Abstract
The PACT Consortium was founded with the overarching goal of creating an international perinatal psychiatry consortium to conduct novel investigations with large sample sizes to understand the genetic signature of perinatal mood disorders. PACT uses a collaborative and team science approach that includes investigators across 19 institutions and seven continents. The large sample sizes allow for statistically rigorous analyses to investigate perinatal psychiatric disorders, with an initial focus on postpartum depression (PPD). Our current aims are to identify clinical subtypes of PPD that contribute diagnostic heterogeneity, and to elucidate the genetic basis of PPD by conducting the first large genome-wide association study of PPD. To accomplish the latter aim, we are partnering with the Major Depressive Disorder Working Group of the Psychiatric Genomics Consortium. To date, our consortium members have recruited 17,912 participants and 11,344 participants have been identified using the PPD ACT mobile app, of which 8,432 are PPD cases. Ultimately, we hope this approach will improve detection, diagnosis, and treatment of women who suffer from perinatal psychiatric disorders.
INTRODUCTION
The postpartum period is a time of increased vulnerability for the onset any psychiatric disorder. The most common disorder among them is postpartum depression (PPD), which affects approximately 500,000 women annually in the US (prevalence 10–15%) (Ko, Rockhill, Tong, Morrow, & Farr, 2017). The risk for a depressive episode is significantly higher during the postpartum period compared to other time periods in a woman’s life (Munk-Olsen et al., 2016). PPD symptoms (e.g., low mood, anhedonia, anxiety, rumination thoughts, and suicidal ideation) can be debilitating for new mothers at a particularly vulnerable time in their lives and a crucial developmental period for the infant (Meltzer-Brody et al., 2018). PPD-related suicide accounts for ~20% of all postpartum deaths, making PPD a leading cause of maternal perinatal mortality (Chesney, Goodwin, & Fazel, 2014; Johannsen et al., 2016). PPD also presents notable risks to newborns including infanticide and reduced maternal sensitivity (Burke, 2003), which can adversely affect emotional regulation and infant attachment leading to adverse neurodevelopmental outcomes for the child (Bremner, Wachs, & Wiley Online Library., 2010; Netsi et al., 2018). Despite the public health issues PPD poses, it is under-studied (Wisner, Moses-Kolko, & Sit, 2010) and its precise etiology is unknown (Brummelte & Galea, 2016). Therefore, the Postpartum depression: Action towards Causes and Treatment (PACT) Consortium was formed to address this critical gap in knowledge. This international research consortium has the overarching mission of rapid progress toward elucidating the causes of postpartum psychiatric illness so that prevention is possible and tailored and personalized effective treatments are developed. This work is vital given the morbidity associated with PPD. Women who experience postpartum psychiatric episodes are at higher mortality risk compared with mothers without psychiatric diagnoses, and the first year after diagnosis represents a time of particularly high relative risk for suicide in this vulnerable group (Johannsen et al., 2016).
DEVELOPMENT OF THE PACT CONSORTIUM
A search for “postpartum depression” in Pubmed shows the number of publications rose from 305 in 2007 to 617 in 2017, more than doubling, indicating more attention is now being given to PPD. However, these numbers are still considerably lower than other psychiatric disorders. For instance, over the same period schizophrenia publications also increased from 4,080 to 5,450 publications and major depressive disorder increased from 4,165 to 5,443 publications. There remains a great need for additional research into PPD as many important questions remain unanswered, which contributes to significant debates within the perinatal mental health community. These topics of discussion include: 1) is PPD a distinct disorder or a subtype of major depressive disorder (MDD), 2) does parturition act as a specific trigger for the onset of a depressive episode, and 3) should diagnostic criteria for PPD be specific to symptom onset during the postpartum period, or should it extend to include onset during pregnancy. On the surface, studying PPD is also an obvious path forward into understanding MDD as a whole: PPD is a more homogenous form of MDD, only females are affected, onset only occurs during reproductive years, and women that suffer with PPD are exposed to the same biopsychosocial event. These important questions demonstrate that there is heterogeneity within PPD that needs to be systematically characterized with further research. What is clear, however, is that the perinatal period is a particularly vulnerable time for psychiatric disorders with potentially adverse outcomes for mother, baby and family.
Tackling these multifaceted issues requires very large sample sizes that are outside the scope for any single research group to address on their own. Therefore, the International Postpartum Depression: Action Towards Causes and Treatment (PACT) Consortium (www.pactforthecure.com) was formed to take a collaborative team science approach to allow for novel investigations with large sample sizes. An overview of PACT accomplishments to date is depicted in Figure 1.
Figure 1.
