Abstract
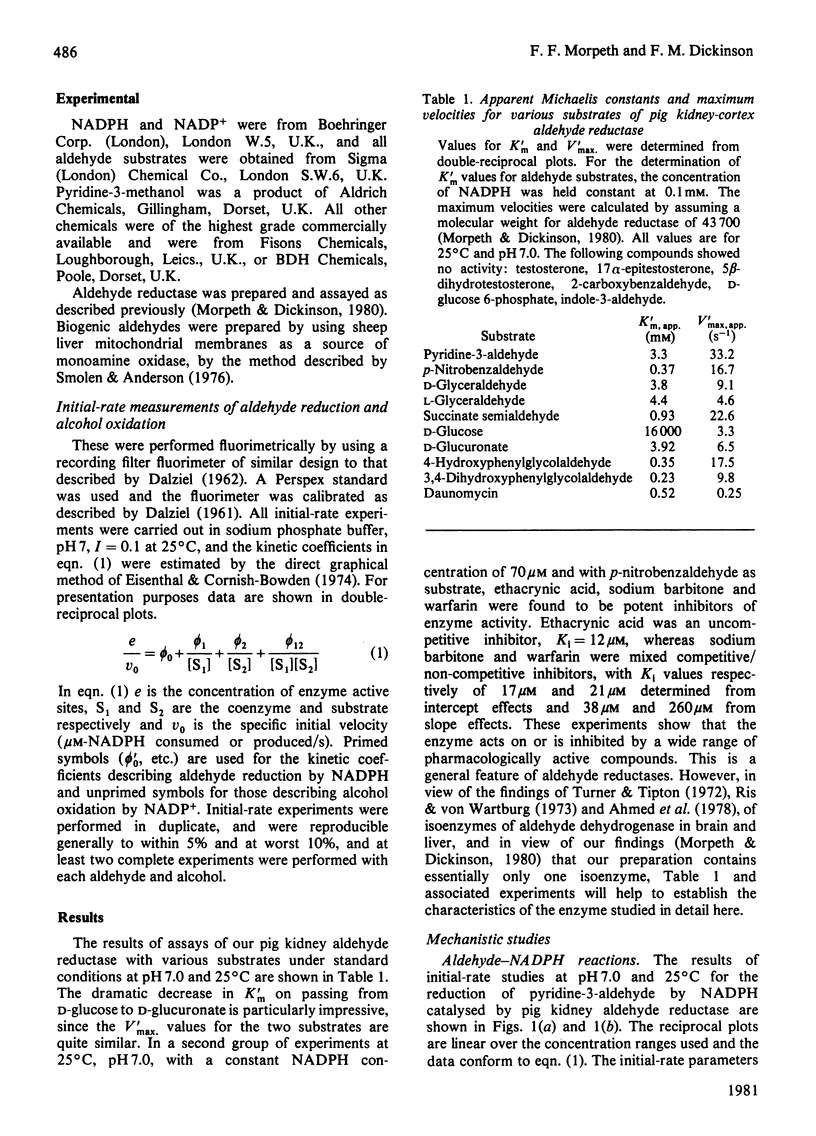
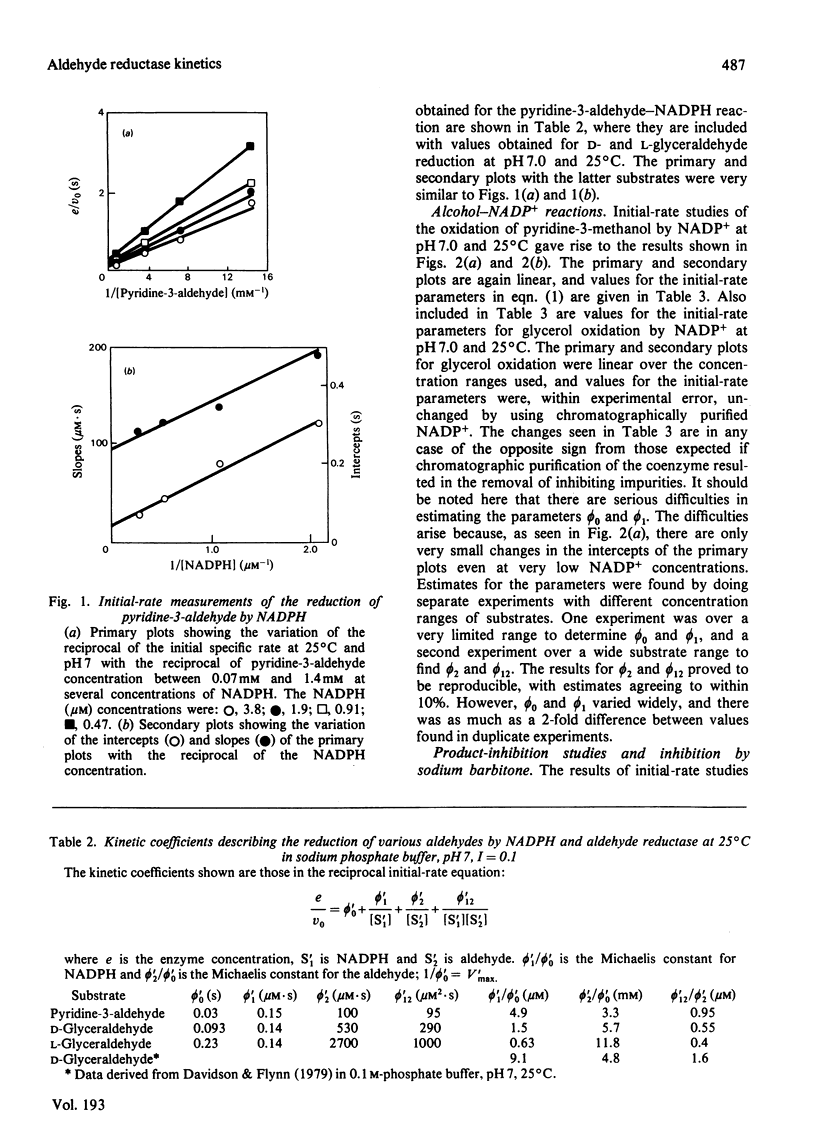
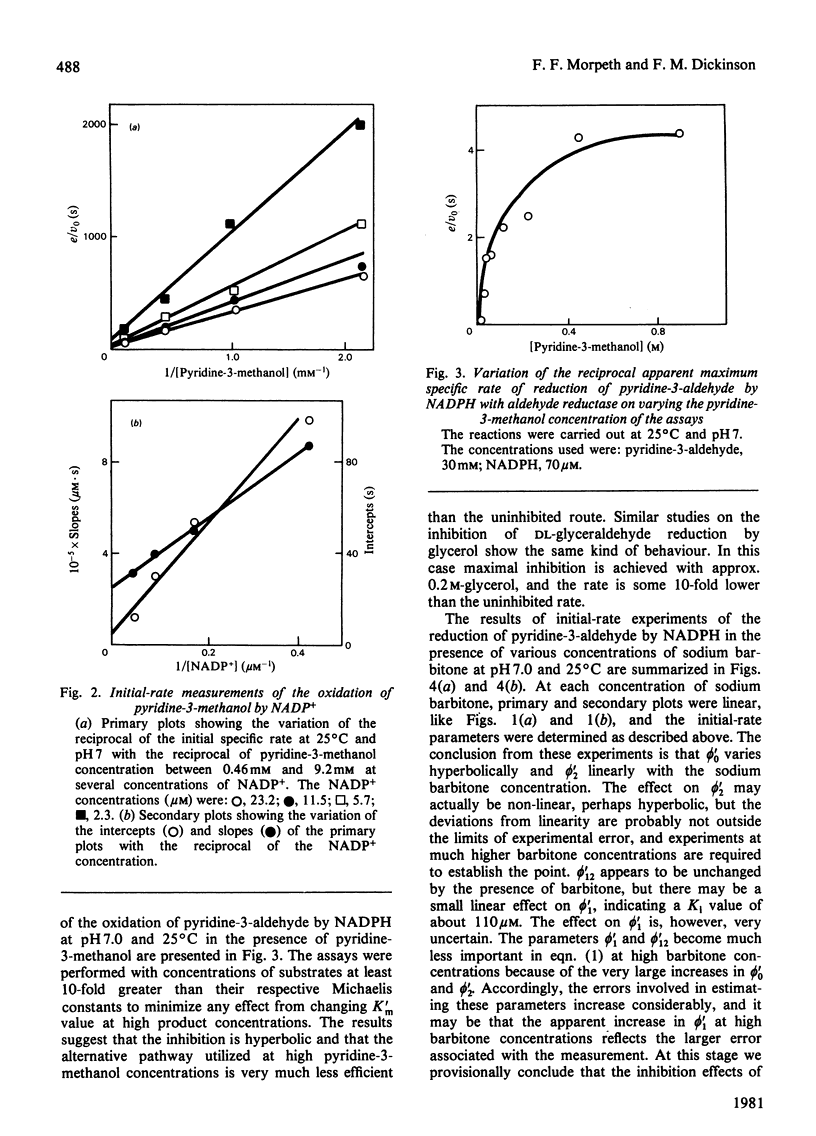
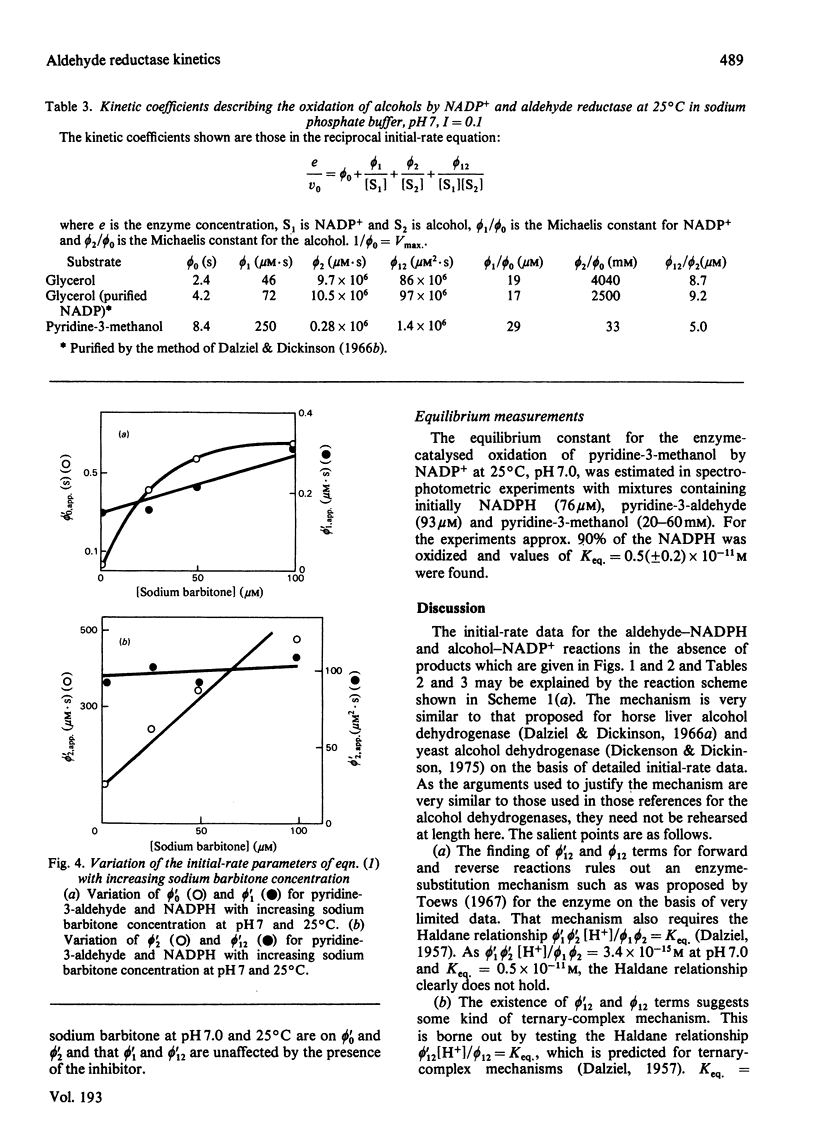
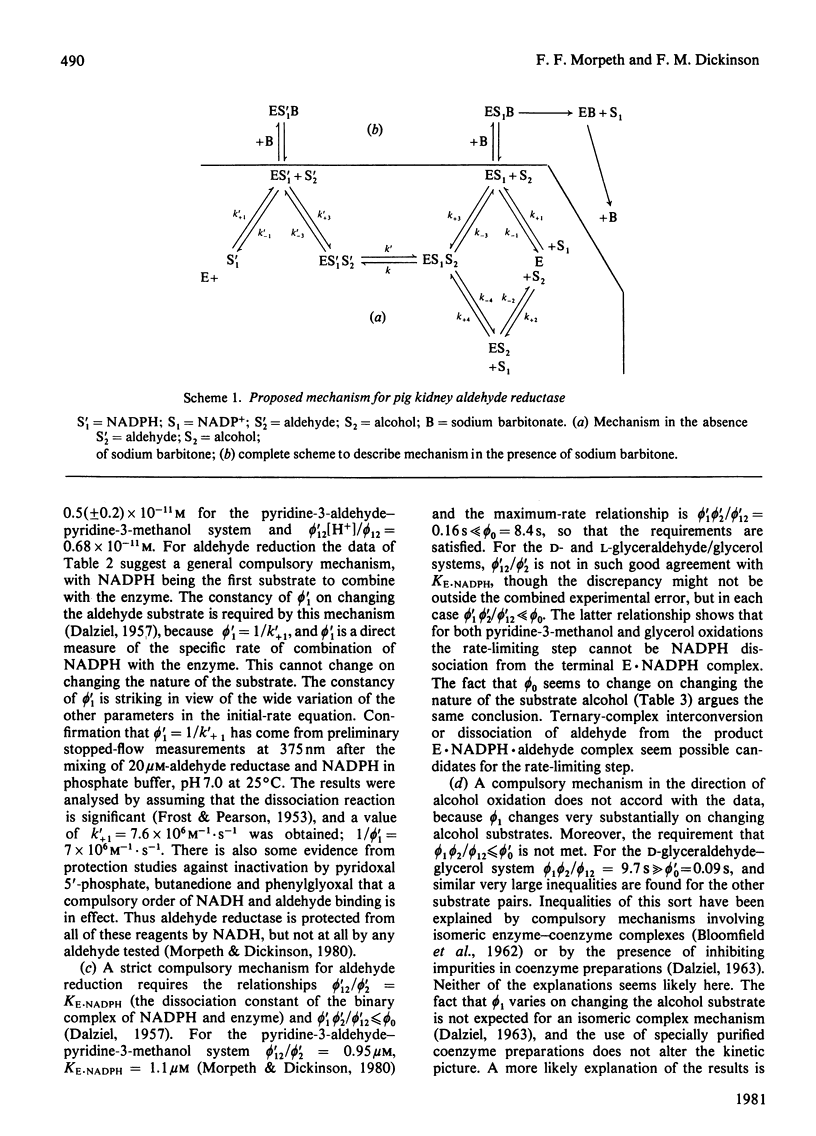
Initial-rate measurements were made of the oxidations of pyridine-3-methanol and glycerol by NADP+ and of the reduction of the corresponding aldehydes by NADPH catalysed by pig kidney aldehyde reductase. In addition, a brief survey of the specificity of the enzyme towards aldehyde substrates and its sensitivity to the inhibitors ethacrynic acid, sodium barbitone and warfarin was made. The detailed kinetic work indicates a compulsory mechanism for aldehyde reduction, with NADPH binding before aldehyde. For alcohol oxidation, however, it is necessary to postulate the formation of kinetically significant amounts of binary complexes of the type enzyme-alcohol to explain the results. Thus, for alcohol oxidation random-order addition of substrates may occur. Inhibition studies of the kinetics of aldehyde reduction in the presence of the corresponding alcohol product provide further evidence for the existence of enzyme-alcohol complexes. Finally, detailed kinetic studies were made of the inhibition of pyridine-3-aldehyde reduction by sodium barbitone. The mechanism of the inhibition is discussed.