Abstract
Bioactive glasses and glass-ceramics exhibit osteoconductivity, which is the ability to form a direct bond with living bone tissue. This property is typically assessed by observing the formation of a hydroxyapatite layer in vitro using simulated body fluid (SBF), a solution designed to mimic the inorganic constituents of human blood plasma. SBF was developed by Kokubo et al. (T. Kokubo, H. Kushitani, S. Sakka, T. Kitsugi and T. Yamamuro, J. Biomed. Mater. Res., 1990, 24, 721–734), and its preparation and storage procedures are precisely regulated according to ISO 23317. However, despite following the regulations, SBF may precipitate during storage owing to local ion concentration fluctuations during preparation because it is a supersaturated solution of hydroxyapatite. The present study is focused on designing a preparation process that enhances the stability of SBF-type biomimetic solutions. Solutions with a nominal composition that is the same as that of SBF were prepared by mixing stock solutions containing calcium or phosphate ions separately. Depending on the preparation procedure, two types of solutions-modified biomimetic solution (mBS) and evolved biomimetic solution (eBS)-were proposed. To evaluate the stability of these solutions, variants with 1.5 times the concentration of the original solutions were prepared, and the prepared solutions were denoted as xSBF, xmBS, and xeBS (where x = 1.0 or 1.5). It was found that the formation of calcium phosphate precipitates in 1.5mBS and 1.5eBS was slower than that in 1.5SBF. Additionally, the precipitate in 1.5eBS exhibited a different morphology from hydroxyapatite precipitate, which may be due to a higher hydrogencarbonate concentration than that in 1.5SBF. eBS demonstrated increased stability and a higher concentration of carbonic acid species (carbonate ions) than SBF, while mBS showed increased stability with a lower carbonate concentration than SBF. The newly proposed routes allow the production of an aqueous solution supersaturated with hydroxyapatite as a conventional SBF that has the potential to evaluate apatite formation via in vitro examination under high initial stability.
Modified acellular solutions are prepared using the liquid–liquid mixing method, with the same nominal ion concentrations as conventional simulated body fluids detailed in ISO 23317.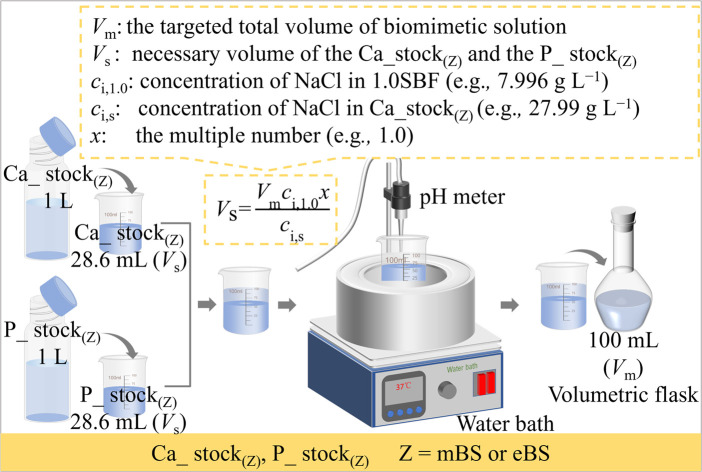
1. Introduction
Bioactive glasses and glass ceramics, such as Bioglass®1–3 and glass-ceramic A–W,4–7 are known as materials that show bone-bonding properties after implantation in bone defects. While these inorganic materials have differences in their compositions and phases compared to those of the inorganic constituents in natural bone tissue, they can make contact with bone tissue without an intervening layer (without encapsulation by collagen fibrous tissues) because the surface of the materials is covered with a bone-like apatite layer after exposure to body fluid.8 The unique property of bioactive materials is osteoconduction that achieves bone regeneration on their surface. The capability of forming a bone-like apatite layer is important to achieve osteoconduction. In order to evaluate apatite formation on materials in the body through in vitro experiments, Kokubo and his colleagues developed an acellular solution that mimics the concentrations of inorganic species in human blood plasma.9 The developed solution is called SBF, which stands for ‘simulated body fluid’. SBF includes inorganic species that are the same as those in human blood plasma, is buffered with tris(hydroxymethyl)aminomethane–hydrochloride (Tris–HCl), and has no proteins or enzymes. SBF is recognized for its usefulness in providing a convenient estimation of the potential of a material to exhibit apatite-forming capability, that is, in vitro calcification on a material surface, and it relates well to osteoconductivity.10 Therefore, research using this SBF is now widespread, even though the experiments do not use proteins or cells. The Elsevier Scopus database for the number of papers from 2018 to 2023 using the keywords ‘simulated’, ‘body’ and ‘fluid’ shows that approximately 1000 original papers are published annually.
The preparation procedure for SBF is standardized in ISO 23317.11 SBF is a supersaturated aqueous solution of hydroxyapatite that is metastable.12,13 Depending on the preparation method, spontaneous precipitation may occur during storage. The local fluctuations in ion concentrations in the solution result from the preparation procedure used to dissolve each chemical reagent, which is expected to be a factor in inducing the spontaneous precipitation of calcium phosphate. Calcium phosphate dissolves more under conditions at lower pH (<5.0) than at higher pH (>9.0). The conventional SBF preparation process involves mixing calcium and phosphate ions at a low pH, then adjusting the pH to 7.4.