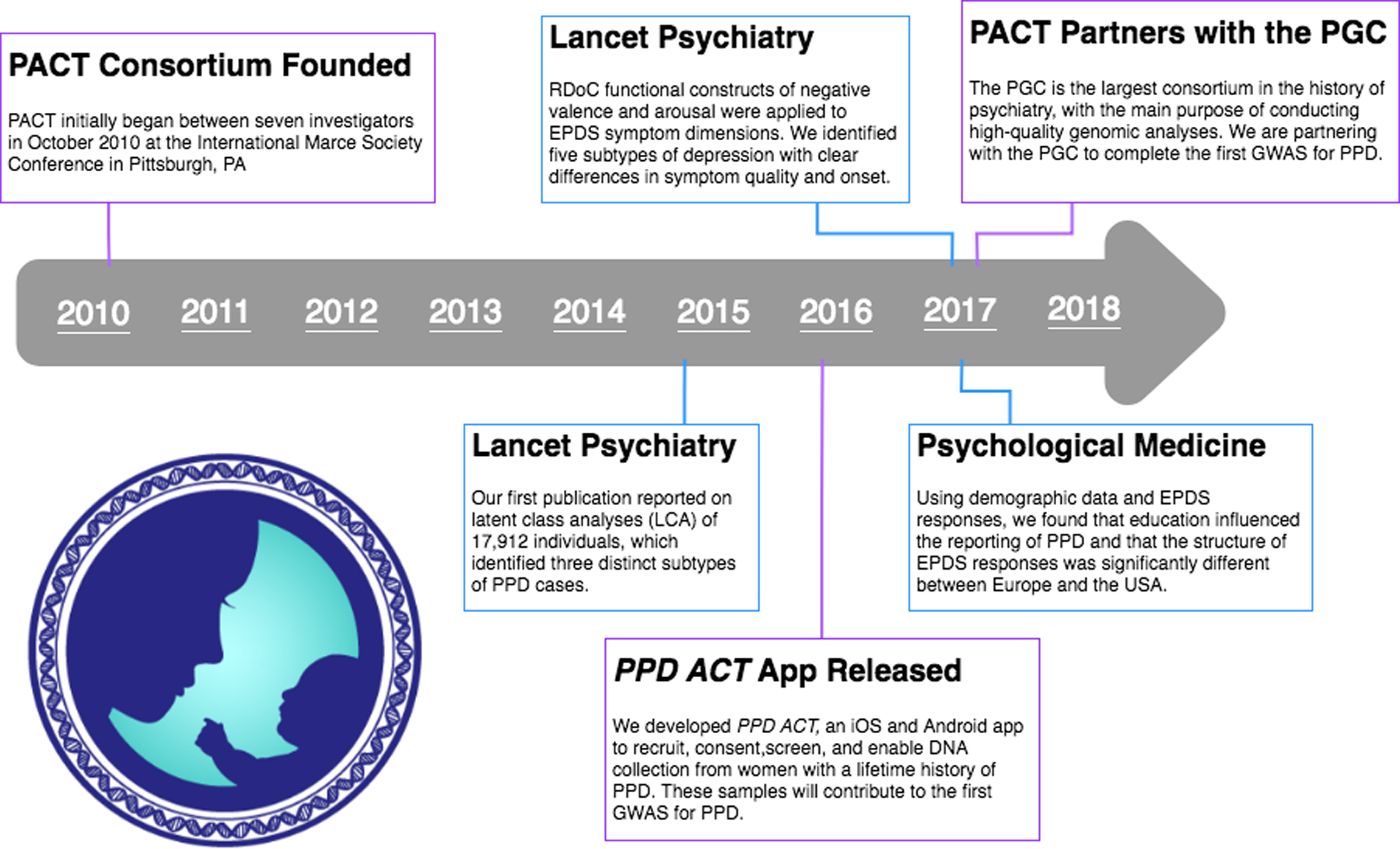
An overview of PACT activities.
ORGANIZATION OF THE PACT CONSORTIUM
PACT initially began following discussions between seven investigators in October 2010 at an open meeting at the International Marce Society Conference in Pittsburgh, PA. Currently, the PACT consortium has investigators from 19 institutions in seven countries. Membership has been extended to investigators or groups who can contribute anonymized individual-level clinical and/or biological data for analyses. Clinical data includes detailed descriptions of the study designs and methods, recruitment, and clinical variables assessed. Biological data includes biospecimens to be used for genomic analyses and/or existing genotyping data with associated clinical data. Participants include women recruited from many different settings including psychiatric clinics, obstetric clinics, primary care, and community advertisements. Individual investigators/studies obtain consent from participants and approval from respective institutional review boards for data sharing. Joining PACT entails reading and agreeing to the rules of conduct detailed in the memorandum of understanding. Assent is indicated by email and effectively constitutes a pledge to behave with integrity. The PACT consortium consists of three committees: the coordinating, phenotyping, and PPD ACT committees.
Coordinating Committee.
The role of the coordinating committee is only to adjudicate issues of relevance to the whole consortium and to plan new directions in an organized manner. The coordinating committee has a non-intrusive and facilitating role, and all other decisions are delegated to the scientists who understand the issues best.
Phenotyping Committee.
