Abstract
Type 2 epithelial cytokines, including thymic stromal lymphopoietin and IL‐33, play central roles in modulation of type 2 immune cells, such as basophils. Basophils are a small subset of granulocytes within the leukocyte population that predominantly exist in the blood. They have non‐redundant roles in allergic inflammation in peripheral tissues such as the lung, skin and gut, where they increase and accumulate at inflammatory lesions and exclusively produce large amounts of IL‐4, a type 2 cytokine. These inflammatory reactions are known to be, to some extent, phenocopies of infectious diseases of ticks and helminths. Recently, biologics related to both type 2 epithelial cytokines and basophils have been approved by the US Food and Drug Administration for treatment of allergic diseases. We summarised the roles of Type 2 epithelial cytokines and basophils in basic science to translational medicine, including recent findings.
Keywords: basophil, IL‐33, thymic stromal lymphopoietin, type 2 immunity
It has been well described that basophils and type 2 epithelial cytokines play important roles in allergic diseases. Based on these findings, significant progress has been made in translational research into these diseases. In this review, we summarise fundamental and novel findings that connect basic and translational research.
Introduction
Basophils are one of the rarest granulocytes in peripheral blood leukocytes. They produce chemical mediators such as histamine and secrete prostaglandins and proinflammatory cytokines, which lead to inflammatory reactions, contribute to allergic inflammation including atopic dermatitis and pulmonary diseases and also protect against helminth infections. 1 , 2 , 3 , 4 , 5 , 6 , 7 , 8 Biologics are medicines that have been isolated from natural sources, although they may be produced by artificial methods. Basophil‐related biologics such as IgE (Omalizumab), IL‐4 receptor α chain (Dupilumab), IL‐5 (Reslizumab and Mepolizumab), IL‐5 receptor α chain (Benralizumab) and IL‐13 (Tralokinumab) have been approved by the US Food and Drug Administration (FDA) for the treatment of allergic diseases (Table 1, Figure 1).
Table 1.
US Food and Drug Administration (FDA) approved biologics related to basophils
| Characteristic | Therapeutic target | Mechanism of action | Indications | Prescribing information |
|---|---|---|---|---|
| Omalizumab (XOLAIR®) | IgE | Binds to free IgE, inhibits binding to FcεRI on mast cells, basophils, and plasmacytoid dendritic cells; FcεRII on dendritic cells and eosinophils | Moderate–severe asthma and allergic asthma; chronic idiopathic urticaria; nasal polyps; food allergies | https://www.gene.com/download/pdf/xolair_prescribing.pdf |
| Dupilumab (DUPIXENT®) | IL‐4Ra | Binds to IL‐4Ra, blocking the downstream effects of both IL‐4 and IL‐13 | Moderate–severe asthma with an eosinophilic phenotype; moderate‐severe atopic dermatitis; chronic RSwNP; eosinophilic esophagitis | https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/761055lbl.pdf |
| Tralokinumab (ADBRYTM) | IL‐13 | Binds to free/circulating IL‐13, thereby inhibiting interaction with its receptor | Moderate–severe atopic dermatitis in adults | https://www.accessdata.fda.gov/drugsatfda_docs/nda/2022/761180Orig1s000lbl.pdf |
| Mepolizumab (NUCALA) | IL‐5 | Binds to free/circulating IL‐5, thereby inhibiting interaction with its receptor | Severe asthma with an eosinophilic phenotype; EGPA | https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/761122s000lbl.pdf |
| Reslizumab (Cinqair) | IL‐5 | Binds to free/circulating IL‐5, thereby inhibiting interaction with its receptor | Severe asthma with an eosinophilic phenotype | https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/761033lbl.pdf |
| Benralizumab (FASENRA®) | IL‐5Ra | Binds to the IL‐5Ra chain, resulting in rapid depletion of eosinophils via antibody‐dependent cellular cytotoxicity | Severe asthma with an eosinophilic phenotype | https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/761070s000lbl.pdf |
| Tezepelumab (TEZSPIRE®) | TSLP | Binds to TSLP, thereby inhibiting interaction with its receptor | Add‐on maintenance treatment in severe asthma | https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/761224s000lbl.pdf |
Figure 1.
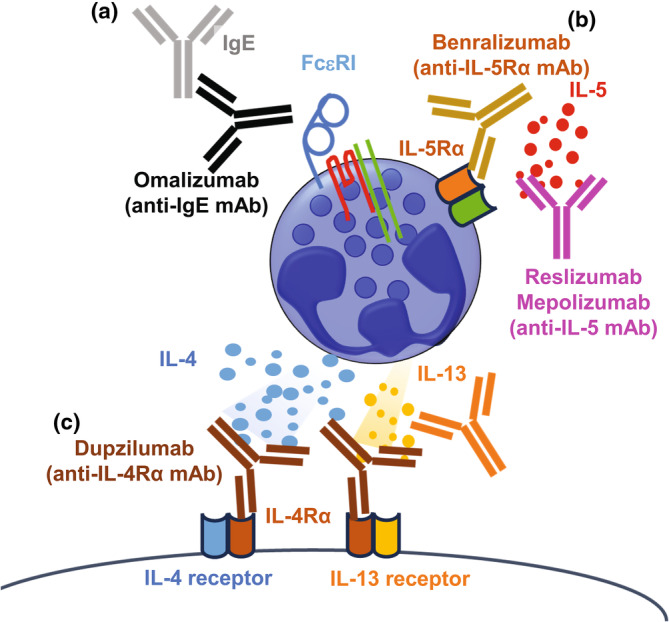
Biologics related to basophils. (a) Omalizumab binds to free IgE to inhibit binding to FcεRI on basophils. (b) Benzralizumab binds to IL‐5Rα and Mepolizumab binds to free IL‐5 to inhibit interaction between IL‐5 receptor and IL‐5. (c) Dupilumab binds to IL‐4Rα to block downstream effects of both IL‐4 and IL‐13. Taralokinumab binds to free IL‐13 to inhibit the interaction with its receptor.
Type 2 epithelial cytokines are produced mainly from epithelial cells, induce type 2 immune responses and are associated with allergic disease and helminth infection. 9 It has been well established that basophils are involved in thymic stromal lymphopoietin (TSLP) and IL‐33‐mediated response to allergens and helminths. 5 , 10 , 11 The role of IL‐25 has also been discussed in relation to the functions of basophils. 12 , 13 Specifically, prior studies have shown that basophils are a source of IL‐25, express IL‐4 and IL‐13 and upregulate surface markers in response to IL‐25 through their IL‐25 receptors; however, another report showed that basophils do not respond to IL‐25. 12 , 14 , 15 Despite these findings, we have not found any clinical trials targeting IL‐25 and its receptor in basophil‐related diseases. In this review, we focus on both basic and clinical research investigating basophils and type 2 epithelial cytokines of TSLP and IL‐33.
Research indicates that basophils display some activities in homeostasis and have immune suppressive functions. 16 , 17 Specifically, mouse basophils produce Amphiregulin, which activates regulatory T cells (Tregs) in mouse models of UVB‐induced suppression of contact hypersensitivity, IL‐4 and other factors that support the resolution of inflammation in atopic skin inflammation, improve myocardial infarction and prime group 2 innate lymphoid cells (ILC2s) in response to neuron‐derived signals for homeostasis of tissue integrity. 18 , 19 , 20 Conversely, human Tregs activate basophils to produce pro‐inflammatory cytokines and upregulate expression of basophil activation markers such as CD69 and CD203c in vitro. 19 , 21 Additionally, the role of basophils has been discussed in the progression of several cancers, but the relationship with TSLP and IL‐33 remains to be discovered. 22 , 23 , 24 Understanding novel functions of basophils may help to better understand the outcomes from biologics related to basophils in clinical trials.
Thymic stromal lymphopoietin
TSLP belongs to the IL‐2 cytokine family, has four α‐helix bundle structures, 25 and is expressed by epithelial cells at the barrier surfaces of organs including the lungs, skin and gastrointestinal tract in both homeostatic and inflammatory conditions. 26 , 27 , 28 , 29 Haematopoietic cells, including dendritic cells, basophils and mast cells, are also sources of TSLP. 30 , 31 , 32 Mechanical injury, infection, inflammatory cytokines and proteases such as trypsin and papain can initiate release of TSLP from epithelial cells. 26 , 33 , 34 Two different isoforms of TSLP are known in humans. The long isoform is upregulated in human bronchial epithelial cells and keratinocytes by stimulation with toll‐like receptor ligands, whereas the short isoform is mostly decreased or unaltered by those stimulations. It is unknown whether mice have multiple isoforms; however, administration of the synthetic short form TSLP reduced house dust mite‐induced inflammatory response in mice and prevented signalling of long form TSLP. 35
The receptor for TSLP is composed of a TSLP Receptor (TSLPR) subunit and IL‐7 receptor α chain. 25 TSLP signalling has both homeostatic and inflammatory functions. TSLP has anti‐apoptotic effects to induce expression of Bcl‐xL in primary human bronchial epithelial cells and Bcl‐2 in both human and mouse breast cancer cells. 36 , 37 Growth of human cervical cancer cells is associated with TSLP autocrine. 38 Additionally, TSLP also contributes to anti‐apoptotic effects on intestinal epithelial cells in both humans and mice through induction of an endogenous inhibitor, secretory leukocyte peptidase inhibitor (SLPI), for neutrophil elastase that contributes to inflammatory bowel diseases. 39 , 40 , 41 , 42 TSLP has also been implicated in regulating Tregs in the skin, thymus and gut, leading to anti‐inflammatory effects in both humans and mice. 43 , 44 , 45 Dendritic cell (DC)‐derived TSLP is important for mouse gut homeostasis. 46 , 47 TSLP signalling on DCs promotes Tregs in the gut to prevent bacteria‐mediated inflammation and TSLP signalling via interaction between DCs and T cells promotes Treg development, resulting in protection against colitis in a mouse model. 45 , 47 Intriguingly, TSLP‐responding Tregs are associated with progression of colorectal cancer in both humans and mice. 48
TSLP also initiates inflammation via type 2 immune responses in haematopoietic cells such as eosinophilia. 49 TSLP signalling induces activation of DCs to differentiate T helper 2 (Th2) cells through upregulation of OX40L. 50 TSLP acts on T cells and B cells to modulate T‐cell differentiation and antibody production, respectively. 51 , 52
In some models, ILC2s are critical for TSLP‐associated allergic inflammation, especially steroid‐resistant allergic airway inflammation. 53 , 54 , 55 , 56 , 57 , 58 Patients with atopic allergic diseases such as AD, asthma, allergic rhinoconjunctivitis and eosinophilic esophagitis (EoE) are known to have dysregulated expression of TSLP linked to genetic variants of TSLP. 59 , 60 Overexpression of TSLP has been reported in AD, Netherton syndrome, asthma, COPD and EoE. 27 , 61 Some sensory neurons expressing TSLPR can drive the itch reaction in allergic diseases such as atopic dermatitis (AD) in response to TSLP. 62 , 63 Blockage of TSLP signalling has been tested in clinical trials in several diseases such as asthma, atopic dermatitis, cat allergy and EoE (Table 2). Tezepelumab (anti‐TSLP antibody) was approved for severe, uncontrolled asthma by the FDA in 2022. 64 Tezepelumab has been tested in a Phase 3 trial (NCT05583227) for efficacy and safety in patients with EoE. In another trial, subcutaneous Tezepelumab did not reach statistically significant improvements in patients with moderate‐to‐severe atopic dermatitis. 65
Table 2.