To prepare SBF, phosphate (i.e., K2HPO4) is dissolved and then mixed with hydrochloric acid solution (HCl aq) to lower the pH once, followed by the addition of CaCl2. Tris(hydroxymethyl)aminomethane (Tris) is then added to bring the pH to around 7.4. Subsequently, a small amount of 1 mol L−1 HCl is added to control the appropriate pH of the solution at 37 °C. Takadama et al. reported that changes in pH occurred during the preparation of SBF.14 The pH of the solution decreased to approximately from 1 to 2 due to the addition of HCl aq, followed by an increase to around 7.6 due to the addition of Tris. Finally, the pH value was adjusted to an appropriate pH of 7.4 under a temperature of 36.5 °C. A similar process is used to prepare ionic compositions with higher concentrations. For instance, 1.5SBF is prepared with an ionic concentration 1.5 times higher than that of SBF to enhance the crystallization of hydroxyapatite on the substrates.15Table 1 gives the nominal composition of SBF and 1.5SBF, as well as those of human blood plasma.16 The pH of the solutions is usually maintained around 7.4 with Tris–HCl buffer system at 37 °C.
Nominal composition of simulated body fluids (SBF) and 1.5SBF with 1.5 times the ionic concentration of SBF. pH of the solutions is buffered to 7.4 with tris(hydroxymethyl)aminomethane–HCl (Tris–HCl buffer) system at 37 °Ca.
| Ion | Concentration/mmol L−1 | ||
|---|---|---|---|
| SBF (=1.0SBF) | 1.5SBF | Human blood plasma | |
| Na+ | 142.0 | 213.0 | 142.0 |
| K+ | 5.0 | 7.5 | 5.0 |
| Mg2+ | 1.5 | 2.3 | 1.5 |
| Ca2+ | 2.5 | 3.8 | 2.5 |
| Cl− | 147.8 | 221.7 | 103.0 |
| HCO3− | 4.2 | 6.3 | 27.0 |
| HPO42− | 1.0 | 1.5 | 1.0 |
| SO42− | 0.5 | 0.8 | 0.5 |
The pH of SBF is adjusted to around 7.4 at 37 °C by containing 50 mmol L−1 of tris(hydroxymethyl)aminomethane (Tris) and approximately 45 mmol L−1 of HCl aq (Tris–HCl buffer system), whilst that of 1.5SBF is adjusted by containing 75 mmol L−1 of Tris and approximately 67.5 mmol L−1 of HCl aq.
Notably, traditional SBF preparation involves dissolving chemical reagents one at a time to achieve a clear solution, but this process causes concerns about the fluctuation of local concentration around the dissolving solid substances, which affects the induction of nucleation of hydroxyapatite. Moreover, the composition of SBF is widely discussed given the lower concentration of hydrogen carbonate (bicarbonate) ion (HCO3−) and the higher concentration of chloride ion than those in human body plasma.17 From the viewpoint of stability during the preparation and storage of SBF, the authors have focused on the preparation and processing of SBF-type solutions with a nominal composition the same as those of SBF but which were prepared by mixing two different stock solutions: one containing calcium ions and the other containing phosphate ions. Following preliminary studies, the authors have introduced and discussed both the modified and evolved methods in brief.18–20 The preparation route by the present authors has a higher potential for stability (i.e., no spontaneous precipitation) than that for SBF. The proposed preparation routes mix two different stock solutions; one contains calcium ions (Ca_stock(Z)) and the other contains phosphates (P_stock(Z)), where Z means the type of prepared biomimetic solutions. The developed biomimetic solutions, hereafter abbreviated as modified biomimetic solution (mBS) and evolved biomimetic solution (eBS), maintain the same nominal ionic concentrations as SBF but are created by mixing the two stock solutions. This method offers the advantage of producing more homogeneous conditions in the final solution, unlike the traditional approach of adding each reagent individually, which can cause local compositional fluctuations during dissolution. There have been debates over the concentration of carbonate ions in SBF.17,21,22 The concentration of HCO3− in SBF is not equal to that in human blood plasma. However, Oyane et al. reported that higher concentrations (around 27 mmol L−1) in solution called r-SBF would lead to supersaturation of the solution with respect to calcite, a type of calcium carbonate,23 and saturation at a concentration of 10 mmol L−1 in the solution called m-SBF. The total concentration of carbonic acid species was measured for discussion on the formation of calcium carbonate crystals. pH changes during the preparation may contribute to the concentration of carbonate species in the prepared solutions. Namely, the addition of NaHCO3 in the preparing solution allows dissolved HCO3− in the solution. The pH of the solution varied the fraction of dissolved carbonic acid species (carbonate ions). The solution's pH dropped below 5, and almost all of the carbonic acid species turned into H2CO3 (shown in ESI #1†), which could be evaporated from the solution. This is shown in the chemical equilibrium below.NaHCO3 ⇄ Na+ + HCO3−HCO3− + H+ ⇄ H2CO3H2CO3 ⇄ H2O + CO2↑
Thus, the concentration of carbonic acid species is well influenced by pH changes during preparation and storage, although the nominal concentration was designed to be 4.2 mmol L−1 in SBF.
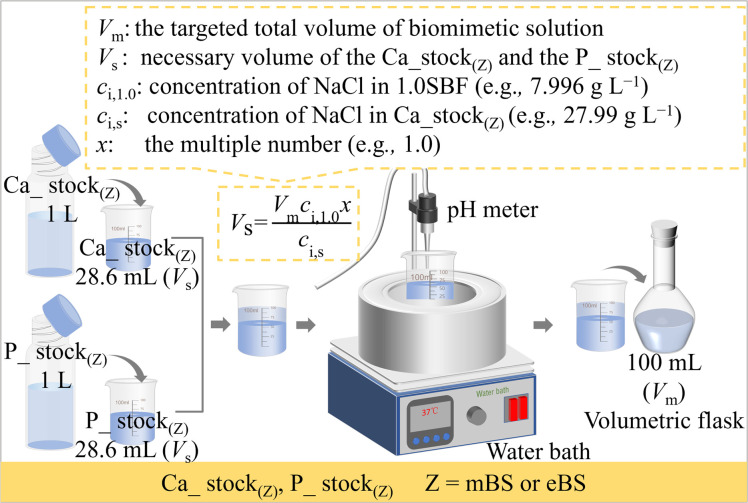
Based on the perspectives described above, the aim of this study was to investigate the influence of the preparation process on solution stability, in contrast to the conventional SBF process. The proposed method gives a unique perspective to the mixed stock solution (Ca_stock(Z) and P_stock(Z), where Z = mBS or eBS). This preparation method also has the advantage of long-term storage until the calcium and phosphate have been mixed, because the solutions may not be supersaturated with respect to hydroxyapatite. Furthermore, various times ionic compositions are easily prepared from the equivalent volume of Ca_stock(Z) and P_stock(Z) by dilution with water up to x = 1.5, as utilizing the equation shown in Fig. 1.