The role of the phenotyping committee is to address the phenotypic characterization and harmonization of available clinical data from PACT members. Harmonization of clinical data is a critical step. Previous examples have shown unless this is done with expertise and rigor, interpretation of subsequent results is not reliable. This is especially true given the multiple diagnostic criteria that may be used in various clinical settings. The Diagnostic and Statistical Manual of Mental Disorders, fifth edition (DSM-V), includes onset of symptoms during pregnancy and for up to four weeks postpartum (American Psychiatric Association. & American Psychiatric Association. DSM-5 Task Force., 2013). In contrast, the International Statistical Classification of Diseases, tenth revision (ICD-10), more strictly defines PPD as onset within six weeks following childbirth. Further, the World Health Organization and the Centers for Disease Control and Prevention extend the period of symptom onset to 12 months postpartum. Given the range of definitions, the phenotyping committee was responsible for developing a common codebook of harmonized diagnostic constructs for comparable results across multiple cohorts. This codebook played a critical role in completing Aim 1 of our consortium agenda (see below).
PPD ACT Committee.
This committee developed and deployed PPD ACT, a novel app-based PPD genetics study (Guintivano et al., 2018). The rationale for developing the first mobile app for psychiatric genetics research was to sufficiently power the first large-scale genome-wide association study (GWAS) for PPD (see Aim 2 below). However, in order to identify a signal and be confident in results, special care was taken to identify cases that are reflective of what is seen clinically. The PPD ACT committee carefully built the app screen by screen and worked closely with app developers to ensure scientific rigor and integrity. Further, the integrity of our screening methods were validated clinically (100% sensitivity) and underwent a second test of a subset of cases to show a high degree of test-retest agreement among cases (85%) (Guintivano et al., 2018). In March 2016, we successfully launched the iOS version of PPD ACT in the United States and Australia. One year later, we expanded to Canada and have released an Android version of the app. At the time of writing, we have identified 8,432 cases, which will contribute to achieving the aims set forth for the consortium.
APPROACH
Much of the research done by the PACT Consortium relies on physician diagnosis and participant self-reporting of PPD symptoms. For this reason we use the lifetime version of the Edinburgh Postnatal Depression Scale (EPDS) (Meltzer-Brody, Boschloo, Jones, Sullivan, & Penninx, 2013) to screen for PPD cases and controls. This version has been validated and builds on the standard EPDS, which is a common and widely used PPD screening instrument (Boyd, Le, & Somberg, 2005; J. L. Cox, Holden, & Sagovsky, 1987; J. L. Cox, Murray, & Chapman, 1993; Hewitt & Gilbody, 2009). The EPDS is a 10-item self-report assessment, focusing on current symptoms, and minimizes confounding of somatic symptoms of MDD with the demands inherent to parenting an infant (e.g., insomnia) (J. L. Cox et al., 1987). Comparatively, the lifetime modified version allows for screening across the lifetime by focusing the participant on their worst perinatal depressive episode. This is ideal for an app-based recruitment of PPD cases so any woman with a history of PPD can participate, not just those in the immediate postpartum period. For both the standard and lifetime versions of the EPDS, PPD symptoms are rated on a scale of 0 – 30 with higher scores indicating greater symptom severity. EPDS scores ≥ 12 are consistent with PPD (Wisner, Parry, & Piontek, 2002).
SPECIFIC RESEARCH AIMS OF THE CONSORTIUM AND CURRENT PROGRESS
As noted above, heterogeneity of PPD symptoms, including timing of symptom onset, poses numerous challenges for elucidating the precise causes of the disorder. Delineating precise combinations of symptoms and subtypes of PPD is the first step to identifying underlying etiology, which is arguably the greatest need for any psychiatric disorder, including PPD. However, these tasks are too great for any single research group to tackle alone. Thousands, or even hundreds of thousands, of participants are needed to adequately power such analyses. Therefore, the PACT consortium was formed to pool available resources and to investigate symptom heterogeneity and genetic causes of PPD.
Aim 1.
The first aim of the PACT consortium was to perform a rigorous investigation of the heterogeneity of PPD in order to identify clinical subtypes. Work for this aim was largely completed using existing data from all PACT members, following rigorous data harmonization (see Phenotyping Committee above). There have been three publications to date examining this first aim.
Our first publication reported on two latent class analyses (LCA) of 17,912 individuals (Postpartum Depression: Action Towards & Treatment, 2015). LCA is a technique that clusters individuals on the basis of responses to create distinct classes. We applied LCA to investigate whether PPD can be stratified into empirically defined subtypes based on EPDS responses. The first LCA included all 17,912 individuals, which included clinically defined cases and controls. From this, three unique classes were identified representing: 1) those who did not self-report anxiety or depression (mean EPDS score 3.3), 2) those with mild symptoms (mean EPDS score 12.3), and 3) those with more severe symptoms (mean EPDS score 20.3). Using LCA to group individuals on the bases of their symptoms (EDPS responses), we identify controls (those with no symptoms) and cases, both mild and severe. A second LCA was performed on PACT cases (n = 4,245) to further delineate subtypes of PPD. This LCA was done using indicator variables (severity of depression, EPDS total score, EPDS anxiety subscale score, complications of pregnancy, obstetric complications, suicidal ideation, and psychiatric history). The result of this case only analysis revealed three distinct classes characterized by severity of symptoms, timing of onset of depressive symptoms, and presence of suicidal thoughts. All classes had high proportions of individuals with psychiatric history.