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmed N. K., Felsted R. L., Bachur N. R. Heterogeneity of anthracycline antibiotic carbonyl reductases in mammalian livers. Biochem Pharmacol. 1978;27(23):2713–2719. doi: 10.1016/0006-2952(78)90047-3. [DOI] [PubMed] [Google Scholar]
- Bosron W. F., Prairie R. L. Triphosphopyridine nucleotide-linked aldehyde reductase. I. Purification and properties of the enzyme from pig kidney cortex. J Biol Chem. 1972 Jul 25;247(14):4480–4485. [PubMed] [Google Scholar]
- Bronaugh R. L., Erwin V. G. Further characterization of a reduced nicotinamide-adenine dinucleotide phosphate-dependent aldehyde reductase from bovine brain. Inhibition by phenothiazine derivatives. Biochem Pharmacol. 1972 May 15;21(10):1457–1464. doi: 10.1016/0006-2952(72)90370-x. [DOI] [PubMed] [Google Scholar]
- DALZIEL K. KINETIC STUDIES OF LIVER ALCOHOL DEHYDROGENASE AND PH EFFECTS WITH COENZYME PREPARATIONS OF HIGH PURITY. J Biol Chem. 1963 Aug;238:2850–2858. [PubMed] [Google Scholar]
- DALZIEL K. Kinetic studies of liver alcohol dehydrogenase. Biochem J. 1962 Aug;84:244–254. doi: 10.1042/bj0840244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DALZIEL K. The preparation and properties