Fig. 1. Schematic of the preparation process of xmBS and xeBS (x = 1.0 or 1.5).
In the present study, to clarify differences of stability among the prepared solutions, solutions with 1.5 times the composition of 1.0SBF, i.e., 1.5SBF, 1.5mBS and 1.5eBS, were also examined, since they are also made to increase their supersaturation of the solution to hydroxyapatite and result in easy observation of spontaneous precipitation after being kept at 37 °C. Total concentrations of carbonic acid species were measured in actual solutions to examine their effects on the stability of the prepared solutions.
2. Experiment
2.1. Chemicals
Chemicals including sodium chloride (NaCl ≥ 99.5%), sodium bicarbonate (NaHCO3 ≥ 99.5%), potassium chloride (KCl ≥ 99.5%), dipotassium hydrogen phosphate trihydrate (K2HPO4·3H2O ≥ 99%), magnesium chloride hexahydrate (MgCl2·6H2O ≥ 98%), calcium chloride (CaCl2 ≥ 95%), sodium sulfate (Na2SO4 ≥ 99%), and tris(hydroxymethyl)aminomethane ((CH2OH)3CNH2 (Tris) ≥ 99.9%) were purchased from Nacalai Tesque Inc., Japan. 1.00 mol L−1 HCl aq was purchased from Kanto Chemical Co., Inc., Japan. MgCl2·6H2O and Tris were stored in a refrigerator at 5 °C and the others were stored at room temperature. All the chemical reagents were used without further purification.
2.2. Preparation of the solutions
The preparation procedure of SBF followed the protocol of ISO 23317. The solid chemical reagents and HCl aq for 1.5SBF were 1.5 times in their amounts of their weight and volume, respectively, as those for SBF, shown in Table 2. In the present study, two types of biomimetic solutions whose chemicals and amounts are reported as those for the preparation of SBF were attempted to be prepared by different processing where two stock solutions were mixed. These solutions for the preparation are named Ca_stock (Z) and P_stock(Z), where Z indicates the type of the finally prepared solution. Ca_stock(Z) and P_stock(Z) contain calcium sources and phosphate sources, respectively. The final prepared solutions were named mBS (modified biomimetic solution) and eBS (evolved biomimetic solution), respectively, but the nominal compositions of the solutions are the same as SBF. Tables 3 and 4 show the amounts of chemical reagents used to prepare the stock solutions for mBS and eBS, respectively. Fig. 1 shows an illustration of the preparation process of mBS and eBS by mixing Ca_stock(Z) and P_stock(Z) (Z = mBS or eBS). This preparation process allows solutions with concentrations up to 1.5 times higher than SBF to be easily obtained. The mBS and eBS can be prepared by mixing Ca_stock(Z) and P_stock(Z) (Z = mBS or eBS) of the same volumes, followed by dilution with appropriate amounts of water to make the solution to xmBS or xeBS, where x means the number of times made to varied concentration, as shown in Fig. 1. Fig. 1 also includes typical calculations for preparing solutions when x is 1.0, but it can be expected to be prepared to different concentrations below the maximum concentration of 1.5 times.
Amounts of chemicals used to prepare 1.0SBF and 1.5SBF, as well as the order in which they were mixed. SBF is denoted as 1.0SBF to make a comparison of concentration with 1.5SBF.
| Order | Chemicals | Amount (for 1000 mL of solution) | |
|---|---|---|---|
| 1.0SBF | 1.5SBF | ||
| 1 | NaCl | 7.996 g | 11.994 g |
| 2 | NaHCO3 | 0.350 g | 0.525 g |
| 3 | KCl | 0.224 g | 0.336 g |
| 4 | K2HPO4·3H2O | 0.228 g | 0.342 g |
| 5 | MgCl2·6H2O | 0.305 g | 0.458 g |
| 6 | 1 mol L−1 HCl | 40 mL | 60 mL |
| 7 | CaCl2 | 0.292 g | 0.438 g |
| 8 | Na2SO4 | 0.071 g | 0.107 g |
| 9 | (CH2OH)3CNH2 | 6.057 g | 9.086 g |
| 10 | 1 mol L−1 HCl | Appropriate amount for adjusting pH | Appropriate amount for adjusting pH |
Amounts of chemicals used to prepare the stock solutions for xmBS (x = 1.0 or 1.5) designed in this study. (a) Ca_stock(mBS) and (b) P_stock(mBS), as well as the order in which they were mixed.