Following the successful identification of PPD subtypes in our large consortium cohort, we wanted to further interrogate these diverse phenotypes, specifically the type and quality of presentation in women with onset during pregnancy versus postpartum. We hypothesized that the underlying causes for onset and quality of symptoms could be driven by underlying pathophysiological mechanisms such as the hormone fluctuations that characterize the perinatal period (Schiller, Meltzer-Brody, & Rubinow, 2015). Therefore, we chose to examine symptom constructs described in the National Institutes of Mental Health (NIMH) research domain criteria (RDoC) (Insel et al., 2010), which can account for co-occurring symptom domains. For this analysis (Putnam et al., 2017), 663 women with information about onset of depressive symptoms and complete prospective data for the EPDS were included. Principal components and common factor analysis were used to identify symptom dimensions within the EPDS. RDoC functional constructs of negative valence and arousal were applied to these EPDS symptom dimensions (depressed mood, anhedonia, and anxiety). Using these three dimensions, we identified five subtypes of depression across the perinatal period: severe anxious depression, moderate anxious depression, anxious anhedonia, pure anhedonia, and resolved depression. These subtypes have clear differences in symptom quality and onset. Those classified as having resolved depression typically had onset in the second or third trimester of pregnancy, with symptoms resolving at the time of EPDS measurement. Further, anxiety and anhedonia were symptom domains with prominent onset in the immediate postpartum period (< 8 weeks). Ultimately, this work shows unique phenotypes exist within the greater umbrella of “perinatal depression,” specifically in terms of symptom combinations, severity, and timing of onset. This further highlights the need for tailored treatments and investigations into the biological underpinnings of each subtype, particularly with onset during pregnancy versus postpartum.
Lastly, we utilized our PACT phenotypic data to interrogate the impact of demographics (education, country of origin, race and ethnicity) on the self-report of postpartum depression using the EPDS (Di Florio et al., 2017). This supports our aim to interrogate the heterogeneity of PPD, not only on the basis of symptoms, but also in terms of environment that can also have a direct impact on patient well-being. To accomplish this, a subset of PACT participants (n = 8,209), were used based on the availability of information on ethnicity/race, education, and individual item data on the EPDS. Ordinal regression and measurement invariance were used to explore the association between culture, operationalized as education, ethnicity/race and continent, and endorsement of depressive symptoms on the EPDS. We found that education influenced the reporting of PPD and that the structure of EPDS responses was significantly different between Europe and the USA. These results have important implications for the delivery of patient-specific clinical care, emphasizing culturally specific and sensitive treatments. In addition, special considerations should be made for education when screening for symptoms and delivering treatment options.
With these three publications (Di Florio et al., 2017; Postpartum Depression: Action Towards & Treatment, 2015; Putnam et al., 2017), we have illustrated the complex symptom heterogeneity found within PPD can be classified into clinically relevant subtypes using various methods. The next steps will be to replicate these findings and to use these phenotypes to inform investigations into the biological underpinnings of PPD.
Aim 2.
The second and current aim of PACT is to elucidate the genetic basis of PPD by conducting large-scale GWAS. Interrogating the genetics of PPD is a critical step into the etiology of the disorder. Genetic variation represents the principal risk factor for psychiatric disorders because exposure begins at the earliest stage of development and can be viewed as the beginning of a causal pathway that manifests as a complex disorder later in life. The GWAS method has been used extensively and described elsewhere (Corvin, Craddock, & Sullivan, 2010; Hayes, 2013).
These advances in technology have led to a new understanding of the genetic architecture of many complex phenotypes, particularly psychiatric disorders. The 2014 Psychiatric Genomics Consortium (PGC) schizophrenia GWAS identified 108 genome-wide significant loci and novel biological pathways for further exploration (Schizophrenia Working Group of the Psychiatric Genomics, 2014) and was among the top 5 most highly cited paper in science that year. In 2017, the PGC completed the largest meta-analysis for MDD to date (Wray, 2018). This effort identified 44 loci that met statistical significance and consistency of effects. SNPs in genes that encode 42 molecular targets of antidepressant medications (Gaspar & Breen, 2017) were highly enriched for smaller MDD associations than expected by chance (MAGMA(de Leeuw, Mooij, Heskes, & Posthuma, 2015) gene-enrichment, p = 8.5×10−10). This connects MDD genomic findings to MDD therapeutics, and suggests the salience of these results for novel lead compound discovery for MDD (Breen et al., 2016). Additionally, the PGC showed genetic risk for MDD was correlated with that for many adult and childhood onset psychiatric disorders, along with educational attainment, body mass, and age at menarche. The genetic basis of lower educational attainment and higher body mass were putatively causal for MDD, whereas MDD and schizophrenia reflected a shared biological etiology. In sum, this work illustrated that MDD is not a distinct entity that neatly demarcates normalcy from pathology but rather a useful clinical construct compellingly associated with a range of adverse outcomes and the end result of a complex process of intertwined genetic and environmental effects.