Clinical trials of biologics targeting TSLP (from ClinicalTrials.gov, accessed 15 November 2024)
| Study title | Study status | Conditions | Biologics | Character of biologics | Phases | Identifier |
|---|---|---|---|---|---|---|
| A study to evaluate the safety and efficacy of GR2002 injection in patients with atopic dermatitis | Not yet recruiting | Atopic dermatitis | GR2002 injection | Anti‐TSLP bispecific antibody | Phase 1 | NCT06175143 |
| First‐in‐human study of SAR443765 in healthy participants and in asthmatic participants | Completed | Asthma | SAR443765 | Bifunctional nanobody blocking TSLP and IL‐13 | Phase 1 | NCT05366764 |
| Single ascending doses study of anti‐interleukin‐7 receptor α monoclonal antibody (GSK2618960) in healthy volunteers | Completed | Autoimmune diseases | GSK2618960 | Anti‐IL‐7 receptor a mAb | Phase 1 | NCT02293161 |
| Anti‐TSLP (AMG 157) plus antigen‐specific immunotherapy for induction of tolerance in individuals with cat allergy | Completed |
Cat allergy Cat hypersensitivity |
AMG 157 | Anti‐TSLP mAb |
Phase 1 Phase 2 |
NCT02237196 |
| A extension clinical study of TQC2731 injection in the treatment of chronic sinusitis with nasal polyps | Recruiting | Chronic rhinosinusitis with nasal polyps | TQC2731 | Anti‐TSLP mAb | Phase 2 | NCT06451640 |
| Tezepelumab in allergic rhinitis and asthma study (TEZARS) | Recruiting | Asthma with allergic rhinitis | Tezepelumab | Anti‐TSLP mAb | Phase 2 | NCT06189742 |
| A clinical study of TQC2731 injection in the treatment of chronic rhinosinusitis with nasal polyps | Recruiting | Chronic sinusitis nasal polyps | TQC2731 injection | Anti‐TSLP mAb | Phase 2 | NCT06036927 |
| Tezepelumab and methacholine airway hyperresponsiveness in participants with mild allergic asthma | Not yet recruiting | Asthma, allergic | Tezepelumab | Anti‐TSLP mAb | Phase 2 | NCT05740748 |
| Effects of blocking TSLP on airway inflammation and the epithelial immune‐response to exacerbation triggers in patients with COPD | Recruiting |
COPD COPD exacerbation COPD bronchitis airway disease|immune system disorder |
Tezepelumab | Anti‐TSLP mAb | Phase 2 | NCT05507242 |
| Evaluate the efficacy and safety of TQC2731 injection in patients with severe asthma | Unknown | Severe asthma | TQC2731 | Anti‐TSLP mAb | Phase 2 | NCT05472324 |
| Effects of anti‐TSLP in patients with asthma | Completed | Asthma | MEDI9929 | Anti‐TSLP mAb | Phase 2 | NCT02698501 |
| Tezepelumab on airway structure and function in patients with uncontrolled moderate‐to‐severe asthma | Recruiting | Asthma | Tezepelumab | Anti‐TSLP mAb | Phase 3 | NCT05280418 |
TSLP and basophils
The role of basophils has been investigated in TSLP‐dependent inflammation in mice, using topical vitamin D3 analogue (MC903)‐induced skin inflammation, which is akin to atopic dermatitis in humans. In this model, basophils lacking expression of IL‐4, Il4 3′ untranslated region (Il4 3′UTR) mice, exhibit impairment of skin inflammation including swelling, reduction of levels of antigen‐specific IgE in serum and production of type 2 cytokines in draining lymph nodes. 66 Human basophils in peripheral blood lack the function of antigen presentation because of low or negative expression of MHC class II and co‐stimulatory molecules, 67 , 68 whereas mouse basophils exhibit antigen presentation and contribute to an increase in Th2 cells. Mouse basophils can acquire the expression MHC class II complexes by trogocytosis, which is the transfer of a part of the cellular membrane including surface proteins via cell‐contact from dendritic cells after treatment of mice with MC903 plus antigen 69 (Figure 2).
Figure 2.
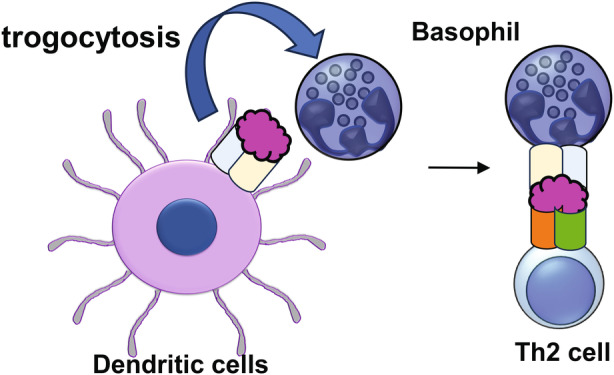
Basophils are acquired to express MHC class II complexes by trogocytosis from dendritic cells after treatment of mice with MC903, leading to induction of Th2 cells.
Basophils are also involved in oral allergen‐induced anaphylactic reactions in mice sensitised with TSLP. 70 Basophils promote differentiation of Th2 cells through production of IL‐4 during Trichinella spiraris (Ts), Heligmosomoides polygyrus (Hp) and Litomosomoides sigmodontis filaria infections. 71 , 72 , 73 In addition, TSLPR‐deficiency but not that of IL‐3R impairs basophils in the draining lymph nodes during Ts infection, leading to reduction of Th2 cells. These suggest that basophil‐derived IL‐4 can enhance Th2 responses in TSLP sensitised mice. In addition, basophils produce IL‐4 to enhance subsequent production of ILC2 in response to TSLP in skin inflammation. 74 However, bone marrow chimera experiments exhibiting TSLPR‐deficiency on basophils did not confer protection from the inflammation in skin or reduce level of IgE in serum in topical MC903‐induced skin inflammation. 5 This experiment was conducted by transferring bone marrow cells from Mcpt8Cre mice, 2 which lack basophils by Cre toxicity only in basophils, into either wild type (WT) mice as a control or TSLPRα−/− mice as basophil‐specific TSLPR‐deficient mice. Furthermore, TSLPR‐floxed mice crossed with Mcpt8 cre , 75 Mcpt8 cre Tslpr fl/fl mice, in a pulmonary inflammation model did not alter the magnitude of the airway inflammation, increase in Th2 cells, or IgE titres in serum after administration of intranasal antigen with topical MC903 treatment in the skin. This suggests that TSLPR on DC and CD4+T cells, but not basophils nor ILC2s, are predominantly reactive to TSLP in this type 2 inflammation. 76 Although the above two independent mouse lines expressing Cre recombinase under gene locus of mouse mast cell protease 8 (mMCP8) have been established, 2 , 75 expression of mMCP8 is not critically restricted to basophils; the expression of mMCP8 has been detected in various cell lineages, including a type of macrophage and inflammatory mast cells. 77 , 78 , 79 , 80 Thus, further studies are needed to clarify the role of basophils and TSLP via methods using mouse lines expressing Cre recombinase in basophils by other genes, such as Cpa3‐Cre mouse 81 or using antibodies for depletion of basophils such as anti‐FcεRiα antibody (MAR‐1) and anti‐CD200R3 Ab (Ba103 and Ba160, which are the same hybridomas as both of them have the same characters including CDR3 sequences and were generated in the single experiment). 1 , 2 , 79 , 80 , 82 , 83
It is unclear whether human basophils can respond to TSLP. The TSLP risk allele for EoE is associated with an increase in frequency of circulating basophils. 84 A different single nucleotide polymorphism of the TSLP allele is also associated with a number of basophils. 85 Two independent groups showed that activated basophils express TSLPR and basophils from patients with allergy upregulate expression of CD203c and release histamine in response to TSLP, which is compatible with IgE‐cross linking and IL‐3. 86 , 87 Rhinovirus infection, a risk factor for asthma exacerbations among atopic patients, also increases the expression of TSLPR on basophils. 88 Furthermore, TSLP promotes the differentiation of human CD34+ cells into basophils and eosinophils, which is also one of the ways to enhance basophil‐mediated allergic responses by TSLP. However, Salabert‐Le Guen et al. 89 showed that human basophils from healthy donors and patients with allergies express TSLPR on the cell surface, but do not express of IL‐7Rα and have altered phosphorylation of STAT5. Gambardella et al. 15 detected production of IL‐4, IL‐13 and CXCL8 from human basophils from healthy donors when in vitro stimulated with IL‐3 and IL‐33, but not TSLP and IL‐25.
