotsky-Himelfarb E, Willett CG, Gregory KM, Gurski LA. Colon cancer, version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2021;19:329–359. doi: 10.6004/jnccn.2021.0012. [DOI] [PubMed] [Google Scholar]
- 4.Benson AB, Venook AP, Al-Hawary MM, Azad N, Chen YJ, Ciombor KK, Cohen S, Cooper HS, Deming D, Garrido-Laguna I, Grem JL, Gunn A, Hecht JR, Hoffe S, Hubbard J, Hunt S, Jeck W, Johung KL, Kirilcuk N, Krishnamurthi S, Maratt JK, Messersmith WA, Meyerhardt J, Miller ED, Mulcahy MF, Nurkin S, Overman MJ, Parikh A, Patel H, Pedersen K, Saltz L, Schneider C, Shibata D, Skibber JM, Sofocleous CT, Stotsky-Himelfarb E, Tavakkoli A, Willett CG, Gregory K, Gurski L. Rectal cancer, version 2.2022, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2022;20:1139–1167. doi: 10.6004/jnccn.2022.0051. [DOI] [PubMed] [Google Scholar]
- 5.Yuan Y, Wang X, Chen G, Wang Y, Sheng W, Li X, Zhou A, Zhang Z, Li G, Cai S, Xu R, Li J, Zhang S. Updates in version 2019 of CSCO guidelines for colorectal cancer from version 2018. Chin J Cancer Res. 2019;31:423–425. doi: 10.21147/j.issn.1000-9604.2019.03.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grothey A, Van Cutsem E, Sobrero A, Siena S, Falcone A, Ychou M, Humblet Y, Bouche O, Mineur L, Barone C, Adenis A, Tabernero J, Yoshino T, Lenz HJ, Goldberg RM, Sargent DJ, Cihon F, Cupit L, Wagner A, Laurent D CORRECT Study Group. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet. 2013;381:303–312. doi: 10.1016/S0140-6736(12)61900-X. [DOI] [PubMed] [Google Scholar]
- 7.Mayer RJ, Van Cutsem E, Falcone A, Yoshino T, Garcia-Carbonero R, Mizunuma N, Yamazaki K, Shimada Y, Tabernero J, Komatsu Y, Sobrero A, Boucher E, Peeters M, Tran B, Lenz HJ, Zaniboni A, Hochster H, Cleary JM, Prenen H, Benedetti F, Mizuguchi H, Makris L, Ito M, Ohtsu A RECOURSE Study Group. Randomized trial of TAS-102 for refractory metastatic colorectal cancer. N Engl J Med. 2015;372:1909–1919. doi: 10.1056/NEJMoa1414325. [DOI] [PubMed] [Google Scholar]
- 8.Li J, Qin S, Xu RH, Shen L, Xu J, Bai Y, Yang L, Deng Y, Chen ZD, Zhong H, Pan H, Guo W, Shu Y, Yuan Y, Zhou J, Xu N, Liu T, Ma D, Wu C, Cheng Y, Chen D, Li W, Sun S, Yu Z, Cao P, Chen H, Wang J, Wang S, Wang H, Fan S, Hua Y, Su W. Effect of fruquintinib vs placebo on overall survival in patients with previously treated metastatic colorectal cancer: the FRESCO randomized clinical trial. JAMA. 2018;319:2486–2496. doi: 10.1001/jama.2018.7855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fukuoka S, Hara H, Takahashi N, Kojima T, Kawazoe A, Asayama M, Yoshii T, Kotani D, Tamura H, Mikamoto Y, Hirano N, Wakabayashi M, Nomura S, Sato A, Kuwata T, Togashi Y, Nishikawa H, Shitara K. Regorafenib plus nivolumab in patients with advanced gastric or colorectal cancer: an open-label, dose-escalation, and dose-expansion phase Ib trial (REGONIVO, EPOC1603) J. Clin. Oncol. 2020;38:2053–2061. doi: 10.1200/JCO.19.03296. [DOI] [PubMed] [Google Scholar]
- 10.Guo Y, Zhang W, Ying J, Zhang Y, Pan Y, Qiu W, Fan Q, Xu Q, Ma Y, Wang G, Guo J, Su W, Fan S, Tan P, Wang Y, Luo Y, Zhou H, Li J. Phase 1b/2 trial of fruquintinib plus sintilimab in treating advanced solid tumours: the dose-escalation and metastatic colorectal cancer cohort in the dose-expansion phases. Eur J Cancer. 2023;181:26–37. doi: 10.1016/j.ejca.2022.12.004. [DOI] [PubMed] [Google Scholar]