In contrast, compared to the progress made for schizophrenia and MDD, genetic studies for PPD are still in the early stages. Small prior genetic epidemiological and linkage studies(Costas et al., 2010; Forty et al., 2006; Mahon et al., 2009; Murphy-Eberenz et al., 2006; Treloar, Martin, Bucholz, Madden, & Heath, 1999) prompted the work of some PACT members to perform a Swedish national study that estimated the heritability of PPD to be 54% and 44% using twin and sibling designs, respectively. In this same population, the heritability of non-perinatal depression was estimated to be 32% (Viktorin et al., 2016). This suggests that approximately one-third of the genetic contribution to perinatal depression was unique and not overlapping with the genetic component of depression not occurring around pregnancy. Another study done by PACT investigators examined the genetic risk for MDD and bipolar disorder in PPD samples (Byrne et al., 2014). This work found empirical genetic evidence for a more important shared genetic etiology between bipolar disorder and PPD than between bipolar disorder and MDD, albeit on small cohorts. An increased heritability, as well as increased genetic overlap with other mood disorders, makes PPD a prime candidate for further genetic discovery efforts.
To date, there has not been a GWAS performed for PPD using modern genomics methods. This provides a great opportunity for team science and the PACT consortium has made conducting the first PPD GWAS a primary focus. Work done for other psychiatric GWAS has shown large sample sizes (ideally hundreds of thousands of cases) are required to identify significant risk loci. In order to reach the required sample sizes, samples collected with the PPD ACT app (Guintivano et al., 2018) will be combined with existing PACT samples, which are being genotyped to complete this aim. However, many more samples are needed for a proper analysis. Consequently, we are partnering with the PGC, in the spirit of collaborative team science, to complete the PPD GWAS. The PGC is the largest consortium in the history of psychiatry, with the main purpose of conducting high-quality genomic analyses. Over the past decade they have completed many GWAS and secondary analyses which have led to biologically, clinically, and therapeutically meaningful insights across multiple psychiatric disorders. As part of our partnership, the longstanding MDD Working Group has convened a PPD special interest group (PPD-SIG). This new working group will be able to identify specific risk loci with a PPD GWAS, and can use this data to estimate genetic heritability, determine the genetic overlap of PPD with other psychiatric disorders, and generate PPD-specific genetic risk scores, potentially allowing for the ability to stratify subtypes of PPD at the genetic level. Through this collaboration, we will have access to large sample sizes, a well-established infrastructure for analysis, and the expertise to interpret findings in clinically and biologically meaningful ways. Over the next five years, we anticipate completion of the first PPD GWAS and an integration of results from identification of PPD subtypes in Aim 1. Together this will allow us to begin answering important questions in the field, namely: 1) how much do PPD and other mood disorders overlap, and 2) is there a genetic difference between PPD symptoms that onset during pregnancy versus postpartum.
SUMMARY OF RESULTS TO DATE
For the past eight years, the PACT Consortium has taken great strides into understanding PPD phenomenology using a team-science approach (Figure 1). Our consortium members have recruited 17,912 participants and 11,344 participants have been identified using the PPD ACT mobile app, of which 8,432 are PPD cases. This unique cohort has allowed us to publish three papers examining the heterogeneity among PPD cases. Additionally, we have shown the promise of mobile technology to recruit broadly for genomics studies. The work to date has provided the necessary foundation for next steps that will examine the genetic signature of PPD.
CLINICAL IMPLICATIONS AND FUTURE DIRECTIONS
PPD is an under-diagnosed disorder, and those who are identified are not adequately treated (E. Q. Cox, Sowa, Meltzer-Brody, & Gaynes, 2016). The severity and common prevalence of PPD demands an improved approach for how we detect, diagnose, and treat women that suffer with this disorder. While we have made significant progress in untangling the heterogeneity of PPD presentations (Aim 1), there remains much work into identifying the genetics behind this disorder.
Ongoing work by individual PACT investigators to obtain genetic samples is the lifeline of the consortium to achieve adequate sample sizes. Without this crucial work, the consortium will not be able to continue making headway in this underrepresented field. Expanding to include more investigators will accelerate our rate of discovery, which ultimately will aid in expedited improvements to patient care.
Once there are identified clinical subtypes of PPD, along with initial genomic risk profiles, we will be able to explore how specific genetic patterns segregate with clinical symptoms. A particular phenotype of interest is timing of onset of symptoms. The debate about the timing of onset has many important implications. Not only does the issue of timing of onset of symptoms have biological implications (i.e., onset of depressive symptoms while hormone levels are very high during pregnancy versus immediately postpartum when there is an abrupt decline of hormones levels following childbirth), but this may also aid in classification of the disorder to determine prognosis and tailored treatment. This approach can assist in the identification of specific risk factors and phenotypic characteristics for any individual woman that suffers from PPD and move the field forward toward a personalized medicine approach of PPD that can help achieve the best long term outcomes. The PACT Consortium wants to be at the forefront of personalized medicine for peripartum psychiatric disorders similar to other fields, such as breast cancer (Cardoso et al., 2016; Sparano et al., 2015), that have made advances by incorporating genomic data with standard clinical practice.
As the field of psychiatric genetics continues to increase our knowledge of complex disorders, the work of PACT in partnership with the PGC is poised to do the same for women who suffer with PPD and other forms of perinatal psychiatric disorders. Ultimately, our long-term success will be judged by whether we are able to improve outcomes for the women and their families impacted by this common and morbid disorder.