Overall, basophils enhance type 2 immune responses, including allergic responses to TSLP stimulation in both humans and mice, although it remains unclear whether those responses of basophils to TSLP are because of initiation through TSLPR on basophils.
IL‐33
IL‐33 is an IL‐1 family cytokine identified by bioinformatic analysis of the human genome. The IL‐1 cytokine family contains 11 cytokines including IL‐1α, IL‐1β, IL‐18 and IL‐33. 90 , 91 Although IL‐1 cytokines are widely expressed in haematopoietic cells, which are associated with inflammatory responses and host defences, 92 , 93 IL‐33 is predominantly produced by epithelial cells such as alveolar type II cells in bronchus and airways, fibroblasts and smooth muscle cells. IL‐33 is hardly detected in mouse blood vessels during homeostasis, whereas human endothelial cells are known to express IL‐33 constitutively and to be a major source of IL‐33 mRNA in inflamed tissues from patients with rheumatoid arthritis, psoriasis and Crohn's disease. 94 , 95 , 96 , 97 In human, bronchial epithelial cells and endothelial cells are the major sources of IL‐33 in human lungs, whereas IL‐33 is mainly expressed in alveolar type 2 cells in mice. 98 , 99 , 100 During mouse lung development, IL‐33, mainly expressed in alveolar type 2 cells, guides the maturation and immunomodulatory functions of alveolar macrophages through activation of basophils. 100 The IL‐33 receptor consists of IL‐1 receptor accessory protein (IL‐1RAP) and suppression of tumorigenicity 2 (ST2). IL‐1RAP is a ubiquitous protein, also associated with IL‐1 receptor type 1 (IL‐1R1) and the IL‐36 receptor. 101 Expression of ST2 is restricted to a subset of T cells, including pathogenic memory Th2 cells (Tpath2), ILC2s, basophils, mast cells, eosinophils and macrophages in humans and mice. 102 , 103 , 104 , 105 , 106 , 107 , 108 , 109 , 110 Although residential Tregs also highly express ST2 compared to circulating Tregs, IL‐33/ST2 axis is dispensable for accumulation and residence in nonlymphoid organs. 111 , 112 The IL‐33/ST2‐IL‐1RAP axis induces myeloid differentiation primary response gene 88 (MyD88), IL‐1 receptor‐associated kinase 1 and 4 and TNF receptor associated factor 6. 113 , 114 The IL‐33 signalling can inhibit proliferation and induce apoptosis in MIA PaCa‐2, a human pancreatic cancer cell line. 115
IL‐33 displays a role in homeostatic and inflammatory responses. Adventitial stromal cells in mouse lung parenchyma also express IL‐33 and TSLP, supporting accumulation and activation of ILC2 cells, which may contribute to subsequent inflammatory reactions. 116 In the bleomycin‐induced lung fibrosis mouse model, airway epithelial cells and alveolar macrophages produce IL‐33 to exacerbate lung fibrosis. 117 IL‐33 signalling contributes to protection against helminth infections in mice such as Heligmosomoides polygylus, L. sigmodontis, Nippostrongylus brasiliensis, Strongyloides ratti and Trichinella spiralis. 108 , 118 , 119 , 120 , 121 , 122 , 123
Elevation of the expression of IL‐33 has been shown in a large number of allergic disorders, including asthma, chronic rhinosinusitis, allergic rhinitis, atopic dermatitis and eosinophilic esophagitis. 124 , 125 , 126 , 127 , 128 , 129 Insulation of cell or tissues, exposure to allergens and infection with nematodes or viruses trigger IL‐33 release in humans and mice. Airborne allergens of the products from fungi including Alternaria alternata and Aspergillus fumigatus, German cockroach and house dust mite contain proteinase activity. Protease allergens can activate proteinase‐activated receptor‐2 (PAR‐2) through oxidative stress and ATP secretion, resulting in IL‐33 release. 107 , 130 , 131 , 132 Laundry detergents and surfactants also have activity to increase oxidative stress that induce expression of IL‐33 from airway epithelial cells. 133 In addition, cigarette smoke induces expression of IL‐33. In the case of COPD, tobacco smoke plus viral damage elevates severity of COPD via increase in IL‐33. 134 IL‐33 expression in epithelial cells and endothelial cells is also increased by other various stimuli including diesel exhaust particles (DEPs), chitin, silica crystals, hydroxypropyl‐b‐cyclodextrin, viral infection and Toll‐like receptor ligands. 135 , 136 , 137 , 138 , 139 , 140 , 141 , 142 Genetic studies have shown significant associations between IL1RL1 and IL33 genetic variants and allergic diseases such as asthma, atopic dermatitis and EoE in humans. 143 , 144 , 145 , 146 , 147 , 148 , 149 , 150 , 151 Anti‐IL‐33 antibodies have been tested as therapies for pulmonary diseases including asthma and COPD as a Phase II trial (Table 3). Treatment with anti‐IL‐33 receptor antibodies (CNTO 7160) blocks IL‐33 signalling in mild asthma; however, clinical benefits were not found for patients in this clinical trial. 152 In the other clinical trial, patients with uncontrolled asthma may have clinical benefits from treatment with anti‐IL‐33 receptor antibodies compared to placebo (GSK37772847), but more studies are needed. 153 Treatment with Etokimab, an IL‐33 antibody, desensitised peanut‐allergic individuals to efficiently tolerate peanut protein with fewer adverse events and reducing Th2 cytokine production and serum IgE for peanuts compared to placebo in Phase 2a of a clinical trial. 154 Furthermore, in a Phase 2a study for patients with moderate‐to‐severe atopic dermatitis, administration of Etokimab improved eczema area and severity index (EASI) 50 and EASI75 scores and reduced eosinophils in peripheral blood. 155
Table 3.
Clinical trials of biologics targeting IL‐33/ST2 (from ClinicalTrials.gov, accessed 15 November 2024)
| Study title | Study status | Conditions | Biologics | Character of biologics | Phases | Identifier |
|---|---|---|---|---|---|---|
| A preliminary study to evaluate PF‐07264660 in healthy participants | Completed | Healthy | PF‐07264660 | Tri‐specific monoclonal antibody targeting IL‐4 IL‐13 and IL‐33 | Phase 1 | NCT05496738 |
| A study to evaluate the safety and tolerability, pharmacokinetics (PK) and pharmacodynamics (PD) of Melrilimab (GSK3772847) in healthy participants | Completed | Asthma healthy volunteers | Melrilimab/GSK3772847 | Anti‐IL‐33 receptor mAb | Phase 1 | NCT04366349 |
| Study of REGN3500 and Dupilumab in patients with asthma | Completed | Asthma, allergic |
REGN3500 Dupilumab |
Anti‐IL‐33 mAb anti‐IL‐4 and ‐13 mAb | Phase 1 | NCT03112577 |
| A proof‐of‐concept study to assess the efficacy, safety and tolerability of Itepekimab (anti‐IL‐33 mAb) in participants with chronic rhinosinusitis without nasal polyps | Not yet recruiting | Chronic rhinosinusitis without nasal polyps | Itepekimab/SAR440340 | Anti‐IL‐33 mAb | Phase 2 | NCT06691113 |
| A proof‐of‐concept study to assess the efficacy, safety and tolerability of Itepekimab (anti‐IL‐33 mAb) in participants with non‐cystic fibrosis bronchiectasis | Recruiting | Bronchiectasis | Itepekimab/SAR440340 | Anti‐IL‐33 mAb | Phase 2 | NCT06280391 |
| Mechanistic study of the effect of Itepekimab on airway inflammation in patients with COPD | Recruiting | COPD | Itepekimab/SAR440340 | Anti‐IL‐33 mAb | Phase 2 | NCT05326412 |
| A phase II, randomised, double‐blind, placebo‐controlled study to assess MEDI3506 in participants with COPD and chronic bronchitis | Completed |
COPD Chronic bronchitis |
MEDI3506 | Anti‐IL‐33 mAb | Phase 2 | NCT04631016 |
| Study to assess the efficacy and safety of MEDI3506 in adults with uncontrolled moderate‐to‐severe asthma | Completed | Asthma | MEDI3506 | Anti‐IL‐33 mAb | Phase 2 | NCT04570657 |
| Anti‐ST2 (MSTT1041A) in COPD (COPD‐ST2OP) | Completed | COPD exacerbation | MSTT1041A | Anti‐ST2 mAb | Phase 2 | NCT03615040 |
