Abstract
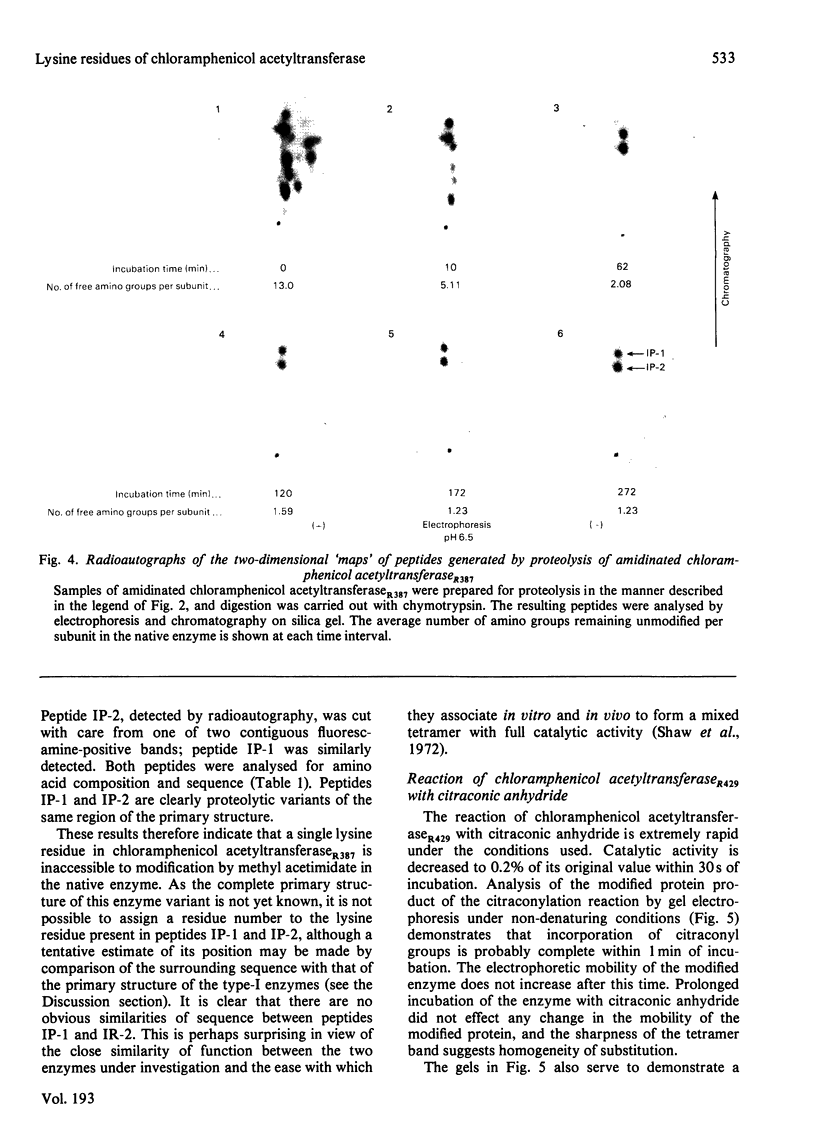
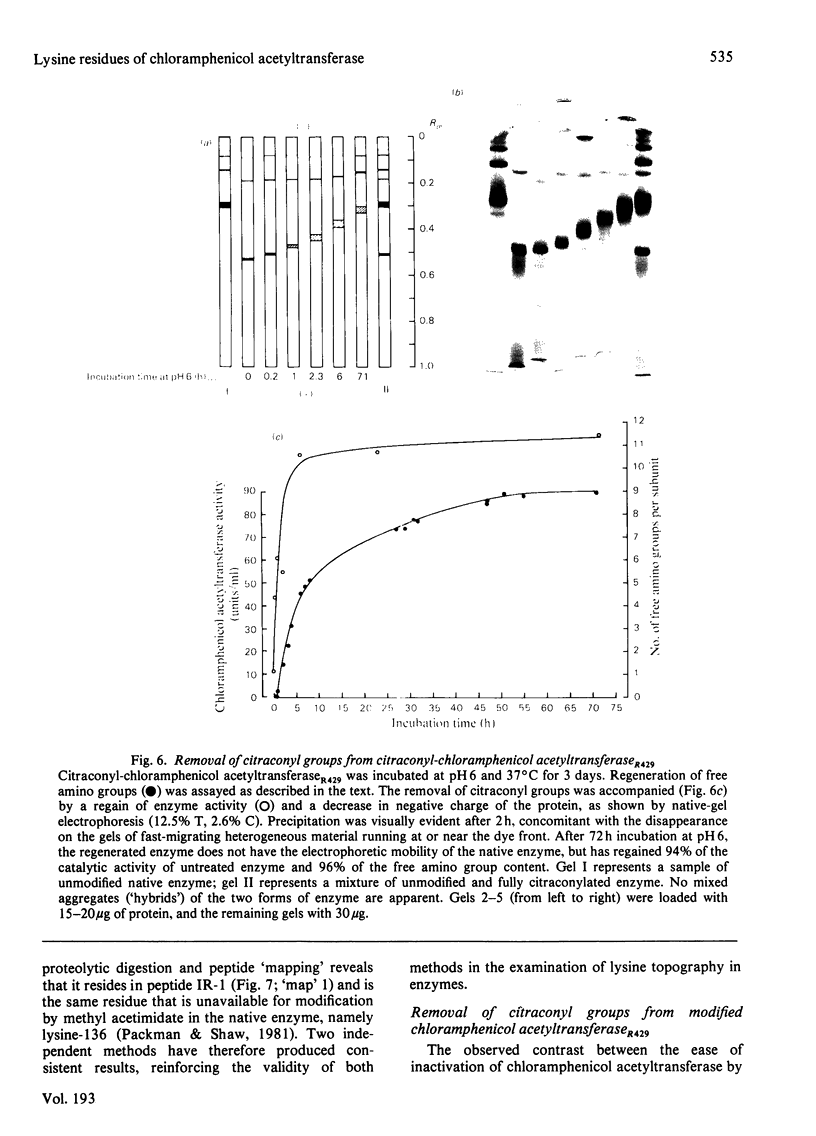
Two variants of chloramphenicol acetyltransferase which are specified by genes on plasmids found in Gram-negative bacteria were subjected to amidination with methyl acetimidate to determine the relative reactivity of surface lysine residues and to search for unreactive or "buried" amino groups which might contribute to stabilization of the native tetramers. Representative examples of the type-I and type-III variants of chloramphenicol acetyltransferase were found to have one lysine residue each in the native state which appears to be inaccessible to methyl acetimidate. The uniquely unreactive residue of the type-I protein is lysine-136, whereas the lysine that is "buried" in the type-III enzyme is provisonally assigned to residue 38 of the prototype sequence. It is suggested that the lysine residue in each case participates in the formation of an ion pair at the intersubunit interface and that the two amino groups in question occupy functionally equivalent positions in the quaternary structures of their respective enzyme variants. Lysine-136 of type-I enzyme is also uniquely unavailable for modification by citraconic anhydride, a reagent used to disrupt the quaternary structure of the native enzyme. Contrary to expectation, exhaustive citraconylation fails to dissociate the tetramer, but does destroy catalytic activity. Removal of citraconyl groups from modified chloramphenicol acetyltransferase is accompanied by a full region of catalytic activity. Analysis of the rate of hydrolysis of citraconyl groups from the modified tetramer by amidination of unblocked amino groups with methyl [14C]acetamidate reveals difference in lability for several of the ten modified lysine residues. Although the unique stability of the quaternary structure of chloramphenicol acetyltransferase may be due to strong hydrophobic interactions, it is argued that lysine-136 may contribute to stability via the formation of an ion pair at the subunit interface.
Full text
PDF












Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bates D. L., Perham R. N., Coggins J. R. Methods for obtaining peptide maps of proteins on a subnanomole scale. Anal Biochem. 1975 Sep;68(1):175–184. doi: 10.1016/0003-2697(75)90692-2. [DOI] [PubMed] [Google Scholar]
- Benveniste R., Davies J. R-factor mediated gentamicin resistance: A new enzyme which modifies aminoglycoside antibiotics. FEBS Lett. 1971 May 20;14(5):293–296. doi: 10.1016/0014-5793(71)80282-x. [DOI] [PubMed] [Google Scholar]
- Browne D. T., Kent S. B. Formation of non-amidine products in the reaction of primary amines with imido esters. Biochem Biophys Res Commun. 1975 Nov 3;67(1):126–132. doi: 10.1016/0006-291x(75)90292-2. [DOI] [PubMed] [Google Scholar]