- 11.Baruch EN, Youngster I, Ben-Betzalel G, Ortenberg R, Lahat A, Katz L, Adler K, Dick-Necula D, Raskin S, Bloch N, Rotin D, Anafi L, Avivi C, Melnichenko J, Steinberg-Silman Y, Mamtani R, Harati H, Asher N, Shapira-Frommer R, Brosh-Nissimov T, Eshet Y, Ben-Simon S, Ziv O, Khan MAW, Amit M, Ajami NJ, Barshack I, Schachter J, Wargo JA, Koren O, Markel G, Boursi B. Fecal microbiota transplant promotes response in immunotherapy-refractory melanoma patients. Science. 2021;371:602–609. doi: 10.1126/science.abb5920. [DOI] [PubMed] [Google Scholar]
- 12.Davar D, Dzutsev AK, McCulloch JA, Rodrigues RR, Chauvin JM, Morrison RM, Deblasio RN, Menna C, Ding Q, Pagliano O, Zidi B, Zhang S, Badger JH, Vetizou M, Cole AM, Fernandes MR, Prescott S, Costa RGF, Balaji AK, Morgun A, Vujkovic-Cvijin I, Wang H, Borhani AA, Schwartz MB, Dubner HM, Ernst SJ, Rose A, Najjar YG, Belkaid Y, Kirkwood JM, Trinchieri G, Zarour HM. Fecal microbiota transplant overcomes resistance to anti-PD-1 therapy in melanoma patients. Science. 2021;371:595–602. doi: 10.1126/science.abf3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhao W, Lei J, Ke S, Chen Y, Xiao J, Tang Z, Wang L, Ren Y, Alnaggar M, Qiu H, Shi W, Yin L, Chen Y. Fecal microbiota transplantation plus tislelizumab and fruquintinib in refractory microsatellite stable metastatic colorectal cancer: an open-label, single-arm, phase II trial (RENMIN-215) EClinicalMedicine. 2023;66:102315. doi: 10.1016/j.eclinm.2023.102315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, Filiberti A, Flechtner H, Fleishman SB, de Haes JC, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85:365–376. doi: 10.1093/jnci/85.5.365. [DOI] [PubMed] [Google Scholar]
- 15.Wong CK, Guo VY, Chen J, Lam CL. Methodological and reporting quality of comparative studies evaluating health-related quality of life of colorectal cancer patients and controls: a systematic review. Dis Colon Rectum. 2016;59:1073–1086. doi: 10.1097/DCR.0000000000000618. [DOI] [PubMed] [Google Scholar]
- 16.Rabin R, de Charro F. EQ-5D: a measure of health status from the EuroQol Group. Ann Med. 2001;33:337–343. doi: 10.3109/07853890109002087. [DOI] [PubMed] [Google Scholar]
- 17.Liu GG, Wu H, Li M, Gao C, Luo N. Chinese time trade-off values for EQ-5D health states. Value Health. 2014;17:597–604. doi: 10.1016/j.jval.2014.05.007. [DOI] [PubMed] [Google Scholar]
- 18.Biller LH, Schrag D. Diagnosis and treatment of metastatic colorectal cancer: a review. JAMA. 2021;325:669–685. doi: 10.1001/jama.2021.0106. [DOI] [PubMed] [Google Scholar]
- 19.Suenaga M, Mizunuma N, Matsusaka S, Shinozaki E, Ozaka M, Ogura M, Yamaguchi T. Phase II study of reintroduction of oxaliplatin for advanced colorectal cancer in patients previously treated with oxaliplatin and irinotecan: RE-OPEN study. Drug Des Devel Ther. 2015;9:3099–3108. doi: 10.2147/DDDT.S85567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang Q, Huang Y, Jiang Z, Wang H, Li W, Zhang B, Xie D. Rechallenge of oxaliplatin-containing regimens in the third- or later-line therapy for patients with heavily treated metastatic