REFERENCES
- American Psychiatric Association., & American Psychiatric Association. DSM-5 Task Force. (2013). Diagnostic and statistical manual of mental disorders : DSM-5 (5th ed.). Washington, D.C.: American Psychiatric Association. [Google Scholar]
- Boyd RC, Le HN, & Somberg R (2005). Review of screening instruments for postpartum depression. Archives of Women’s Mental Health, 8(3), 141–153. doi: 10.1007/s00737-005-0096-6 [DOI] [PubMed] [Google Scholar]
- Breen G, Li Q, Roth BL, O’Donnell P, Didriksen M, Dolmetsch R, . . . Edenberg HJ (2016). Translating genome-wide association findings into new therapeutics for psychiatry. Nature Neuroscience, 19(11), 1392–1396. doi: 10.1038/nn.4411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner JG, Wachs TD, & Wiley Online Library. (2010). The Wiley-Blackwell handbook of infant development . Vol. 2 (2nd ed.). Chichester, West Sussex: Wiley-Blackwell. [Google Scholar]
- Brummelte S, & Galea LA (2016). Postpartum depression: Etiology, treatment and consequences for maternal care. Hormones and Behavior, 77, 153–166. doi: 10.1016/j.yhbeh.2015.08.008 [DOI] [PubMed] [Google Scholar]
- Burke L (2003). The impact of maternal depression on familial relationships. International Review of Psychiatry, 15(3), 243–255. doi: 10.1080/0954026031000136866 [DOI] [PubMed] [Google Scholar]
- Byrne EM, Carrillo-Roa T, Penninx BW, Sallis HM, Viktorin A, Chapman B, . . . Wray NR (2014). Applying polygenic risk scores to postpartum depression. Archives of Women’s Mental Health. doi: 10.1007/s00737-014-0428-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardoso F, van’t Veer LJ, Bogaerts J, Slaets L, Viale G, Delaloge S, . . . Investigators M (2016). 70-Gene Signature as an Aid to Treatment Decisions in Early-Stage Breast Cancer. New England Journal of Medicine, 375(8), 717–729. doi: 10.1056/NEJMoa1602253 [DOI] [PubMed] [Google Scholar]
- Chesney E, Goodwin GM, & Fazel S (2014). Risks of all-cause and suicide mortality in mental disorders: a meta-review. World Psychiatry, 13(2), 153–160. doi: 10.1002/wps.20128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corvin A, Craddock N, & Sullivan PF (2010). Genome-wide association studies: a primer. Psychological Medicine, 40(7), 1063–1077. doi: 10.1017/S0033291709991723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costas J, Gratacos M, Escaramis G, Martin-Santos R, de Diego Y, Baca-Garcia E, . . . Sanjuan J (2010). Association study of 44 candidate genes with depressive and anxiety symptoms in post-partum women. Journal of Psychiatric Research, 44(11), 717–724. doi: 10.1016/j.jpsychires.2009.12.012 [DOI] [PubMed] [Google Scholar]
- Cox EQ, Sowa NA, Meltzer-Brody SE, & Gaynes BN (2016). The Perinatal Depression Treatment Cascade: Baby Steps Toward Improving Outcomes. Journal of Clinical Psychiatry, 77(9), 1189–1200. doi: 10.4088/JCP.15r10174 [DOI] [PubMed] [Google Scholar]
- Cox JL, Holden JM, & Sagovsky R (1987). Detection of postnatal depression. Development of the 10-item Edinburgh Postnatal Depression Scale. British Journal of Psychiatry, 150, 782–786. [DOI] [PubMed] [Google Scholar]
- Cox JL, Murray D, & Chapman G (1993). A controlled study of the onset, duration and prevalence of postnatal depression. British Journal of Psychiatry, 163, 27–31. [DOI] [PubMed] [Google Scholar]
- de Leeuw CA, Mooij JM, Heskes T, & Posthuma D (2015). MAGMA: generalized gene-set analysis of GWAS data. PLoS Computational Biology, 11(4), e1004219. doi: 10.1371/journal.pcbi.1004219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Florio A, Putnam K, Altemus M, Apter G, Bergink V, Bilszta J, . . . Meltzer-Brody S (2017). The impact of education, country, race and ethnicity on the self-report of postpartum depression using the Edinburgh Postnatal Depression Scale. Psychological Medicine, 47(5), 787–799. doi: 10.1017/S0033291716002087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forty L, Jones L, Macgregor S, Caesar S, Cooper C, Hough A, . . . Jones I (2006). Familiality of postpartum depression in unipolar disorder: results of a family study. American Journal of Psychiatry, 163(9), 1549–1553. doi: 10.1176/ajp.2006.163.9.1549 [DOI] [PubMed] [Google Scholar]