- Butler P. J., Harris J. I., Hartley B. S., Lebeman R. The use of maleic anhydride for the reversible blocking of amino groups in polypeptide chains. Biochem J. 1969 May;112(5):679–689. doi: 10.1042/bj1120679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon H. B., Perham R. N. Reversible blocking of amino groups with citraconic anhydride. Biochem J. 1968 Sep;109(2):312–314. doi: 10.1042/bj1090312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitton J. E., Shaw W. V. Comparison of chloramphenicol acetyltransferase variants in staphylococci. Purification, inhibitor studies and N-terminal sequences. Biochem J. 1979 Feb 1;177(2):575–582. doi: 10.1042/bj1770575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons I., Perham R. N. The reaction of aldolase with 2-methylmaleic anhydride. Biochem J. 1970 Mar;116(5):843–849. doi: 10.1042/bj1160843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HJERTEN S. "Molecular sieve" chromatography on polyacrylamide gels, prepared according to a simplified method. Arch Biochem Biophys. 1962 Sep;Suppl 1:147–151. [PubMed] [Google Scholar]
- Habeeb A. F. Determination of free amino groups in proteins by trinitrobenzenesulfonic acid. Anal Biochem. 1966 Mar;14(3):328–336. doi: 10.1016/0003-2697(66)90275-2. [DOI] [PubMed] [Google Scholar]
- Hartley B. S. Strategy and tactics in protein chemistry. Biochem J. 1970 Oct;119(5):805–822. doi: 10.1042/bj1190805f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartman F. C., Wold F. Cross-linking of bovine pancreatic ribonuclease A with dimethyl adipimidate. Biochemistry. 1967 Aug;6(8):2439–2448. doi: 10.1021/bi00860a021. [DOI] [PubMed] [Google Scholar]
- Hedges R. W. R factors from Proteus mirabilis and P. vulgaris. J Gen Microbiol. 1975 Apr;87(2):301–311. doi: 10.1099/00221287-87-2-301. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lambert J. M., Perham R. N., Coggins J. R. Intramolecular ionic interactions of lysine residues and a possible folding domain in fructose diphosphate aldolase. Biochem J. 1977 Jan 1;161(1):63–71. doi: 10.1042/bj1610063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert J. M., Perham R. N. Folding domains and intramolecular ionic interactions of lysine residues in glyceraldehyde 3-phosphate dehydrogenase. Biochem J. 1977 Jan 1;161(1):49–62. doi: 10.1042/bj1610049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liddell J. M., Shaw W. V., Swan I. D. Preliminary crystallographic data for a chloramphenicol acetyltransferase from Escherichia coli. J Mol Biol. 1978 Sep 5;124(1):285–286. doi: 10.1016/0022-2836(78)90160-2. [DOI] [PubMed] [Google Scholar]
- Offord R. E. Electrophoretic mobilities of peptides on paper and their use in the determination of amide groups. Nature. 1966 Aug 6;211(5049):591–593. doi: 10.1038/211591a0. [DOI] [PubMed] [Google Scholar]