| Order | Chemicals | Amount for 1000 mL of solution |
|---|---|---|
| (a) Ca_stock(mBS) | ||
| 1 | NaCl | 27.99 g |
| 2 | NaHCO3 | 1.225 g |
| 3 | KCl | 0.784 g |
| 4 | MgCl2·6H2O | 1.067 g |
| 5 | 1 mol L−1 HCl | 140 mL |
| 6 | CaCl2 | 1.022 g |
| 7 | Na2SO4 | 0.248 g |
| (b) P_stock (mBS) | ||
| 1 | K2HPO4·3H2O | 0.798 g |
| 2 | (CH2OH)3CNH2 | 21.20 g |
Amounts of chemicals used to prepare the stock solutions for xeBS (x = 1.0 or 1.5) designed in this study. (a) Ca_stock(eBS) and (b) P_stock(eBS), as well as the order in which they were mixed.
| Order | Chemicals | Amount for 1000 mL of solution |
|---|---|---|
| (a) Ca_stock(eBS) | ||
| 1 | NaCl | 27.99 g |
| 2 | KCl | 0.784 g |
| 3 | MgCl2·6H2O | 1.067 g |
| 4 | 1 mol L−1 HCl | 140 mL |
| 5 | CaCl2 | 1.022 g |
| 6 | Na2SO4 | 0.248 g |
| 7 | (CH2OH)3CNH2 | 21.20 g |
| (b) P_stock (eBS) | ||
| 1 | K2HPO4·3H2O | 0.798 g |
| 2 | NaHCO3 | 1.225 g |
2.3. Evaluation of the solutions
The pH of the prepared solutions was measured with a pH meter (F-71, HORIBA, Ltd., Japan). To assess the contents of carbonate ions in the solutions, total carbonic acid concentrations of the solutions were measured at room temperature by a portable carbon dioxide meter (CGP-31, DKK-TOA Corp., Japan) after the sample solutions were treated with ionic strength adjustment buffer (ISA-CO, DKK-TOA Co., Japan) to make their pH below 4.
The stabilities of the solutions 1.0SBF, 1.5SBF, 1.0mBS, 1.5mBS, 1.0eBS, and 1.5eBS were examined by the formation of precipitates of the solution after being kept at 37 °C. The beginning point of set up at 37 °C was defined as “0 days”. Commercial slide glasses were used as a reference substrate for the stability test, which should be inert in the examined solution. The slide glass (S1127, Matsunami Glass Ind., Ltd, Japan) was purchased and cut into a piece with a size of 12 mm × 12 mm × 1 mm, followed by polishing using abrasive papers in the order of #400, #800, #1000, #1200, #2000 and #4000. One piece of the polished slide glass was placed in a plastic centrifuge tube, where it was immersed in 35 mL examined solution. The lid of the centrifuge tube was then closed, and its temperature was maintained at 37 °C in an incubator. After being kept for a certain period up to 14 days, visual observation was made to determine the formation of precipitates in the tubes. Then the slide glass was removed from the solution, cleaned in water with ultrasonication, rinsed with ultrapure water, and dried at 40 °C for 24 hours (h).
Based on the stability tests of the six types of solutions, typical precipitates were prepared and analyzed by powder X-ray diffraction (XRD) and scanning electron microscopic (SEM) observation. To obtain enough amounts for powder XRD and SEM observation, 1000 mL of representative solution (i.e., 1.5mBS and 1.5eBS in the present study) was kept in a plastic beaker at 37 °C for 14 days. The precipitates were analyzed after washing and vacuum filtration with ultrapure water a few times. The obtained powder was dried in an oven at 40 °C for 24 h. The powder XRD patterns were obtained in the range of 2θ from 5° to 60° (MiniFlex600, Rigaku Co., Japan) with CuKα irradiation. SEM observations under high vacuum mode were made at an accelerating voltage of 15 kV (JCM-7000, JEOL Co., Japan).
3. Results
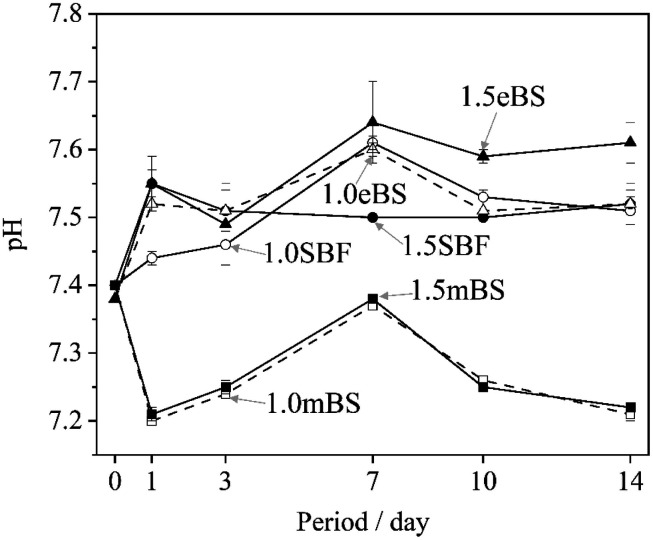
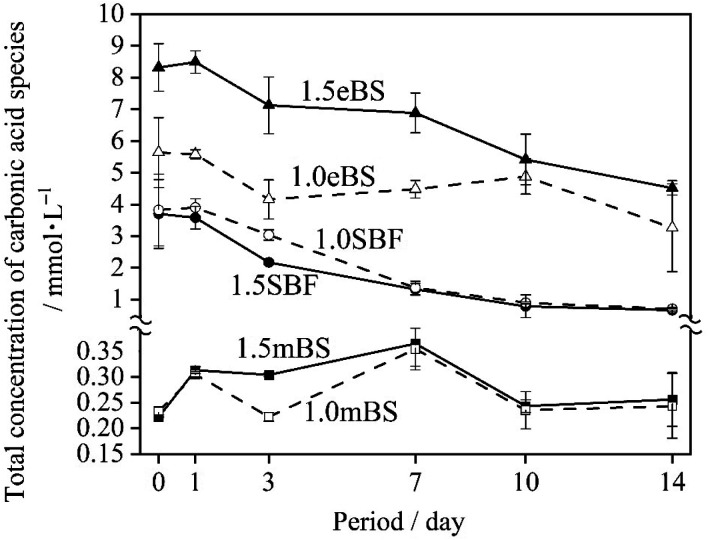
Table 5 gives the measured pH of Ca_stock(Z) and P_stock(Z) (Z = mBS or eBS) at 37 °C. The stock solutions have markedly different pH between mBS and eBS. To make the buffering effect of Tris gentler during the mixing of the solutions, P_stock(mBS) was poured into Ca_stock(mBS) while Ca_stock(eBS) was poured into P_stock(eBS). Changes in the pH of solutions 1.0SBF, 1.5SBF, 1.0mBS, 1.5mBS, 1.0eBS and 1.5eBS due to being kept at 37 °C for various periods are shown in Fig. 2. The pH of the solution at the beginning of being maintained (0 days) was around 7.4. After 1 day, xSBF and xeBS (x = 1.0 or 1.5) showed a tendency to slightly increase, while xmBS showed a slight tendency to decrease. Fig. 3 shows the concentrations of the total carbonic acid species in the solution being maintained at 37 °C for various periods. The concentrations of total carbonic acid species in the prepared solutions (0 days) showed an increase in the order of xmBS < xSBF < xeBS (x = 1.0 or 1.5).