of crystalline alcohol dehydrogenase from liver. Biochem J. 1961 Aug;80:440–445. doi: 10.1042/bj0800440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalziel K., Dickinson F. M. The kinetics and mechanism of liver alcohol dehydrogenase with primary and secondary alcohols as substrates. Biochem J. 1966 Jul;100(1):34–46. doi: 10.1042/bj1000034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson W. S., Flynn T. G. Kinetics and mechanism of action of aldehyde reductase from pig kidney. Biochem J. 1979 Feb 1;177(2):595–601. doi: 10.1042/bj1770595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickenson C. J., Dickinson F. M. A study of the pH- and temperature-dependence of the reactions of yeast alcohol dehydrogenase with ethanol, acetaldehyde and butyraldehyde as substrates. Biochem J. 1975 May;147(2):303–311. doi: 10.1042/bj1470303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickenson C. J., Dickinson F. M. Inhibition by ethanol, acetaldehyde and trifluoroethanol of reactions catalysed by yeast and horse liver alcohol dehydrogenases. Biochem J. 1978 Jun 1;171(3):613–627. doi: 10.1042/bj1710613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenthal R., Cornish-Bowden A. The direct linear plot. A new graphical procedure for estimating enzyme kinetic parameters. Biochem J. 1974 Jun;139(3):715–720. doi: 10.1042/bj1390715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erwin V. G., Tabakoff B., Bronaugh R. L. Inhibition of a reduced nicotinamide adenine dinucleotide phosphate-linked aldehyde reductase from bovine brain by barbiturates. Mol Pharmacol. 1971 Mar;7(2):169–176. [PubMed] [Google Scholar]
- Morpeth F. F., Dickinson F. M. Some properties of pig kidney-cortex aldehyde reductase. Biochem J. 1980 Nov 1;191(2):619–626. doi: 10.1042/bj1910619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettersson G. Dalziel rate behaviour in ternary-complex mechanisms for enzyme reactions involving two substrates. Biochim Biophys Acta. 1972 Jul 13;276(1):1–11. doi: 10.1016/0005-2744(72)90002-2. [DOI] [PubMed] [Google Scholar]
- Pettersson G. The kinetics of substrate-competitive inhibition in ternary-complex mechanisms for enzyme reactions involving two substrates. Eur J Biochem. 1974 Jul 1;46(1):1–4. doi: 10.1111/j.1432-1033.1974.tb03590.x. [DOI] [PubMed] [Google Scholar]
- Plapp B. V. On calculation of rate and dissociation constants from kinetic constants for the Ordered Bi Bi mechanism of liver alcohol dehydrogenase. Arch Biochem Biophys. 1973 May;156(1):112–114. doi: 10.1016/0003-9861(73)90347-0. [DOI] [PubMed] [Google Scholar]