| Proof‐of‐concept study to assess the efficacy, safety and tolerability of SAR440340 (anti‐IL‐33 mAb) in patients with moderate‐to‐severe chronic obstructive pulmonary disease (COPD) | Completed | COPD | SAR440340 | Anti‐IL‐33 mAb | Phase 2 | NCT03546907 |
| Efficacy and safety study of GSK3772847 in subjects with moderately severe asthma | Completed | Asthma | GSK3772847 | Anti‐IL‐33 receptor mAb | Phase 2 | NCT03207243 |
| A study to learn about two study medicines (PF‐07275315 And PF‐07264660) in people who have moderate to severe atopic dermatitis | Recruiting | Atopic dermatitis | PF‐07264660 | Tri‐specific monoclonal antibody targeting IL‐4 IL‐13 and IL‐33 | Phase 2 | NCT05995964 |
| Efficacy and safety of Tozorakimab in patients hospitalised for viral lung infection requiring supplemental oxygen | Recruiting | Viral lung infection and acute respiratory failure | Tozorakimab/MEDI3506 | Anti‐IL‐33 mAb | Phase 3 | NCT05624450 |
| Study to assess the efficacy, safety, and tolerability of SAR440340/REGN3500/Itepekimab in chronic obstructive pulmonary disease (COPD) | Active not recruiting | COPD | Itepekimab/SAR440340 | Anti‐IL‐33 mAb | Phase 3 | NCT04751487 |
| Study to assess the efficacy, safety, and tolerability of SAR440340/REGN3500/Itepekimab in chronic obstructive pulmonary disease (COPD) | Active not recruiting | COPD | Itepekimab/SAR440340 | Anti‐IL‐33 mAb | Phase 3 | NCT04701983 |
IL‐33 and basophils
Basophils and mast cells, which are both granulocytes, are associated with allergic disease in mice and humans expressing ST2, a component of the IL‐33 receptor. 156 Treatment with IL‐33 activates basophils, leading to enhancement of the degranulation and production of pro‐inflammatory cytokines such as IL‐4, and IL‐13 in mouse. 157 , 158 IL‐33 itself also activates human basophils to degranulate and secrete various cytokines such as IL‐1b, IL‐4, IL‐5, IL‐6, IL‐8, IL‐13 and granulocyte‐macrophage stimulating factor. 158 , 159 , 160 IL‐33 activates NF‐kB and p38 MAP‐kinase on human basophils. 159 Intriguingly, treatment with long‐acting muscarinic antagonist alters the IL‐33‐induced IL‐4 production from basophils in both humans and mice, leading to amelioration of eosinophilic inflammation. 161 It has been shown that the expression of IL‐33 is elevated in allergic inflammation such as in atopic dermatitis and gastrointestinal inflammation. 148 , 162 , 163 In IL‐33 transgenic mice, basophils activate ILC2 cells to induce inflammatory processes such as atopic dermatitis. 164 IL‐33 induces activation of mouse basophils through their own ST2 receptor in epicutaneous sensitisation‐induced experimental eosinophilic esophagitis. 165 Humanised mouse models show that allergen plus IgE‐induced activation of human basophils and can confer allergic gut inflammation through production of platelet‐activating factor (PAF) and histamine. 166
In a mouse model of protease‐induced pulmonary inflammation, ILC2 and Th2 cells play a critical role in development phases of eosinophilic inflammation in response to type 2 epithelial cytokines; TSLP is important for Ovalbmin (OVA) immunisation‐induced Th2 cell responses, whereas IL‐33 activates ILC2 cells to promote Th2 cell responses. 167 The similarities between ILC2 and Th2 cells have been studied in chromatin levels. 168 In this case, IL‐4 from Th2 cells increase IL‐33‐induced ILC2 responses (Figure 3). In a similar context, basophils produce IL‐4 in response to IL‐33 to increase the production of type 2 cytokines, such as IL‐5 and IL‐13 and the chemokine CCL11 from ILC2s, resulting in elevation of eosinophilic airway inflammation. 169 , 170 It has been demonstrated that phosphodiesterase (PDE) 4 is involved in IL‐3 and IL‐33‐induced phosphorylation of ERK and subsequent IL‐4 production from mouse basophils in oxazolone‐induced skin inflammation. 171 In addition, treatment with long‐acting muscarinic antagonist alters this IL‐33‐induced IL‐4 production from basophils in both humans and mice, leading to amelioration of eosinophilic inflammation. 161 On the contrary, mast cells suppress pulmonary inflammation induced by treatment with papain through production of IL‐2 to promote Tregs in an IL‐33‐dependent manner. 107 These results suggest that the role of basophils is distinct from mast cells in IL‐33‐contributed airway inflammation. Collectively, since basophils have unique pathological roles in IL‐33‐contributed allergic diseases, it would be worth targeting both basophils and mast cells in potential therapies for IL‐33‐contributed allergic diseases.
Figure 3.
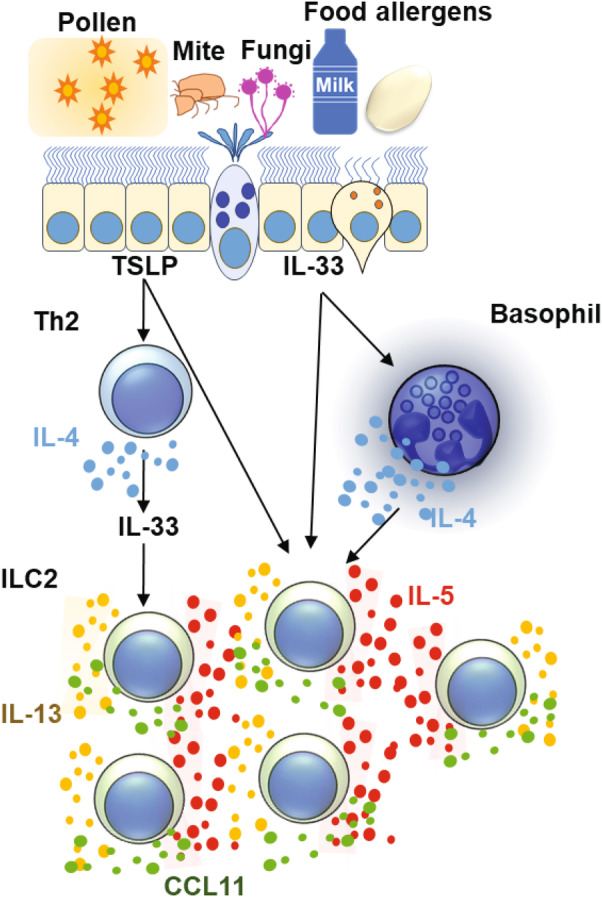
A model of type 2 airway inflammation. Various stimulations induce secretion of type 2 epithelial cytokines from epithelial cells in lung, subsequent activation of basophils and Th2 cells induce expansion and activation of ILC2 cells through production of IL‐4.
Future research directions
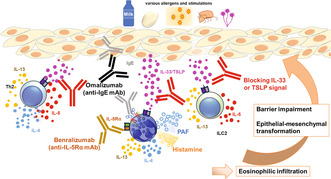
The studies summarised in this review indicate that type 2 epithelial cytokines of TSLP and IL‐33 play a role as positive modulators in activation of basophils both directly and also indirectly, leading to exacerbation of allergic reactions. In addition, anti‐IgE antibody therapy (Omalizumab) has preventive effects in food allergy (NCT03881696). 172 Results suggest that synergistic effects may be possible if we block both basophils and type 2 epithelial cytokines in allergic diseases. For example, a combination of antibodies for type 2 epithelial cytokines using anti‐IgE mAb (Omalizumab) or anti‐IL‐5Rα mAb (Benralizumab) treatment, which reduces circulating basophils in EoE 173 (Figure 4). A tri‐specific monoclonal antibody targeting IL‐4, IL‐13 and IL‐33 (PF‐07264660) and a bifunctional nanobody blocking TSLP and IL‐13 (SAR443765) are in this context. Administration of anti‐IL‐3 Rα (CD123) mAb (Talacotuzumab) also reduces the frequency of basophils in the blood 174 and reports suggest that the level of production of IL‐3 from phytohemagglutinin‐stimulated peripheral blood mononuclear cells is correlated with improvement of lung function in pre‐school children with asthma. 175 These future studies will provide novel therapeutic approaches to help patients suffering from allergic diseases.
Figure 4.
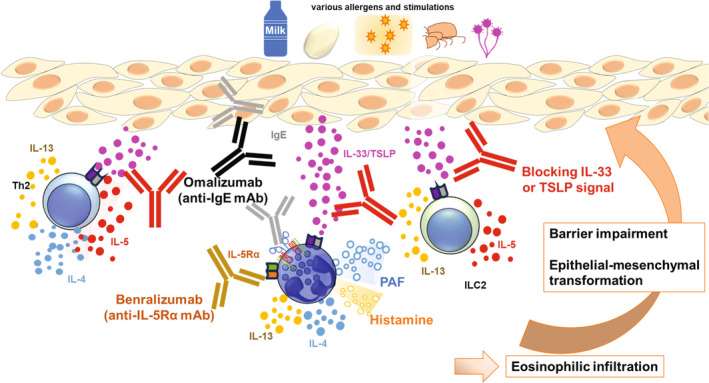
A proposed model of combination therapy with anti‐IL‐33/ST2 mAb or anti‐TSLP mAb plus blocking basophils for gastrointestinal inflammation. Because both type 2 epithelial cytokines and IgE complexes stimulate basophils to exacerbate gut inflammation and Th2 immune responses, it might be beneficial to block both type 2 epithelial cytokines and basophils. 84 , 165 , 166
Conclusion
We have summarised the role of basophils and type 2 epithelial cytokines, specifically TSLP and IL‐33, in allergic diseases and helminth infections in humans and mice. We hope this manuscript will help clinicians and scientists studying translational and basic science.
Conflict of interest
The authors declare no conflict of interest.
Author contributions
Kazushige Obata‐Ninomiya: Conceptualization; data curation; formal analysis; funding acquisition; investigation; supervision; validation; visualization; writing – original draft; writing – review and editing. Tharmalingam Jayaraman: Visualization. Steven F Ziegler: reading and advice.
Acknowledgments
We thank O Doyle, T Lawson and A Hocking for writing support; R Penn, Z Bishop, F Barry, M Woldetensae and R Snodgrass for technical support. We also thank Professor H Karasuyama and Dr C Pellefigues for contributing critical comments for this manuscript.