- Gaspar HA, & Breen G (2017). Pathways analyses of schizophrenia GWAS focusing on known and novel drug targets. bioRxiv. doi: 10.1101/091264 [DOI] [Google Scholar]
- Guintivano J, Krohn H, Lewis C, Byrne EM, Henders AK, Ploner A, . . . Meltzer-Brody S (2018). PPD ACT: An App-based Genetic Study of Postpartum Depression. Translational Psychiatry(Under Review). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes B (2013). Overview of Statistical Methods for Genome-Wide Association Studies (GWAS). Methods in Molecular Biology, 1019, 149–169. doi: 10.1007/978-1-62703-447-0_6 [DOI] [PubMed] [Google Scholar]
- Hewitt CE, & Gilbody SM (2009). Is it clinically and cost effective to screen for postnatal depression: a systematic review of controlled clinical trials and economic evidence. BJOG: An International Journal of Obstetrics and Gynaecology, 116(8), 1019–1027. doi: 10.1111/j.1471-0528.2009.02148.x [DOI] [PubMed] [Google Scholar]
- Insel T, Cuthbert B, Garvey M, Heinssen R, Pine DS, Quinn K, . . . Wang P (2010). Research domain criteria (RDoC): toward a new classification framework for research on mental disorders. American Journal of Psychiatry, 167(7), 748–751. doi: 10.1176/appi.ajp.2010.09091379 [DOI] [PubMed] [Google Scholar]
- Johannsen BM, Larsen JT, Laursen TM, Bergink V, Meltzer-Brody S, & Munk-Olsen T (2016). All-Cause Mortality in Women With Severe Postpartum Psychiatric Disorders. American Journal of Psychiatry, 173(6), 635–642. doi: 10.1176/appi.ajp.2015.14121510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko JY, Rockhill KM, Tong VT, Morrow B, & Farr SL (2017). Trends in Postpartum Depressive Symptoms - 27 States, 2004, 2008, and 2012. MMWR: Morbidity and Mortality Weekly Report, 66(6), 153–158. doi: 10.15585/mmwr.mm6606a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahon PB, Payne JL, MacKinnon DF, Mondimore FM, Goes FS, Schweizer B, . . . Potash JB (2009). Genome-wide linkage and follow-up association study of postpartum mood symptoms. American Journal of Psychiatry, 166(11), 1229–1237. doi: 10.1176/appi.ajp.2009.09030417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meltzer-Brody S, Boschloo L, Jones I, Sullivan PF, & Penninx BW (2013). The EPDS-Lifetime: assessment of lifetime prevalence and risk factors for perinatal depression in a large cohort of depressed women. Archives of Women’s Mental Health, 16(6), 465–473. doi: 10.1007/s00737-013-0372-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meltzer-Brody S, Howard LM, Bergink V, Vigod S, Jones I, Munk-Olsen T, . . . Milgrom J (2018). Postpartum psychiatric disorders. Nat Rev Dis Primers, 4, 18022. doi: 10.1038/nrdp.2018.22 [DOI] [PubMed] [Google Scholar]
- Munk-Olsen T, Maegbaek ML, Johannsen BM, Liu X, Howard LM, di Florio A, . . . Meltzer-Brody S (2016). Perinatal psychiatric episodes: a population-based study on treatment incidence and prevalence. Transl Psychiatry, 6(10), e919. doi: 10.1038/tp.2016.190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy-Eberenz K, Zandi PP, March D, Crowe RR, Scheftner WA, Alexander M, . . . Levinson DF (2006). Is perinatal depression familial? Journal of Affective Disorders, 90(1), 49–55. doi: 10.1016/j.jad.2005.10.006 [DOI] [PubMed] [Google Scholar]
- Netsi E, Pearson RM, Murray L, Cooper P, Craske MG, & Stein A (2018). Association of Persistent and Severe Postnatal Depression With Child Outcomes. JAMA Psychiatry, 75(3), 247–253. doi: 10.1001/jamapsychiatry.2017.4363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postpartum Depression: Action Towards, C., & Treatment, C. (2015). Heterogeneity of postpartum depression: a latent class analysis. Lancet Psychiatry, 2(1), 59–67. doi: 10.1016/S2215-0366(14)00055-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putnam KT, Wilcox M, Robertson-Blackmore E, Sharkey K, Bergink V, Munk-Olsen T, . . . Treatment C (2017). Clinical phenotypes of perinatal depression and time of symptom onset: analysis of data from an international consortium. Lancet Psychiatry, 4(6), 477–485. doi: 10.1016/S2215-0366(17)30136-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiller CE, Meltzer-Brody S, & Rubinow DR (2015). The role of reproductive hormones in postpartum depression. CNS Spectr, 20(1), 48–59. doi: 10.1017/S1092852914000480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schizophrenia