- Packman L. C., Shaw W. V. The use of naturally occurring hybrid variants of chloramphenicol acetyltransferase to investigate subunit contacts. Biochem J. 1981 Feb 1;193(2):541–552. doi: 10.1042/bj1930541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plapp B. V., Kim J. C. Determination of epsilon-acetimidyllysine in proteins. Anal Biochem. 1974 Nov;62(1):291–294. doi: 10.1016/0003-2697(74)90390-x. [DOI] [PubMed] [Google Scholar]
- Reynolds J. H. Acetimidation of bovine pancreatic ribonuclease A. Biochemistry. 1968 Sep;7(9):3131–3135. doi: 10.1021/bi00849a016. [DOI] [PubMed] [Google Scholar]
- Shaw W. V. Chloramphenicol acetyltransferase from chloramphenicol-resistant bacteria. Methods Enzymol. 1975;43:737–755. doi: 10.1016/0076-6879(75)43141-x. [DOI] [PubMed] [Google Scholar]
- Shaw W. V., Packman L. C., Burleigh B. D., Dell A., Morris H. R., Hartley B. S. Primary structure of a chloramphenicol acetyltransferase specified by R plasmids. Nature. 1979 Dec 20;282(5741):870–872. doi: 10.1038/282870a0. [DOI] [PubMed] [Google Scholar]
- Shaw W. V., Sands L. C., Datta N. Hybridization of variants of chloramphenicol acetyltransferase specified by fi + and fi - R factors. Proc Natl Acad Sci U S A. 1972 Oct;69(10):3049–3053. doi: 10.1073/pnas.69.10.3049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw W. V. The enzymatic acetylation of chloramphenicol by extracts of R factor-resistant Escherichia coli. J Biol Chem. 1967 Feb 25;242(4):687–693. [PubMed] [Google Scholar]
- Udenfriend S., Stein S., Böhlen P., Dairman W., Leimgruber W., Weigele M. Fluorescamine: a reagent for assay of amino acids, peptides, proteins, and primary amines in the picomole range. Science. 1972 Nov 24;178(4063):871–872. doi: 10.1126/science.178.4063.871. [DOI] [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]
- WALEY S. G., WATSON J. The action of trypsin on polylysine. Biochem J. 1953 Sep;55(2):328–337. doi: 10.1042/bj0550328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods K. R., Wang K. T. Separation of dansyl-amino acids by polyamide layer chromatography. Biochim Biophys Acta. 1967 Feb 21;133(2):369–370. doi: 10.1016/0005-2795(67)90078-5. [DOI] [PubMed] [Google Scholar]
- Zaidenzaig Y., Fitton J. E., Packman L. C., Shaw W. V. Characterization and comparison of chloramphenicol acetyltransferase variants. Eur J Biochem. 1979 Oct 15;100(2):609–618. doi: 10.1111/j.1432-1033.1979.tb04208.x. [DOI] [PubMed] [Google Scholar]
- Zaidenzaig Y., Shaw W. V. Affinity and hydrophobic chromatography of three variants of chloramphenicol acetyltransferases specified by R factors in Escherichia coli. FEBS Lett. 1976 Mar 1;62(3):266–271. doi: 10.1016/0014-5793(76)80072-5. [DOI] [PubMed] [Google Scholar]
- Zaidenzaig Y., Shaw W. V. The reactivity of sulfhydryl groups at the active site of an F-factor--specified variant of chloramphenicol acetyltransferase. Eur J Biochem. 1978 Feb;83(2):553–562. doi: 10.1111/j.1432-1033.1978.tb12123.x. [DOI] [PubMed] [Google Scholar]