References
- 1. Obata K, Mukai K, Tsujimura Y et al. Basophils are essential initiators of a novel type of chronic allergic inflammation. Blood 2007; 110: 913–920. [DOI] [PubMed] [Google Scholar]
- 2. Ohnmacht C, Schwartz C, Panzer M, Schiedewitz I, Naumann R, Voehringer D. Basophils orchestrate chronic allergic dermatitis and protective immunity against helminths. Immunity 2010; 33: 364–374. [DOI] [PubMed] [Google Scholar]
- 3. Obata‐Ninomiya K, Ishiwata K, Tsutsui H et al. The skin is an important bulwark of acquired immunity against intestinal helminths. J Exp Med 2013; 210: 2583–2595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Nei Y, Obata‐Ninomiya K, Tsutsui H et al. GATA‐1 regulates the generation and function of basophils. Proc Natl Acad Sci USA 2013; 110: 18620–18625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Schwartz C, Eberle JU, Hoyler T, Diefenbach A, Lechmann M, Voehringer D. Opposing functions of thymic stromal lymphopoietin‐responsive basophils and dendritic cells in a mouse model of atopic dermatitis. J Allergy Clin Immunol 2016; 138: 1443–1446.e1448. [DOI] [PubMed] [Google Scholar]
- 6. Webb LM, Oyesola OO, Fruh SP et al. The notch signaling pathway promotes basophil responses during helminth‐induced type 2 inflammation. J Exp Med 2019; 216: 1268–1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wiebe D, Limberg MM, Gray N, Raap U. Basophils in pruritic skin diseases. Front Immunol 2023; 14: 1213138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Poto R, Loffredo S, Marone G et al. Basophils beyond allergic and parasitic diseases. Front Immunol 2023; 14: 1190034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Roan F, Obata‐Ninomiya K, Ziegler SF. Epithelial cell‐derived cytokines: more than just signaling the alarm. J Clin Invest 2019; 129: 1441–1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chan BCL, Lam CWK, Tam LS, Wong CK. IL33: roles in allergic inflammation and therapeutic perspectives. Front Immunol 2019; 10: 364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Saluja R, Ketelaar ME, Hawro T, Church MK, Maurer M, Nawijn MC. The role of the IL‐33/IL‐1RL1 axis in mast cell and basophil activation in allergic disorders. Mol Immunol 2015; 63: 80–85. [DOI] [PubMed] [Google Scholar]
- 12. Salter BM, Oliveria JP, Nusca G et al. IL‐25 and IL‐33 induce type 2 inflammation in basophils from subjects with allergic asthma. Respir Res 2016; 17: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yuan Q, Peng N, Xiao F et al. New insights into the function of interleukin‐25 in disease pathogenesis. Biomark Res 2023; 11: 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wang YH, Angkasekwinai P, Lu N et al. IL‐25 augments type 2 immune responses by enhancing the expansion and functions of TSLP‐DC‐activated Th2 memory cells. J Exp Med 2007; 204: 1837–1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gambardella AR, Poto R, Tirelli V et al. Differential effects of alarmins on human and mouse basophils. Front Immunol 2022; 13: 894163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chirumbolo S. State‐of‐the‐art review about basophil research in immunology and allergy: is the time right to treat these cells with the respect they deserve? Blood Transfus 2012; 10: 148–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pellefigues C, Naidoo K, Mehta P et al. Basophils promote barrier dysfunction and resolution in the atopic skin. J Allergy Clin Immunol 2021; 148: 799–812.e710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Meulenbroeks C, van Weelden H, Schwartz C et al. Basophil‐derived amphiregulin is essential for UVB irradiation‐induced immune suppression. J Invest Dermatol 2015; 135: 222–228. [DOI] [PubMed] [Google Scholar]
- 19. Sicklinger F, Meyer IS, Li X et al. Basophils balance healing after myocardial infarction via IL‐4/IL‐13. J Clin Invest 2021; 131: e136778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Inclan‐Rico JM, Ponessa JJ, Valero‐Pacheco N et al. Basophils prime group 2 innate lymphoid cells for neuropeptide‐mediated inhibition. Nat Immunol 2020; 21: 1181–1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sharma M, Das M, Stephen‐Victor E et al. Regulatory T cells induce activation rather than suppression of human basophils. Sci Immunol 2018; 3: eaan0829. [DOI] [PubMed] [Google Scholar]
- 22. Poto R, Gambardella AR, Marone G et al. Basophils from allergy to cancer. Front Immunol 2022; 13: 1056838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Marone G, Schroeder JT, Mattei F et al. Is there a role for basophils in cancer? Front Immunol 2020; 11: 2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. LaMarche NM, Hegde S, Park MD et al. An IL‐4 signalling axis in bone marrow drives pro‐tumorigenic myelopoiesis. Nature 2024; 625: 166–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Friend SL, Hosier S, Nelson A, Foxworthe D, Williams DE, Farr A. A thymic stromal cell line supports in vitro development of surface IgM+ B cells and produces a novel growth factor affecting B and T lineage cells. Exp Hematol 1994; 22: 321–328. [PubMed] [Google Scholar]
- 26. Soumelis V, Reche PA, Kanzler H et al. Human epithelial cells trigger dendritic cell mediated allergic inflammation by producing TSLP. Nat Immunol 2002; 3: 673–680. [DOI] [PubMed] [Google Scholar]
- 27. Ying S, O'Connor B, Ratoff J et al. Thymic stromal lymphopoietin expression is increased in asthmatic airways and correlates with expression of Th2‐attracting chemokines and disease severity. J Immunol 2005; 174: 8183–8190. [DOI] [PubMed] [Google Scholar]
- 28. Rochman Y, Leonard WJ. The role of thymic stromal lymphopoietin in CD8+ T cell homeostasis. J Immunol 2008; 181: 7699–7705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Taylor BC, Zaph C, Troy AE et al. TSLP regulates intestinal immunity and inflammation in mouse models of helminth infection and colitis. J Exp Med 2009; 206: 655–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sokol CL, Chu NQ, Yu S, Nish SA, Laufer TM, Medzhitov R. Basophils function as antigen‐presenting cells for an allergen‐induced T helper type 2 response. Nat Immunol 2009; 10: 713–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kashyap M, Rochman Y, Spolski R, Samsel L, Leonard WJ. Thymic stromal lymphopoietin is produced by dendritic cells. J Immunol 2011; 187: 1207–1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Moon PD, Kim HM. Thymic stromal lymphopoietin is expressed and produced by caspase‐1/NF‐kappaB pathway in mast cells. Cytokine 2011; 54: 239–243. [DOI] [PubMed] [Google Scholar]
- 33. Kato A, Favoreto S Jr, Avila PC, Schleimer RP. TLR3‐ and Th2 cytokine‐dependent production of thymic stromal lymphopoietin in human airway epithelial cells. J Immunol 2007; 179: 1080–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Allakhverdi Z, Comeau MR, Jessup HK et al. Thymic stromal lymphopoietin is released by human epithelial cells in response to microbes, trauma, or inflammation and potently activates mast cells. J Exp Med 2007; 204: 253–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Dong H, Hu Y, Liu L et al. Distinct roles of short and long thymic stromal lymphopoietin isoforms in house dust mite‐induced asthmatic airway epithelial barrier disruption. Sci Rep 2016; 6: 39559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Shubin NJ, Clauson M, Niino K et al. Thymic stromal lymphopoietin protects in a model of airway damage and inflammation via regulation of caspase‐1 activity and apoptosis inhibition. Mucosal Immunol 2020; 13: 584–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kuan EL, Ziegler SF. A tumor‐myeloid cell axis, mediated via the cytokines IL‐1alpha and TSLP, promotes the progression of breast cancer. Nat Immunol 2018; 19: 366–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Xie F, Liu LB, Shang WQ et al. The infiltration and functional regulation of eosinophils induced by TSLP promote the proliferation of cervical cancer cell. Cancer Lett 2015; 364: 106–117. [DOI] [PubMed] [Google Scholar]
- 39. Schmid M, Fellermann K, Fritz P, Wiedow O, Stange EF, Wehkamp J. Attenuated induction of epithelial and leukocyte serine antiproteases elafin and secretory leukocyte protease inhibitor in Crohn's disease. J Leukoc Biol 2007; 81: 907–915. [DOI] [PubMed] [Google Scholar]
- 40. Curciarello R, Sobande T, Jones S et al. Human neutrophil elastase proteolytic activity in ulcerative colitis favors the loss of function of therapeutic monoclonal antibodies. J Inflamm Res 2020; 13: 233–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Motta JP, Magne L, Descamps D et al. Modifying the protease, antiprotease pattern by elafin overexpression protects mice from colitis. Gastroenterology 2011; 140: 1272–1282. [DOI] [PubMed] [Google Scholar]
- 42. Reardon C, Lechmann M, Brustle A et al. Thymic stromal lymphopoetin‐induced expression of the endogenous inhibitory enzyme SLPI mediates recovery from colonic inflammation. Immunity 2011; 35: 223–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kashiwagi M, Hosoi J, Lai JF et al. Direct control of regulatory T cells by keratinocytes. Nat Immunol 2017; 18: 334–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hanabuchi S, Ito T, Park WR et al. Thymic stromal lymphopoietin‐activated plasmacytoid dendritic cells induce the generation of FOXP3+ regulatory T cells in human thymus. J Immunol 2010; 184: 2999–3007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Mosconi I, Geuking MB, Zaiss MM et al. Intestinal bacteria induce TSLP to promote mutualistic T‐cell responses. Mucosal Immunol 2013; 6: 1157–1167. [DOI] [PubMed] [Google Scholar]