Working Group of the Psychiatric Genomics, C. (2014). Biological insights from 108 schizophrenia-associated genetic loci. Nature, 511(7510), 421–427. doi: 10.1038/nature13595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparano JA, Gray RJ, Makower DF, Pritchard KI, Albain KS, Hayes DF, . . . Sledge GW (2015). Prospective Validation of a 21-Gene Expression Assay in Breast Cancer. New England Journal of Medicine, 373(21), 2005–2014. doi: 10.1056/NEJMoa1510764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treloar SA, Martin NG, Bucholz KK, Madden PA, & Heath AC (1999). Genetic influences on post-natal depressive symptoms: findings from an Australian twin sample. Psychological Medicine, 29(3), 645–654. [DOI] [PubMed] [Google Scholar]
- Viktorin A, Meltzer-Brody S, Kuja-Halkola R, Sullivan PF, Landen M, Lichtenstein P, & Magnusson PK (2016). Heritability of Perinatal Depression and Genetic Overlap With Nonperinatal Depression. American Journal of Psychiatry, 173(2), 158–165. doi: 10.1176/appi.ajp.2015.15010085 [DOI] [PubMed] [Google Scholar]
- Wisner KL, Moses-Kolko EL, & Sit DK (2010). Postpartum depression: a disorder in search of a definition. Archives of Women’s Mental Health, 13(1), 37–40. doi: 10.1007/s00737-009-0119-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisner KL, Parry BL, & Piontek CM (2002). Clinical practice. Postpartum depression. New England Journal of Medicine, 347(3), 194–199. doi: 10.1056/NEJMcp011542 [DOI] [PubMed] [Google Scholar]
- Wray NRR, S.; Mattheisen M; Trzaskowski M; Byrne EM; Abdellaoui A; Adams MJ; Agerbo E; Air TM; Andlauer TFM; Bacanu S; Bækvad-Hansen M; Beekman ATF; Bigdeli TB; Binder ER; Blackwood DHR; Bryois J; Buttenschøn HN; Bybjerg-Grauholm J; Cai N; Castelao E; Christensen JH; Clarke T; Coleman JRI; Colodro-Conde L; Couvy-Duchesne B; Craddock N; Crawford GE; Crowley CA; Dashti HS; Davies G; Deary IJ; Degenhardt F; Derks EM; Direk N; Dolan CV; Dunn EC; Eley TC; Eriksson N; Escott-Price V; Kiadeh FFH; Finucane HK; Forstner AJ; Frank J; Gaspar HA; Gill M; Giusti-Rodríguez P; Goes FS; Gordon SD; Grove J; Hall LS; Hansen CS; Hansen TF; Herms S; Hickie IB; Hoffmann P; Homuth G; Horn C; Hottenga J; Hougaard DM; Hu M; Hyde CL; Ising M; Jansen R; Jin F; Jorgenson E; Knowles JA; Kohane IS; Kraft J; Kretzschmar WW; Krogh J; Kutalik Z; Lane JM; Li Y; Li Y; Lind PA; Liu X; Lu L; MacIntyre DJ; MacKinnon DF; Maier RM; Maier W; Marchini J; Mbarek H; McGrath P; McGuffin P; Medland SE; Mehta D; Middeldorp CM; Mihailov E; Milaneschi Y; Milani L; Mondimore FM; Montgomery GW; Mostafavi S; Mullins N; Nauck M; Ng B; Nivard MG; Nyholt DR; O’Reilly PF; Oskarsson H; Owen MJ; Painter JN; Pedersen CB; Pedersen MG; Peterson RE; Pettersson E; Peyrot WJ; Pistis G; Posthuma D; Purcell SM; Quiroz JA; Qvist P; Rice JP; Riley BP; Rivera M; Mirza SS; Saxena R; Schoevers R; Schulte EC; Shen L; Shi J; Shyn SI; Sigurdsson E; Sinnamon GCB; Smit JH; Smith DJ; Stefansson H; Steinberg S; Stockmeier CA; Streit F; Strohmaier J; Tansey KE; Teismann H; Teumer A; Thompson W; Thomson PA; Thorgeirsson TE; Tian C; Traylor M; Treutlein J; Trubetskoy V; Uitterlinden AG; Umbricht D; Van der Auwera S; van Hemert AM; Viktorin A; Visscher PM; Wang Y; Webb BT; Weinsheimer SM; Wellmann J; Willemsen G; Witt SH; Wu Y; Xi HS; Yang J; Zhang F; eQTLGen Consortium; 23andMe Research Team; Arolt V; Baune BT; Berger K; Boomsma DI; Cichon S; Dannlowski U; de Geus EJC; DePaulo JR; Domenici E; Domschke K; Esko T; Grabe HJ; Hamilton SP; Hayward C; Heath AC; Hinds DA; Kendler KS; Kloiber S; Lewis G; Li QS; Lucae S; Madden PAF; Magnusson PK; Martin NG; McIntosh AM; Metspalu A; Mors O; Mortensen PB; Müller-Myhsok B; Nordentoft M; Nöthen MM; O’Donovan MC; Paciga SA; Pedersen NL; Penninx BWJH; Perlis RH; Porteous DJ; Potash JB; Preisig M; Rietschel M; Schaefer C; Schulze TG; Smoller JW; Stefansson K; Tiemeier H; Uher R; Völzke H; Weissman MM; Werge T; Winslow AR; Lewis CM; Levinson DF; Breen G; Børglum AD; Sullivan PF; for the Major Depressive Disorder Working Group of the Psychiatric Genomics Consortium. (2018). Genome-wide association analyses identify 44 risk variants and refine the genetic architecture of major depressive disorder. Nature Genetics, In press. [DOI] [PMC free article] [PubMed] [Google Scholar]