pH of the prepared stock solutions measured at 37 °C.
| Solution | pH | |
|---|---|---|
| mBS | Ca_stock(mBS) | 0.72 ± 0.06 |
| P_ stock(mBS) | 10.01 ± 0.07 | |
| eBS | Ca_stock(eBS) | 7.28 ± 0.15 |
| P_ stock(eBS) | 8.40 ± 0.04 |
Fig. 2. Changes in the pH value of the solutions xSBF, xmBS and xeBS, where x is 1.0 or 1.5, after being kept for various periods. ○: 1.0SBF, ●: 1.5SBF, □: 1.0mBS, ■: 1.5mBS, △: 1.0eBS, and ▲: 1.5eBS.
Fig. 3. The total concentration of carbonic acid species in each biomimetic solution as a function of time kept at 37 °C. The measurement was conducted at 25 °C after the solutions were treated with ionic strength adjustment buffer to adjust their pH below 4. ○: 1.0SBF, ●: 1.5SBF, □: 1.0mBS, ■: 1.5mBS, △: 1.0eBS, and▲: 1.5eBS.
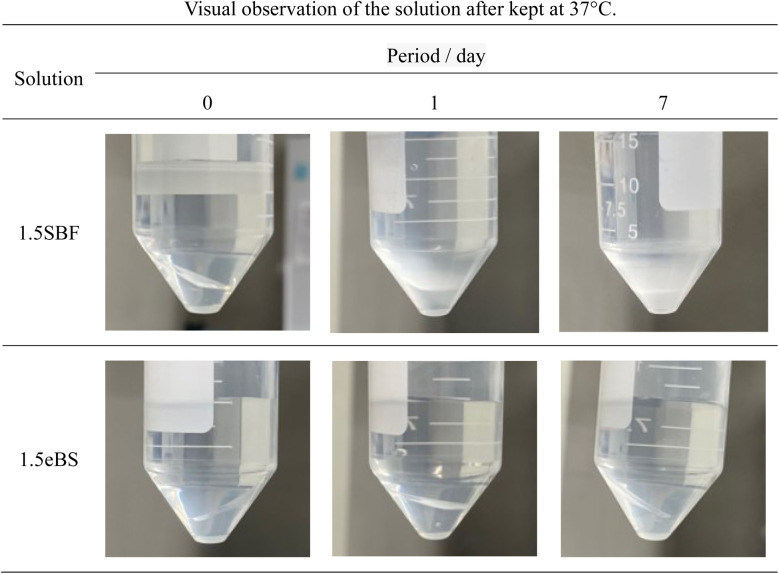
For SBF, both 1.0SBF and 1.5SBF had similar initial total concentrations of carbonic acid species around 4 mmol L−1, which gradually decreased to about 1 mmol L−1. In contrast, 1.0mBS and 1.5mBS showed lower initial concentrations of carbonic acid species, around 0.3 mmol L−1 on day 0, compared to 1.0SBF and 1.5SBF. Meanwhile, 1.0eBS and 1.5eBS exhibited higher initial concentrations of carbonic acid species on day 0 than 1.0SBF, 1.5SBF, 1.0mBS, and 1.5mBS. Notably, the initial concentration of total carbonic acid species in 1.5eBS was significantly higher than in 1.0eBS, and the concentration in 1.5eBS decreased as the storage period increased. A similar trend was observed in 1.0eBS, with concentrations in both solutions stabilizing at around 4 mmol L−1. When stored at 37 °C, 1.5SBF showed noticeable precipitation within one day, while 1.5eBS remained clear after seven days, as shown in Fig. 4.
Fig. 4. Typical appearance of the solutions in a centrifuge tube containing slide glass after stored for various periods. Appearances examined are given in ESI #2.†.