- Ris M. M., von Wartburg J. P. Heterogeneity of NADPH-dependent aldehyde reductase from human and rat brain. Eur J Biochem. 1973 Aug 1;37(1):69–77. doi: 10.1111/j.1432-1033.1973.tb02958.x. [DOI] [PubMed] [Google Scholar]
- SILVERSTEIN E., BOYER P. D. EQUILIBRIUM REACTION RATES AND THE MECHANISMS OF LIVER AND YEAST ALCOHOL DEHYDROGENASE. J Biol Chem. 1964 Nov;239:3908–3914. [PubMed] [Google Scholar]
- Smolen A., Anderson A. D. Partial purification and characterization of a reduced nicotinamide adenine dinucleotide phosphate-linked aldehyde reductase from heart. Biochem Pharmacol. 1976 Feb 1;25(3):317–323. doi: 10.1016/0006-2952(76)90221-5. [DOI] [PubMed] [Google Scholar]
- Toews C. J. The kinetics and reaction mechanism of the nicotinamide-adenine dinucleotide phosphate-specific glycerol dehydrogenase of rat skeletal muscle. Biochem J. 1967 Dec;105(3):1067–1073. doi: 10.1042/bj1051067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner A. J., Tipton K. F. The characterization of two reduced nicotinamide-adenine dinucleotide phosphate-linked aldehyde reductases from pig brain. Biochem J. 1972 Dec;130(3):765–772. doi: 10.1042/bj1300765. [DOI] [PMC free article] [PubMed] [Google Scholar]