- 46. Messerschmidt JL, Azin M, Dempsey KE, Demehri S. TSLP/dendritic cell axis promotes CD4+ T cell tolerance to the gut microbiome. JCI Insight 2023; 8: e160690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Spadoni I, Iliev ID, Rossi G, Rescigno M. Dendritic cells produce TSLP that limits the differentiation of Th17 cells, fosters Treg development, and protects against colitis. Mucosal Immunol 2012; 5: 184–193. [DOI] [PubMed] [Google Scholar]
- 48. Obata‐Ninomiya K, de Jesus Carrion S, Hu A, Ziegler SF. Emerging role for thymic stromal lymphopoietin‐responsive regulatory T cells in colorectal cancer progression in humans and mice. Sci Transl Med 2022; 14: eabl6960. [DOI] [PubMed] [Google Scholar]
- 49. Rochman Y, Kotliar M, Ben‐Baruch Morgenstern N, Barski A, Wen T, Rothenberg ME. TSLP shapes the pathogenic responses of memory CD4+ T cells in eosinophilic esophagitis. Sci Signal 2023; 16: eadg6360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ito T, Wang YH, Duramad O et al. TSLP‐activated dendritic cells induce an inflammatory T helper type 2 cell response through OX40 ligand. J Exp Med 2005; 202: 1213–1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Domeier PP, Rahman ZSM, Ziegler SF. B cell‐ and T cell‐intrinsic regulation of germinal centers by thymic stromal lymphopoietin signaling. Sci Immunol 2023; 8: eadd9413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Kitajima M, Lee HC, Nakayama T, Ziegler SF. TSLP enhances the function of helper type 2 cells. Eur J Immunol 2011; 41: 1862–1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Kim BS, Siracusa MC, Saenz SA et al. TSLP elicits IL‐33‐independent innate lymphoid cell responses to promote skin inflammation. Sci Transl Med 2013; 5: 170ra116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Verma M, Liu S, Michalec L, Sripada A, Gorska MM, Alam R. Experimental asthma persists in IL‐33 receptor knockout mice because of the emergence of thymic stromal lymphopoietin‐driven IL‐9+ and IL‐13+ type 2 innate lymphoid cell subpopulations. J Allergy Clin Immunol 2018; 142: 793–803.e798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Stier MT, Bloodworth MH, Toki S et al. Respiratory syncytial virus infection activates IL‐13‐producing group 2 innate lymphoid cells through thymic stromal lymphopoietin. J Allergy Clin Immunol 2016; 138: 814–824.e811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Duerr CU, McCarthy CD, Mindt BC et al. Type I interferon restricts type 2 immunopathology through the regulation of group 2 innate lymphoid cells. Nat Immunol 2016; 17: 65–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Kabata H, Moro K, Fukunaga K et al. Thymic stromal lymphopoietin induces corticosteroid resistance in natural helper cells during airway inflammation. Nat Commun 2013; 4: 2675. [DOI] [PubMed] [Google Scholar]
- 58. Liu S, Verma M, Michalec L et al. Steroid resistance of airway type 2 innate lymphoid cells from patients with severe asthma: the role of thymic stromal lymphopoietin. J Allergy Clin Immunol 2018; 141: 257–268.e256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Cianferoni A, Spergel J. The importance of TSLP in allergic disease and its role as a potential therapeutic target. Expert Rev Clin Immunol 2014; 10: 1463–1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Ziegler SF. Thymic stromal lymphopoietin and allergic disease. J Allergy Clin Immunol 2012; 130: 845–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Shikotra A, Choy DF, Ohri CM et al. Increased expression of immunoreactive thymic stromal lymphopoietin in patients with severe asthma. J Allergy Clin Immunol 2012; 129: 104–111.e101–109. [DOI] [PubMed] [Google Scholar]
- 62. Wilson SR, The L, Batia LM et al. The epithelial cell‐derived atopic dermatitis cytokine TSLP activates neurons to induce itch. Cell 2013; 155: 285–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Oetjen LK, Mack MR, Feng J et al. Sensory neurons co‐opt classical immune signaling pathways to mediate chronic itch. Cell 2017; 171: 217–228.e213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Menzies‐Gow A, Corren J, Bourdin A et al. Tezepelumab in adults and adolescents with severe, Uncontrolled asthma. N Engl J Med 2021; 384: 1800–1809. [DOI] [PubMed] [Google Scholar]
- 65. Simpson EL, Parnes JR, She D et al. Tezepelumab, an anti‐thymic stromal lymphopoietin monoclonal antibody, in the treatment of moderate to severe atopic dermatitis: a randomized phase 2a clinical trial. J Am Acad Dermatol 2019; 80: 1013–1021. [DOI] [PubMed] [Google Scholar]
- 66. Hussain M, Borcard L, Walsh KP et al. Basophil‐derived IL‐4 promotes epicutaneous antigen sensitization concomitant with the development of food allergy. J Allergy Clin Immunol 2018; 141: 223–234.e225. [DOI] [PubMed] [Google Scholar]
- 67. Sharma M, Hegde P, Aimanianda V et al. Circulating human basophils lack the features of professional antigen presenting cells. Sci Rep 2013; 3: 1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Voskamp AL, Prickett SR, Mackay F, Rolland JM, O'Hehir RE. MHC class II expression in human basophils: induction and lack of functional significance. PLoS One 2013; 8: e81777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Miyake K, Shiozawa N, Nagao T, Yoshikawa S, Yamanishi Y, Karasuyama H. Trogocytosis of peptide‐MHC class II complexes from dendritic cells confers antigen‐presenting ability on basophils. Proc Natl Acad Sci USA 2017; 114: 1111–1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Han H, Thelen TD, Comeau MR, Ziegler SF. Thymic stromal lymphopoietin‐mediated epicutaneous inflammation promotes acute diarrhea and anaphylaxis. J Clin Invest 2014; 124: 5442–5452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Giacomin PR, Siracusa MC, Walsh KP et al. Thymic stromal lymphopoietin‐dependent basophils promote Th2 cytokine responses following intestinal helminth infection. J Immunol 2012; 189: 4371–4378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Schwartz C, Turqueti‐Neves A, Hartmann S, Yu P, Nimmerjahn F, Voehringer D. Basophil‐mediated protection against gastrointestinal helminths requires IgE‐induced cytokine secretion. Proc Natl Acad Sci USA 2014; 111: E5169–E5177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Torrero MN, Hubner MP, Larson D, Karasuyama H, Mitre E. Basophils amplify type 2 immune responses, but do not serve a protective role, during chronic infection of mice with the filarial nematode Litomosoides sigmodontis . J Immunol 2010; 185: 7426–7434. [DOI] [PubMed] [Google Scholar]
- 74. Kim BS, Wang K, Siracusa MC et al. Basophils promote innate lymphoid cell responses in inflamed skin. J Immunol 2014; 193: 3717–3725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Sullivan BM, Liang HE, Bando JK et al. Genetic analysis of basophil function in vivo . Nat Immunol 2011; 12: 527–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Kabata H, Flamar AL, Mahlakoiv T et al. Targeted deletion of the TSLP receptor reveals cellular mechanisms that promote type 2 airway inflammation. Mucosal Immunol 2020; 13: 626–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Pellefigues C, Mehta P, Prout MS et al. The Basoph8 mice enable an unbiased detection and a conditional depletion of basophils. Front Immunol 2019; 10: 2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Satoh T, Nakagawa K, Sugihara F et al. Identification of an atypical monocyte and committed progenitor involved in fibrosis. Nature 2017; 541: 96–101. [DOI] [PubMed] [Google Scholar]
- 79. Derakhshan T, Samuchiwal SK, Hallen N et al. Lineage‐specific regulation of inducible and constitutive mast cells in allergic airway inflammation. J Exp Med 2021; 218: e20200321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Alvarado‐Vazquez PA, Cardenas EI, Das A, Hallgren J. Depletion of Mcpt8‐expressing cells reduces lung mast cells in mice with experimental asthma. Allergy 2023; 78: 1363–1366. [DOI] [PubMed] [Google Scholar]
- 81. Lilla JN, Chen CC, Mukai K et al. Reduced mast cell and basophil numbers and function in Cpa3‐Cre; Mcl‐1fl/fl mice. Blood 2011; 118: 6930–6938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Sokol CL, Barton GM, Farr AG, Medzhitov R. A mechanism for the initiation of allergen‐induced T helper type 2 responses. Nat Immunol 2008; 9: 310–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Sawaguchi M, Tanaka S, Nakatani Y et al. Role of mast cells and basophils in IgE responses and in allergic airway hyperresponsiveness. J Immunol 2012; 188: 1809–1818. [DOI] [PubMed] [Google Scholar]
- 84. Noti M, Wojno ED, Kim BS et al. Thymic stromal lymphopoietin‐elicited basophil responses promote eosinophilic esophagitis. Nat Med 2013; 19: 1005–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Astle WJ, Elding H, Jiang T et al. The allelic landscape of human blood cell trait variation and links to common complex disease. Cell 2016; 167: 1415–1429.e1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Siracusa MC, Saenz SA, Hill DA et al. TSLP promotes interleukin‐3‐independent basophil haematopoiesis and type 2 inflammation. Nature 2011; 477: 229–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Salter BM, Oliveria JP, Nusca G et al. Thymic stromal lymphopoietin activation of basophils in patients with allergic asthma is IL‐3 dependent. J Allergy Clin Immunol 2015; 136: 1636–1644. [DOI] [PubMed] [Google Scholar]
- 88. Agrawal R, Wisniewski J, Yu MD et al. Infection with human rhinovirus 16 promotes enhanced IgE responsiveness in basophils of atopic asthmatics. Clin Exp Allergy 2014; 44: 1266–1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Salabert‐Le Guen N, Hemont C, Delbove A et al. Thymic stromal lymphopoietin does not activate human basophils. J Allergy Clin Immunol 2018; 141: 1476–1479.e1476. [DOI] [PubMed] [Google Scholar]