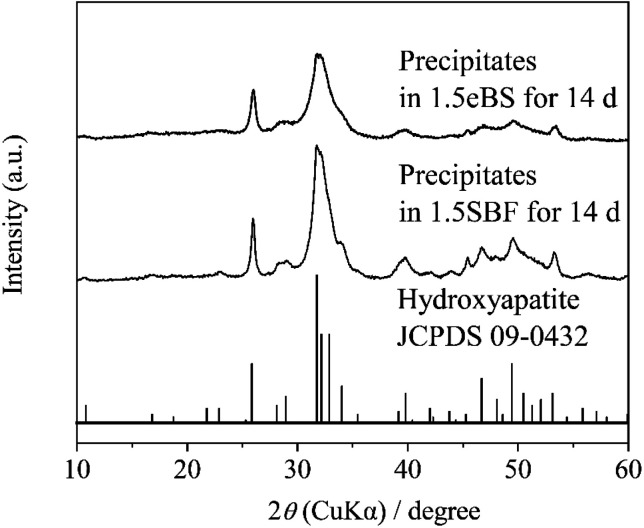
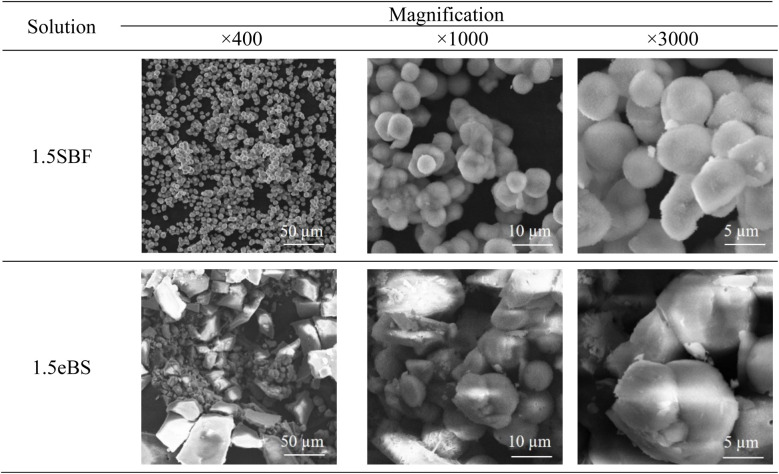
All the appearances examined are shown in ESI (ESI #2),† and the results are summarized in Table 6. 1.5SBF shows precipitation within 1 day after the solution was kept at 37 °C, while 1.0SBF showed the solution is clear up to 14 days. In the case of 1.5mBS, the solution remained clear up to 14 days and 1.5eBS up to 7 days. Both 1.0mBS and 1.0eBS maintained clarity up to 14 days. To characterize the precipitates formed in the solutions, 1000 mL of 1.5SBF and 1.5eBS were kept at 37 °C for 14 days, followed by analysis with XRD and SEM observation of the collected precipitates. Fig. 5 shows the powder XRD patterns of the precipitates formed in 1.5SBF and 1.5eBS. The precipitate of 1.5SBF is characterized as hydroxyapatite with broad diffraction peaks, and the precipitate of 1.5eBS consists of the same type of hydroxyapatite. The broad peaks assigned to hydroxyapatite seem to be similar to those in bone minerals. The morphology of the precipitates was analyzed by SEM for both samples as shown in Fig. 6. The precipitates formed in 1.5SBF showed spherical particles which are composed of small (nanometre-sized fine) products. These should be hydroxyapatite precipitates formed during the holding periods. On the other hand, the precipitates in 1.5eBS look to be a cubic-structured product with spherical particles. While the XRD pattern does not reveal a crystalline phase except for the hydroxyapatite phase, small amounts of another phase should have been formed with the cubic-structured morphology but were too small to be detected.
Results of precipitation in the solutions after stored at 37 °C for various periods. +: precipitation was observed. −: precipitation was not observed.
| Solution | Period/day | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 14 | |
| 1.0SBF | − | − | − | − | − | − | − | − | − |
| 1.5SBF | − | + | + | + | + | + | + | + | + |
| 1.0mBS | − | − | − | − | − | − | − | − | − |
| 1.5mBS | − | − | − | − | − | − | − | − | − |
| 1.0eBS | − | − | − | − | − | − | − | − | − |
| 1.5eBS | − | − | − | − | − | − | − | − | + |
Fig. 5. Powder X-ray diffraction (XRD) patterns of precipitates in 1.5SBF and 1.5eBS after stored at 37 °C for 14 days.
Fig. 6. SEM images of the spontaneous precipitates formed in 1.5SBF (upper) and 1.5eBS.
4. Discussion
4.1. Formation of precipitates from solution
The evaluation using SBF is to determine the potential of hydroxyapatite formation on the surface of materials in an aqueous solution that mimics human blood plasma without the contribution of biological factors such as proteins or cells. Therefore, the aqueous solution which is focused on in the present study is purposely called “biomimetic solution (BS)”. The 1.0mBS and 1.0eBS are solutions designed so that the ionic concentration in the preparation is equal to that of SBF (=1.0SBF). Therefore, these solutions should be treated as supersaturated and metastable solutions with respect to hydroxyapatite, just like SBF. The observations summarized in Table 6 indicate that spontaneous precipitation did not occur within 14 days for all three xSBF, xmBS, and xeBS where x equals 1.0. Precipitation was observed after 1 day in the 1.5SBF, where all ion concentrations were increased by 1.5 times, whereas no precipitation was observed after 14 days in the 1.5mBS and after 7 days in the 1.5eBS. Specifically, the aqueous solution prepared by mixing from the stock solution demonstrated high stability against precipitation in SBF. Oyane et al. reported the existence of calcium phosphate clusters under SBF conditions.23,24 An increased degree of supersaturation concerning hydroxyapatite would promote the formation of crystals from clusters. The local increase in ion concentration with fluctuations during the preparation process may contribute to the increase in the size and number of calcium phosphate clusters that promote precipitation formation. Thus, the spontaneously formed crystals were hydroxyapatite with similar characteristics to bone minerals as seen by detection with the XRD pattern shown in Fig. 5 and observation of the morphology of precipitated particles shown in Fig. 6.