colorectal cancer. Onco Targets Ther. 2018;11:2467–2473. doi: 10.2147/OTT.S154220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Andre T, Shiu KK, Kim TW, Jensen BV, Jensen LH, Punt C, Smith D, Garcia-Carbonero R, Benavides M, Gibbs P, de la Fouchardiere C, Rivera F, Elez E, Bendell J, Le DT, Yoshino T, Van Cutsem E, Yang P, Farooqui MZH, Marinello P, Diaz LA Jr KEYNOTE-177 Investigators. Pembrolizumab in microsatellite-instability-high advanced colorectal cancer. N Engl J Med. 2020;383:2207–2218. doi: 10.1056/NEJMoa2017699. [DOI] [PubMed] [Google Scholar]
- 22.Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, Kudo M, Breder V, Merle P, Kaseb AO, Li D, Verret W, Xu DZ, Hernandez S, Liu J, Huang C, Mulla S, Wang Y, Lim HY, Zhu AX, Cheng AL IMbrave150 Investigators. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020;382:1894–1905. doi: 10.1056/NEJMoa1915745. [DOI] [PubMed] [Google Scholar]
- 23.Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Rutkowski P, Lao CD, Cowey CL, Schadendorf D, Wagstaff J, Dummer R, Ferrucci PF, Smylie M, Hogg D, Hill A, Marquez-Rodas I, Haanen J, Guidoboni M, Maio M, Schoffski P, Carlino MS, Lebbe C, McArthur G, Ascierto PA, Daniels GA, Long GV, Bastholt L, Rizzo JI, Balogh A, Moshyk A, Hodi FS, Wolchok JD. Five-year survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med. 2019;381:1535–1546. doi: 10.1056/NEJMoa1910836. [DOI] [PubMed] [Google Scholar]
- 24.Paz-Ares L, Luft A, Vicente D, Tafreshi A, Gumus M, Mazieres J, Hermes B, Cay Senler F, Csoszi T, Fulop A, Rodriguez-Cid J, Wilson J, Sugawara S, Kato T, Lee KH, Cheng Y, Novello S, Halmos B, Li X, Lubiniecki GM, Piperdi B, Kowalski DM KEYNOTE-407 Investigators. Pembrolizumab plus chemotherapy for squamous non-small-cell lung cancer. N Engl J Med. 2018;379:2040–2051. doi: 10.1056/NEJMoa1810865. [DOI] [PubMed] [Google Scholar]
- 25.Diaz LA Jr, Shiu KK, Kim TW, Jensen BV, Jensen LH, Punt C, Smith D, Garcia-Carbonero R, Benavides M, Gibbs P, de la Fourchardiere C, Rivera F, Elez E, Le DT, Yoshino T, Zhong WY, Fogelman D, Marinello P, Andre T KEYNOTE-177 Investigators. Pembrolizumab versus chemotherapy for microsatellite instability-high or mismatch repair-deficient metastatic colorectal cancer (KEYNOTE-177): final analysis of a randomised, open-label, phase 3 study. Lancet Oncol. 2022;23:659–670. doi: 10.1016/S1470-2045(22)00197-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lenz HJ, Van Cutsem E, Luisa Limon M, Wong KYM, Hendlisz A, Aglietta M, Garcia-Alfonso P, Neyns B, Luppi G, Cardin DB, Dragovich T, Shah U, Abdullaev S, Gricar J, Ledeine JM, Overman MJ, Lonardi S. First-line nivolumab plus low-dose ipilimumab for microsatellite instability-high/mismatch repair-deficient metastatic colorectal cancer: the phase II CheckMate 142 study. J. Clin. Oncol. 2022;40:161–170. doi: 10.1200/JCO.21.01015. [DOI] [PubMed] [Google Scholar]
- 27.Wang F, Jin Y, Wang M, Luo HY, Fang WJ, Wang YN, Chen YX, Huang RJ, Guan WL, Li JB, Li YH, Wang FH, Hu XH, Zhang YQ, Qiu MZ, Liu LL, Wang ZX, Ren C, Wang DS, Zhang DS, Wang ZQ, Liao WT, Tian L, Zhao Q, Xu RH. Combined anti-PD-1, HDAC inhibitor and anti-VEGF for MSS/pMMR colorectal cancer: a randomized phase 2 trial. Nat Med. 2024;30:1035–1043. doi: 10.1038/s41591-024-02813-1. [DOI] [PubMed] [Google Scholar]