- 90. Fields JK, Gunther S, Sundberg EJ. Structural basis of IL‐1 family cytokine signaling. Front Immunol 2019; 10: 1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Gonzalez L, Rivera K, Andia ME, Martinez Rodriguez G. The IL‐1 family and its role in atherosclerosis. Int J Mol Sci 2022; 24: 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Dinarello CA. Interleukin‐1. Cytokine Growth Factor Rev 1997; 8: 253–265. [DOI] [PubMed] [Google Scholar]
- 93. Palomo J, Dietrich D, Martin P, Palmer G, Gabay C. The interleukin (IL)‐1 cytokine family – balance between agonists and antagonists in inflammatory diseases. Cytokine 2015; 76: 25–37. [DOI] [PubMed] [Google Scholar]
- 94. Baekkevold ES, Roussigne M, Yamanaka T et al. Molecular characterization of NF‐HEV, a nuclear factor preferentially expressed in human high endothelial venules. Am J Pathol 2003; 163: 69–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Carriere V, Roussel L, Ortega N et al. IL‐33, the IL‐1‐like cytokine ligand for ST2 receptor, is a chromatin‐associated nuclear factor in vivo . Proc Natl Acad Sci USA 2007; 104: 282–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Pichery M, Mirey E, Mercier P et al. Endogenous IL‐33 is highly expressed in mouse epithelial barrier tissues, lymphoid organs, brain, embryos, and inflamed tissues: in situ analysis using a novel Il‐33‐LacZ gene trap reporter strain. J Immunol 2012; 188: 3488–3495. [DOI] [PubMed] [Google Scholar]
- 97. Balato A, Lembo S, Mattii M et al. IL‐33 is secreted by psoriatic keratinocytes and induces pro‐inflammatory cytokines via keratinocyte and mast cell activation. Exp Dermatol 2012; 21: 892–894. [DOI] [PubMed] [Google Scholar]
- 98. Moussion C, Ortega N, Girard JP. The IL‐1‐like cytokine IL‐33 is constitutively expressed in the nucleus of endothelial cells and epithelial cells in vivo: a novel ‘alarmin’? PLoS One 2008; 3: e3331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Christianson CA, Goplen NP, Zafar I et al. Persistence of asthma requires multiple feedback circuits involving type 2 innate lymphoid cells and IL‐33. J Allergy Clin Immunol 2015; 136: 59–68.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Cohen M, Giladi A, Gorki AD et al. Lung single‐cell signaling interaction map reveals basophil role in macrophage imprinting. Cell 2018; 175: 1031–1044.e1018. [DOI] [PubMed] [Google Scholar]
- 101. Boraschi D, Italiani P, Weil S, Martin MU. The family of the interleukin‐1 receptors. Immunol Rev 2018; 281: 197–232. [DOI] [PubMed] [Google Scholar]
- 102. Moro K, Yamada T, Tanabe M et al. Innate production of TH2 cytokines by adipose tissue‐associated c‐kit+Sca‐1+ lymphoid cells. Nature 2010; 463: 540–544. [DOI] [PubMed] [Google Scholar]
- 103. Stolarski B, Kurowska‐Stolarska M, Kewin P, Xu D, Liew FY. IL‐33 exacerbates eosinophil‐mediated airway inflammation. J Immunol 2010; 185: 3472–3480. [DOI] [PubMed] [Google Scholar]
- 104. Neill DR, McKenzie AN. Nuocytes and beyond: new insights into helminth expulsion. Trends Parasitol 2011; 27: 214–221. [DOI] [PubMed] [Google Scholar]
- 105. Bonilla WV, Frohlich A, Senn K et al. The alarmin interleukin‐33 drives protective antiviral CD8+ T cell responses. Science 2012; 335: 984–989. [DOI] [PubMed] [Google Scholar]
- 106. Endo Y, Hirahara K, Iinuma T et al. The interleukin‐33‐p38 kinase axis confers memory T helper 2 cell pathogenicity in the airway. Immunity 2015; 42: 294–308. [DOI] [PubMed] [Google Scholar]
- 107. Morita H, Arae K, Unno H et al. An interleukin‐33‐mast cell‐interleukin‐2 axis suppresses papain‐induced allergic inflammation by promoting regulatory T cell numbers. Immunity 2015; 43: 175–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Obata‐Ninomiya K, Ishiwata K, Nakano H et al. CXCR6+ST2+ memory T helper 2 cells induced the expression of major basic protein in eosinophils to reduce the fecundity of helminth. Proc Natl Acad Sci USA 2018; 115: E9849–E9858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Cayrol C, Duval A, Schmitt P et al. Environmental allergens induce allergic inflammation through proteolytic maturation of IL‐33. Nat Immunol 2018; 19: 375–385. [DOI] [PubMed] [Google Scholar]
- 110. Magat JM, Thomas JL, Dumouchel JP, Murray F, Li WX, Li J. Endogenous IL‐33 and its autoamplification of IL‐33/ST2 pathway play an important role in asthma. J Immunol 2020; 204: 1592–1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Hemmers S, Schizas M, Rudensky AY. T reg cell‐intrinsic requirements for ST2 signaling in health and neuroinflammation. J Exp Med 2021; 218: e20201234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Spath S, Roan F, Presnell SR, Hollbacher B, Ziegler SF. Profiling of Tregs across tissues reveals plasticity in ST2 expression and hierarchies in tissue‐specific phenotypes. iScience 2022; 25: 104998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Schmitz J, Owyang A, Oldham E et al. IL‐33, an interleukin‐1‐like cytokine that signals via the IL‐1 receptor‐related protein ST2 and induces T helper type 2‐associated cytokines. Immunity 2005; 23: 479–490. [DOI] [PubMed] [Google Scholar]
- 114. Funakoshi‐Tago M, Tago K, Hayakawa M et al. TRAF6 is a critical signal transducer in IL‐33 signaling pathway. Cell Signal 2008; 20: 1679–1686. [DOI] [PubMed] [Google Scholar]
- 115. Fang Y, Zhao L, Xiao H et al. IL‐33 acts as a foe to MIA PaCa‐2 pancreatic cancer. Med Oncol 2017; 34: 23. [DOI] [PubMed] [Google Scholar]
- 116. Dahlgren MW, Jones SW, Cautivo KM et al. Adventitial stromal cells define group 2 innate lymphoid cell tissue niches. Immunity 2019; 50: 707–722.e706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Li D, Guabiraba R, Besnard AG et al. IL‐33 promotes ST2‐dependent lung fibrosis by the induction of alternatively activated macrophages and innate lymphoid cells in mice. J Allergy Clin Immunol 2014; 134: 1422–1432.e1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Yasuda K, Muto T, Kawagoe T et al. Contribution of IL‐33‐activated type II innate lymphoid cells to pulmonary eosinophilia in intestinal nematode‐infected mice. Proc Natl Acad Sci USA 2012; 109: 3451–3456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Scalfone LK, Nel HJ, Gagliardo LF et al. Participation of MyD88 and interleukin‐33 as innate drivers of Th2 immunity to Trichinella spiralis . Infect Immun 2013; 81: 1354–1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Hung LY, Lewkowich IP, Dawson LA et al. IL‐33 drives biphasic IL‐13 production for noncanonical type 2 immunity against hookworms. Proc Natl Acad Sci USA 2013; 110: 282–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Ajendra J, Specht S, Neumann AL et al. ST2 deficiency does not impair type 2 immune responses during chronic filarial infection but leads to an increased microfilaremia due to an impaired splenic microfilarial clearance. PLoS One 2014; 9: e93072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Coakley G, McCaskill JL, Borger JG et al. Extracellular vesicles from a helminth parasite suppress macrophage activation and constitute an effective vaccine for protective immunity. Cell Rep 2017; 19: 1545–1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Meiners J, Reitz M, Rudiger N et al. IL‐33 facilitates rapid expulsion of the parasitic nematode Strongyloides ratti from the intestine via ILC2‐ and IL‐9‐driven mast cell activation. PLoS Pathog 2020; 16: e1009121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Prefontaine D, Lajoie‐Kadoch S, Foley S et al. Increased expression of IL‐33 in severe asthma: evidence of expression by airway smooth muscle cells. J Immunol 2009; 183: 5094–5103. [DOI] [PubMed] [Google Scholar]
- 125. Meephansan J, Tsuda H, Komine M, Tominaga S, Ohtsuki M. Regulation of IL‐33 expression by IFN‐gamma and tumor necrosis factor‐alpha in normal human epidermal keratinocytes. J Invest Dermatol 2012; 132: 2593–2600. [DOI] [PubMed] [Google Scholar]
- 126. Shaw JL, Fakhri S, Citardi MJ et al. IL‐33‐responsive innate lymphoid cells are an important source of IL‐13 in chronic rhinosinusitis with nasal polyps. Am J Respir Crit Care Med 2013; 188: 432–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Imai Y, Yasuda K, Sakaguchi Y et al. Skin‐specific expression of IL‐33 activates group 2 innate lymphoid cells and elicits atopic dermatitis‐like inflammation in mice. Proc Natl Acad Sci USA 2013; 110: 13921–13926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Doherty TA, Baum R, Newbury RO et al. Group 2 innate lymphocytes (ILC2) are enriched in active eosinophilic esophagitis. J Allergy Clin Immunol 2015; 136: 792–794.e793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Lam M, Hull L, Imrie A et al. Interleukin‐25 and interleukin‐33 as mediators of eosinophilic inflammation in chronic rhinosinusitis. Am J Rhinol Allergy 2015; 29: 175–181. [DOI] [PubMed] [Google Scholar]
- 130. Jeong SK, Kim HJ, Youm JK et al. Mite and cockroach allergens activate protease‐activated receptor 2 and delay epidermal permeability barrier recovery. J Invest Dermatol 2008; 128: 1930–1939. [DOI] [PubMed] [Google Scholar]
- 131. Uchida M, Anderson EL, Squillace DL et al. Oxidative stress serves as a key checkpoint for IL‐33 release by airway epithelium. Allergy 2017; 72: 1521–1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Yee MC, Nichols HL, Polley D et al. Protease‐activated receptor‐2 signaling through beta‐arrestin‐2 mediates Alternaria alkaline serine protease‐induced airway inflammation. Am J Physiol Lung Cell Mol Physiol 2018; 315: L1042–L1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Saito K, Orimo K, Kubo T et al. Laundry detergents and surfactants induced eosinophilic airway inflammation by increasing IL‐33 expression and activating ILC2s. Allergy 2023; 78: 1878–1892. [DOI] [PubMed] [Google Scholar]