4.2. Effect of preparation routine on carbonate ion contents in the prepared solution
The contents of carbonate species dissolved in SBF and its derivatives have been argued about, and Oyane et al. reported that carbonate contents of 27 mmol L−1 mimic human blood plasma, making the solution supersaturated with respect to calcite,23 a type of calcium carbonate (CaCO3), which formed a calcite cluster and gradually grew. Moreover, they reported that 10 mmol L−1 of carbonate species allowed close to saturation conditions without the formation of calcite. Therefore, it is important to set the carbonate concentration at 10 mmol L−1 or less to avoid the precipitation of calcium carbonate. In the present study, carbonate contents were determined with the total concentration of carbonic acid species in the solution. As shown in Fig. 3, after preparation (0 day) of the concentrations, the tendency was 1.0mBS ≈ 1.5mBS < 1.0SBF ≈ 1.5SBF < 1.0eBS < 1.5eBS.
On the preparation procedure of 1.0mBS and 1.5mBS, NaHCO3-containing Ca_Stock(mBS) was kept under low pH around 0.72. During this process, carbonic acid species are removed from the solution, and the pH should be kept at around 7.4. Therefore, mBS is regarded to have lower contents of carbonate species approximately ranging from 0.20 to 0.30 mmol L−1 in both 1.0mBS and 1.5mBS for an equilibrium condition under ambient atmosphere with adsorption of carbon dioxide. In the cases of 1.0SBF and 1.5SBF, both of the solutions showed almost similar total concentrations of carbonic acid species after the preparation (0 day) ranging from 3 mmol L−1 to 4 mmol L−1. In the preparation process of 1.0SBF and 1.5SBF, the pH is lowered after NaHCO3 dissolves, which reduces the amount of carbonic acid in the solution, and then the pH is raised to around 7.4, which brings the concentration of carbonic acid species according to the equilibrium pressure. In contrast, 1.0eBS and 1.5eBS had higher concentrations of total carbonic acid species than 1.0SBF and 1.5SBF. In the preparation process of 1.0eBS and 1.5eBS, NaHCO3 dissolves in P_stock(eBS) at a pH of around 8.4, which leads to high amounts of dissolved carbonic acid species. The total concentrations of carbonic acid species gradually decreased with increasing retention time, according to the equilibrium pressure.
Even for 1.5eBS, the solution appeared clear for up to 7 days, compared to 1.5SBF. This means that the instability of 1.5SBF is not attributed to the higher concentration of carbonic acid species. Oyane et al. reported the formation of a calcium carbonate cluster in a similar type of SBF-derivative solution under the condition of 27 mmol L−1 of carbonic acids, while 10 mmol L−1 are saturated conditions with respect to calcite.24 As seen in SEM (Fig. 6), slight amounts of cubic-structured precipitates were observed and presumed to calcite in the precipitates in 1.5eBS kept for 14 days. Still, the formation of calcite would affect the formation of hydroxyapatite.
4.3. Trends of change in pH of the solutions
The Tris–HCl system buffered the pH changes of the solutions, so they were limited. However, Fig. 2 shows the trend of the changes, which is linked to the amount of carbonic acid species and the formation of hydroxyapatite. There is a slight decrease from the initial pH for 1.0mBS and 1.5mBS, where the carbonate ion concentrations are low and comparable. This may be due to the absorption of carbon dioxide gas from the surrounding atmosphere, resulting in a decrease in pH. As for 1.5SBF, it precipitated hydroxyapatite after being kept for 1 day. The increase in pH predicted the release of carbon dioxide gas from the solution. However, for 1.5SBF, decrease in pH due to hydroxyapatite formation also occurred, and therefore the degree of balanced changes in pH should be considered.
As previously mentioned, the homogeneity of the solution during the formation of calcium phosphate clusters primarily contributes to the stability of SBF. The method of mixing two different stock solutions is attractive because it improves the traditional preparation method of mixing solids one by one. Changes in the concentration of carbon dioxide gas during the solution preparation process contribute little if the concentration is below the saturation concentration. The proposed method of mixing the two stock solutions can increase the solution's uniformity, resulting in a stable biomimetic solution. It is important to note that large fluctuations in pH during the process of mixing the two stock solutions may cause fluctuations in the concentration of carbonic acid species, so it is preferable to select conditions with little pH fluctuation. The method using stock solutions would allow easy storage because calcium phosphate is not produced before the calcium stock solution and phosphate stock solution are mixed. Furthermore, mixing both solutions in the same volume and changing the degree of dilution can easily produce solutions with different concentrations. Therefore, solutions with different degrees of supersaturation with respect to hydroxyapatite can be synthesized. The solutions will contribute to biomimetic synthesis even in an environment that is not similar to human blood plasma.
5. Conclusions
In this work, biomimetic solutions were developed by new preparation routes using mixing two stock solutions. Solutions with a nominal composition the same as that of SBF were prepared by mixing stock solutions containing either calcium or phosphate ions separately. To evaluate the stability of these solutions, variants with 1.5 times the concentration of the original solutions were also examined. Including conventional SBF, solutions denoted as xSBF, xmBS, and xeBS (where x = 1.0 or 1.5) were prepared. It was found that the formation of calcium phosphate precipitates in 1.5mBS and 1.5eBS was slower than in 1.5SBF. Additionally, the precipitate in 1.5eBS contained a different, cubic-structured morphology from the hydroxyapatite precipitate. This may be calcite contributed from a higher hydrogen carbonate concentration than in 1.5SBF. The preparation process for eBS endowed higher stability and a higher concentration of carbonate ions than that for SBF, while mBS had higher stability and a lower concentration of carbonate ions than SBF. Biomimetic solutions were prepared with lower or higher carbonic acid species than SBF, which acts as an aqueous solution supersaturated with respect to hydroxyapatite and has the potential to evaluate apatite formation by in vitro examination.