- 28.Barlesi F, Garon EB, Kim DW, Felip E, Han JY, Kim JH, Ahn MJ, Fidler MJ, Gubens MA, de Castro G Jr, Surmont V, Li Q, Deitz AC, Lubiniecki GM, Herbst RS. Health-related quality of life in KEYNOTE-010: a phase II/III study of pembrolizumab versus docetaxel in patients with previously treated advanced, programmed death ligand 1-expressing NSCLC. J Thorac Oncol. 2019;14:793–801. doi: 10.1016/j.jtho.2019.01.016. [DOI] [PubMed] [Google Scholar]
- 29.Overman MJ, Lonardi S, Wong KYM, Lenz HJ, Gelsomino F, Aglietta M, Morse MA, Van Cutsem E, McDermott R, Hill A, Sawyer MB, Hendlisz A, Neyns B, Svrcek M, Moss RA, Ledeine JM, Cao ZA, Kamble S, Kopetz S, Andre T. Durable clinical benefit with nivolumab plus ipilimumab in DNA mismatch repair-deficient/microsatellite instability-high metastatic colorectal cancer. J. Clin. Oncol. 2018;36:773–779. doi: 10.1200/JCO.2017.76.9901. [DOI] [PubMed] [Google Scholar]
- 30.Van Cutsem E, Kato K, Ajani J, Shen L, Xia T, Ding N, Zhan L, Barnes G, Kim SB. Tislelizumab versus chemotherapy as second-line treatment of advanced or metastatic esophageal squamous cell carcinoma (RATIONALE 302): impact on health-related quality of life. ESMO Open. 2022;7:100517. doi: 10.1016/j.esmoop.2022.100517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vaughn DJ, Bellmunt J, Fradet Y, Lee JL, Fong L, Vogelzang NJ, Climent MA, Petrylak DP, Choueiri TK, Necchi A, Gerritsen W, Gurney H, Quinn DI, Culine S, Sternberg CN, Mai Y, Li H, Perini RF, Bajorin DF, de Wit R. Health-related quality-of-life analysis from KEYNOTE-045: a phase III study of pembrolizumab versus chemotherapy for previously treated advanced urothelial cancer. J. Clin. Oncol. 2018;36:1579–1587. doi: 10.1200/JCO.2017.76.9562. [DOI] [PubMed] [Google Scholar]
- 32.Liu Y, Zhang C. The role of human gammadelta T cells in anti-tumor immunity and their potential for cancer immunotherapy. Cells. 2020;9:1206. doi: 10.3390/cells9051206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pean P, Nouhin J, Ratana M, Madec Y, Borand L, Marcy O, Laureillard D, Fernandez M, Barre-Sinoussi F, Weiss L, Scott-Algara D. High activation of gammadelta T cells and the gammadelta2(pos) T-cell subset is associated with the onset of tuberculosis-associated immune reconstitution inflammatory syndrome, ANRS 12153 CAPRI NK. Front Immunol. 2019;10:2018. doi: 10.3389/fimmu.2019.02018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Junqueira C, Polidoro RB, Castro G, Absalon S, Liang Z, Sen Santara S, Crespo A, Pereira DB, Gazzinelli RT, Dvorin JD, Lieberman J. gammadelta T cells suppress Plasmodium falciparum blood-stage infection by direct killing and phagocytosis. Nat Immunol. 2021;22:347–357. doi: 10.1038/s41590-020-00847-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li HK, Wu TS, Kuo YC, Hsiao CW, Yang HP, Lee CY, Leng PJ, Chiang YJ, Cheng ZF, Yang SH, Lin YL, Chen LY, Chen CS, Chen YJ, Hsiao SC, Tang SW. A novel allogeneic rituximab-conjugated gamma delta T cell therapy for the treatment of relapsed/refractory B-cell lymphoma. Cancers (Basel) 2023;15:4844. doi: 10.3390/cancers15194844. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.