- 134. Kearley J, Silver JS, Sanden C et al. Cigarette smoke silences innate lymphoid cell function and facilitates an exacerbated type I interleukin‐33‐dependent response to infection. Immunity 2015; 42: 566–579. [DOI] [PubMed] [Google Scholar]
- 135. Arae K, Ikutani M, Horiguchi K et al. Interleukin‐33 and thymic stromal lymphopoietin, but not interleukin‐25, are crucial for development of airway eosinophilia induced by chitin. Sci Rep 2021; 11: 5913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Brandt EB, Bolcas PE, Ruff BP, Khurana Hershey GK. IL33 contributes to diesel pollution‐mediated increase in experimental asthma severity. Allergy 2020; 75: 2254–2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Unno H, Arae K, Matsuda A et al. Critical role of IL‐33, but not IL‐25 or TSLP, in silica crystal‐mediated exacerbation of allergic airway eosinophilia. Biochem Biophys Res Commun 2020; 533: 493–500. [DOI] [PubMed] [Google Scholar]
- 138. Weng CM, Wang CH, Lee MJ et al. Aryl hydrocarbon receptor activation by diesel exhaust particles mediates epithelium‐derived cytokines expression in severe allergic asthma. Allergy 2018; 73: 2192–2204. [DOI] [PubMed] [Google Scholar]
- 139. Kobari S, Kusakabe T, Momota M et al. IL‐33 is essential for adjuvant effect of hydroxypropyl‐beta‐cyclodexrin on the protective intranasal influenza vaccination. Front Immunol 2020; 11: 360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Han M, Rajput C, Hong JY et al. The innate cytokines IL‐25, IL‐33, and TSLP cooperate in the induction of type 2 innate lymphoid cell expansion and mucous metaplasia in rhinovirus‐infected immature mice. J Immunol 2017; 199: 1308–1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Wu YH, Lai AC, Chi PY et al. Pulmonary IL‐33 orchestrates innate immune cells to mediate respiratory syncytial virus‐evoked airway hyperreactivity and eosinophilia. Allergy 2020; 75: 818–830. [DOI] [PubMed] [Google Scholar]
- 142. Emi‐Sugie M, Shoda T, Futamura K et al. Robust production of IL‐33 and TSLP by lung endothelial cells in response to low‐dose dsRNA stimulation. J Allergy Clin Immunol 2020; 146: 1449–1452.e1442. [DOI] [PubMed] [Google Scholar]
- 143. Bonnelykke K, Sleiman P, Nielsen K et al. A genome‐wide association study identifies CDHR3 as a susceptibility locus for early childhood asthma with severe exacerbations. Nat Genet 2014; 46: 51–55. [DOI] [PubMed] [Google Scholar]
- 144. Grotenboer NS, Ketelaar ME, Koppelman GH, Nawijn MC. Decoding asthma: translating genetic variation in IL33 and IL1RL1 into disease pathophysiology. J Allergy Clin Immunol 2013; 131: 856–865. [DOI] [PubMed] [Google Scholar]
- 145. Gudbjartsson DF, Bjornsdottir US, Halapi E et al. Sequence variants affecting eosinophil numbers associate with asthma and myocardial infarction. Nat Genet 2009; 41: 342–347. [DOI] [PubMed] [Google Scholar]
- 146. Moffatt MF, Gut IG, Demenais F et al. A large‐scale, consortium‐based genomewide association study of asthma. N Engl J Med 2010; 363: 1211–1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Ramasamy A, Kuokkanen M, Vedantam S et al. Genome‐wide association studies of asthma in population‐based cohorts confirm known and suggested loci and identify an additional association near HLA. PLoS One 2012; 7: e44008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Savinko T, Matikainen S, Saarialho‐Kere U et al. IL‐33 and ST2 in atopic dermatitis: expression profiles and modulation by triggering factors. J Invest Dermatol 2012; 132: 1392–1400. [DOI] [PubMed] [Google Scholar]
- 149. Savenije OE, Mahachie John JM, Granell R et al. Association of IL33‐IL‐1 receptor‐like 1 (IL1RL1) pathway polymorphisms with wheezing phenotypes and asthma in childhood. J Allergy Clin Immunol 2014; 134: 170–177. [DOI] [PubMed] [Google Scholar]
- 150. Kottyan LC, Davis BP, Sherrill JD et al. Genome‐wide association analysis of eosinophilic esophagitis provides insight into the tissue specificity of this allergic disease. Nat Genet 2014; 46: 895–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Smith SR, Gillard JT, Kustka AB et al. Transcriptional orchestration of the global cellular response of a model pennate diatom to diel light cycling under iron limitation. PLoS Genet 2016; 12: e1006490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Nnane I, Frederick B, Yao Z et al. The first‐in‐human study of CNTO 7160, an anti‐interleukin‐33 receptor monoclonal antibody, in healthy subjects and patients with asthma or atopic dermatitis. Br J Clin Pharmacol 2020; 86: 2507–2518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Crim C, Stone S, Millar V et al. IL‐33 receptor inhibition in subjects with uncontrolled asthma: a randomized, placebo‐controlled trial. J Allergy Clin Immunol Glob 2022; 1: 198–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Chinthrajah S, Cao S, Liu C et al. Phase 2a randomized, placebo‐controlled study of anti‐IL‐33 in peanut allergy. JCI Insight 2019; 4: e131347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Chen YL, Gutowska‐Owsiak D, Hardman CS et al. Proof‐of‐concept clinical trial of etokimab shows a key role for IL‐33 in atopic dermatitis pathogenesis. Sci Transl Med 2019; 11: eaax2945. [DOI] [PubMed] [Google Scholar]
- 156. Chhiba KD, Hsu CL, Berdnikovs S, Bryce PJ. Transcriptional heterogeneity of mast cells and basophils upon activation. J Immunol 2017; 198: 4868–4878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Kondo Y, Yoshimoto T, Yasuda K et al. Administration of IL‐33 induces airway hyperresponsiveness and goblet cell hyperplasia in the lungs in the absence of adaptive immune system. Int Immunol 2008; 20: 791–800. [DOI] [PubMed] [Google Scholar]
- 158. Suzukawa M, Iikura M, Koketsu R et al. An IL‐1 cytokine member, IL‐33, induces human basophil activation via its ST2 receptor. J Immunol 2008; 181: 5981–5989. [DOI] [PubMed] [Google Scholar]
- 159. Pecaric‐Petkovic T, Didichenko SA, Kaempfer S, Spiegl N, Dahinden CA. Human basophils and eosinophils are the direct target leukocytes of the novel IL‐1 family member IL‐33. Blood 2009; 113: 1526–1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Smithgall MD, Comeau MR, Yoon BR, Kaufman D, Armitage R, Smith DE. IL‐33 amplifies both Th1‐ and Th2‐type responses through its activity on human basophils, allergen‐reactive Th2 cells, iNKT and NK cells. Int Immunol 2008; 20: 1019–1030. [DOI] [PubMed] [Google Scholar]
- 161. Matsuyama T, Machida K, Motomura Y et al. Long‐acting muscarinic antagonist regulates group 2 innate lymphoid cell‐dependent airway eosinophilic inflammation. Allergy 2021; 76: 2785–2796. [DOI] [PubMed] [Google Scholar]
- 162. Han H, Roan F, Johnston LK, Smith DE, Bryce PJ, Ziegler SF. IL‐33 promotes gastrointestinal allergy in a TSLP‐independent manner. Mucosal Immunol 2018; 11: 394–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Trier AM, Mack MR, Fredman A et al. IL‐33 signaling in sensory neurons promotes dry skin itch. J Allergy Clin Immunol 2022; 149: 1473–1480.e1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Imai Y, Yasuda K, Nagai M et al. IL‐33‐induced atopic dermatitis‐like inflammation in mice is mediated by group 2 innate lymphoid cells in concert with basophils. J Invest Dermatol 2019; 139: 2185–2194.e2183. [DOI] [PubMed] [Google Scholar]
- 165. Venturelli N, Lexmond WS, Ohsaki A et al. Allergic skin sensitization promotes eosinophilic esophagitis through the IL‐33‐basophil axis in mice. J Allergy Clin Immunol 2016; 138: 1367–1380.e1365. [DOI] [PubMed] [Google Scholar]
- 166. Weigmann B, Schughart N, Wiebe C et al. Allergen‐induced IgE‐dependent gut inflammation in a human PBMC‐engrafted murine model of allergy. J Allergy Clin Immunol 2012; 129: 1126–1135. [DOI] [PubMed] [Google Scholar]
- 167. Gurram RK, Wei D, Yu Q et al. Crosstalk between ILC2s and Th2 cells varies among mouse models. Cell Rep 2023; 42: 112073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Shih HY, Sciume G, Mikami Y et al. Developmental acquisition of regulomes underlies innate lymphoid cell functionality. Cell 2016; 165: 1120–1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Kamijo S, Takeda H, Tokura T et al. IL‐33‐mediated innate response and adaptive immune cells contribute to maximum responses of protease allergen‐induced allergic airway inflammation. J Immunol 2013; 190: 4489–4499. [DOI] [PubMed] [Google Scholar]
- 170. Motomura Y, Morita H, Moro K et al. Basophil‐derived interleukin‐4 controls the function of natural helper cells, a member of ILC2s, in lung inflammation. Immunity 2014; 40: 758–771. [DOI] [PubMed] [Google Scholar]
- 171. Takahashi K, Miyake K, Ito J et al. Topical application of a PDE4 inhibitor ameliorates atopic dermatitis through inhibition of basophil IL‐4 production. J Invest Dermatol 2024; 144: 1048–1057.e8. [DOI] [PubMed] [Google Scholar]
- 172. Wood RA, Togias A, Sicherer SH et al. Omalizumab for the treatment of multiple food allergies. N Engl J Med 2024; 390: 889–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Laviolette M, Gossage DL, Gauvreau G et al. Effects of benralizumab on airway eosinophils in asthmatic patients with sputum eosinophilia. J Allergy Clin Immunol 2013; 132: 1086–1096.e1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Oon S, Huynh H, Tai TY et al. A cytotoxic anti‐IL‐3Ralpha antibody targets key cells and cytokines implicated in systemic lupus erythematosus. JCI Insight 2016; 1: e86131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Kolle J, Zimmermann T, Kiefer A et al. Targeted deletion of Interleukin‐3 results in asthma exacerbations. iScience 2022; 25: 104440. [DOI] [PMC free article] [PubMed] [Google Scholar]