Data availability
Data are available upon request from the authors.
Author contributions
Conceptualization, C. O.; methodology, W. D., Y. H. and C. O.; validation, Y. M., K. S. and Y. L.; formal analysis, Y. M. and K. S.; investigation, W. D. and Y. H.; resources, C. O.; data curation, W. D. and Y. M.; writing—original draft preparation, W. D.; writing—review and editing, W. D., Y. M., Y. L., Y. H., J. N., K. S. and C. O.; visualization, W. D.; supervision, C. O.; project administration, C. O.; funding acquisition, W. D. and C. O. All authors have read and agreed to the published version of the manuscript.
Conflicts of interest
The authors declare no conflict of interest.
Supplementary Material
Acknowledgments
This work was supported by KAKENHI of Japan (21H02027 and 22K19894) and JST SPRING, grant number JPMJSP2125. The author (W. D.) would like to take this opportunity to thank the “THERS make New Standards Program for the Next Generation Researchers”.
Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4ra07739c
References
- Hench L. L. J. Am. Ceram. Soc. 1991;74:1487–1510. [Google Scholar]
- Hench L. L. J. Mater. Sci.:Mater. Med. 2006;17:967–978. doi: 10.1007/s10856-006-0432-z. [DOI] [PubMed] [Google Scholar]
- Greenspan D. Biomed. Glas. 2019;5:178–184. [Google Scholar]
- Kokubo T. Shigematsu M. Nagashima Y. Tashiro M. Nakamura T. Yamamuro T. Higashi S. Bull. Inst. Chem. Res., Kyoto Univ. 1982;60:260–268. [Google Scholar]
- Kokubo T. Biomaterials. 1991;12:155–163. doi: 10.1016/0142-9612(91)90194-f. [DOI] [PubMed] [Google Scholar]
- Kokubo T. Kim H.-M. Kawashita M. Nakamura T. J. Mater. Sci.:Mater. Med. 2004;15:99–107. doi: 10.1023/b:jmsm.0000011809.36275.0c. [DOI] [PubMed] [Google Scholar]
- Kokubo T. J. R. Soc. Interface. 2009;6:S267–S268. doi: 10.1098/rsif.2009.0079.focus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtsuki C. J. Ceram. Soc. Jpn. 2013;121:129–134. [Google Scholar]
- Kokubo T. Kushitani H. Sakka S. Kitsugi T. Yamamuro T. J. Biomed. Mater. Res. 1990;24:721–734. doi: 10.1002/jbm.820240607. [DOI] [PubMed] [Google Scholar]
- Baino F. Yamaguchi S. Biomimetics. 2020;5:57. doi: 10.3390/biomimetics5040057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ISO 23317:2014(en), Implants for surgery – In vitro evaluation for apatite–forming ability of implant materials, https://www.iso.org/obp/ui/en/#iso:std:iso:23317:ed–3:v1:en, accessed August 11, 2024
- Neuman W. F. and Neuman M. W., Chemical Dynamics of Bone Mineral, University of Chicago, Chicago, 1958 [Google Scholar]
- Ohtsuki C. Kokubo T. Yamamuro T. J. Non-Cryst. Solids. 1992;143:84–92. [Google Scholar]
- Takadama H. Hashimoto M. Mizuno M. Kokubo T. Phosphorus Res. Bull. 2004;17:119–125. [Google Scholar]
- Ohtsuki C. Kamitakahara M. Miyazaki T. J. Tissue Eng. Regener. Med. 2007;1:33–38. doi: 10.1002/term.3. [DOI] [PubMed] [Google Scholar]
- Gamble J., Chemical Anatomy, Physiology and Pathology of Extracellular Fluid, Harvard University Press, Cambridge, 6th edn, 1967 [Google Scholar]
- Bohner M. Lemaitre J. Biomater. 2009;30:2175–2179. doi: 10.1016/j.biomaterials.2009.01.008. [DOI] [PubMed] [Google Scholar]
- Ohtsuki C., Dong W., Long Y., Hayashi Y., Suzuki K., and Nakamura J., in The 37th International Korea–Japan Seminar on Ceramics K–J Ceramics, November 15–18, 2023, Konjiam Resort, Korea, 2023 [Google Scholar]
- Dong W., Hayashi Y., Mishra A., Long Y., Suzuki K., and Ohtsuki C., in The 42nd Annual Meeting of the Research Society for Orthopaedic Biomaterials, December 2, 2023, Plaza Doushin, Tsu, Mie, Tsu, 2023 [Google Scholar]
- Suzuki K., Ohtsuki C., Dong W., Long Y., Nakamura J. and Matsukawa Y., in 12th World Biomaterials Congress (WBC2024), May 26–31, 2024, EXCO, Daegu, Korea, 2024 [Google Scholar]
- Pan H. Zhao X. Darvell B. W. Lu W. W. Acta Biomater. 2010;6:4181–4188. doi: 10.1016/j.actbio.2010.05.013. [DOI] [PubMed] [Google Scholar]
- Mozafari M. Banijamali S. Baino F. Kargozar S. Hill R. G. Acta Biomater. 2019;91:35–47. doi: 10.1016/j.actbio.2019.04.039. [DOI] [PubMed] [Google Scholar]
- Oyane A. Kim H.-M. Furuya T. Kokubo T. Miyazaki T. Nakamura T. J. Biomed. Mater. Res., Part A. 2003;65A:188–195. doi: 10.1002/jbm.a.10482. [DOI] [PubMed] [Google Scholar]
- Oyane A. Onuma K. Ito A. Kim H.-M. Kokubo T. Nakamura T. J. Biomed. Mater. Res., Part A. 2003;64:339–348. doi: 10.1002/jbm.a.10426. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available upon request from the authors.