Abstract
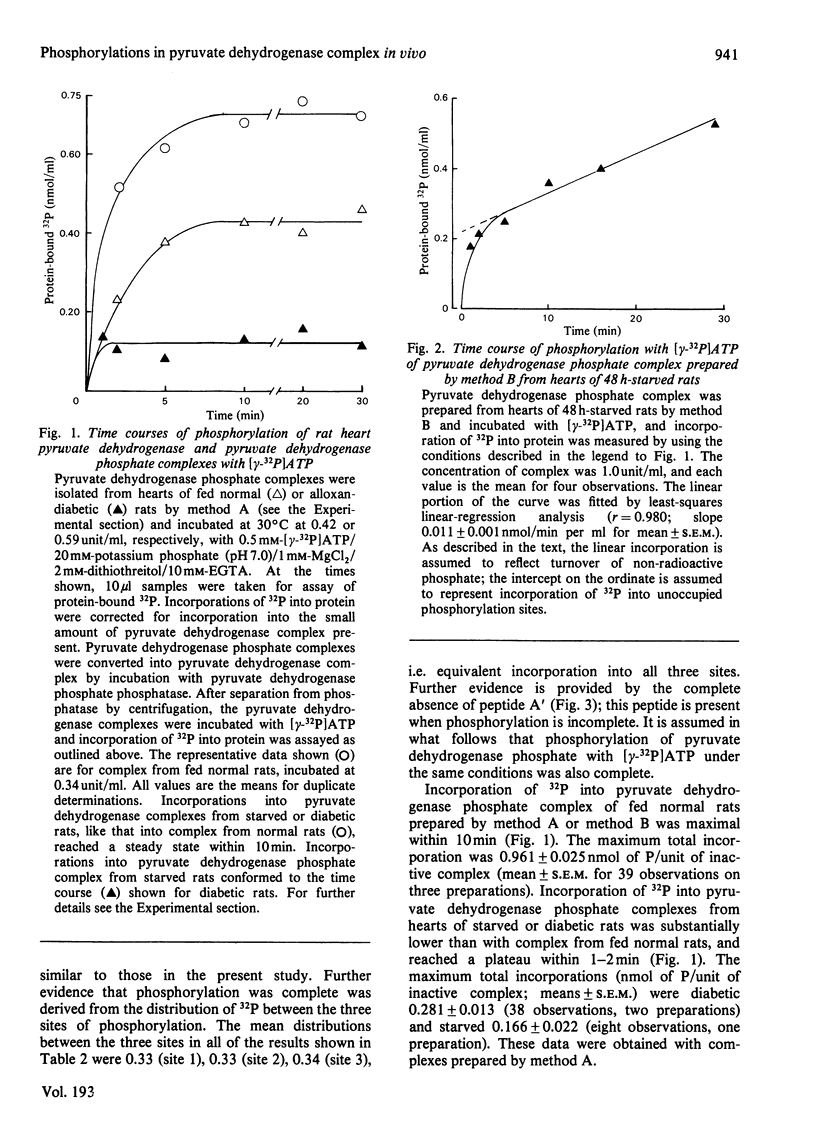
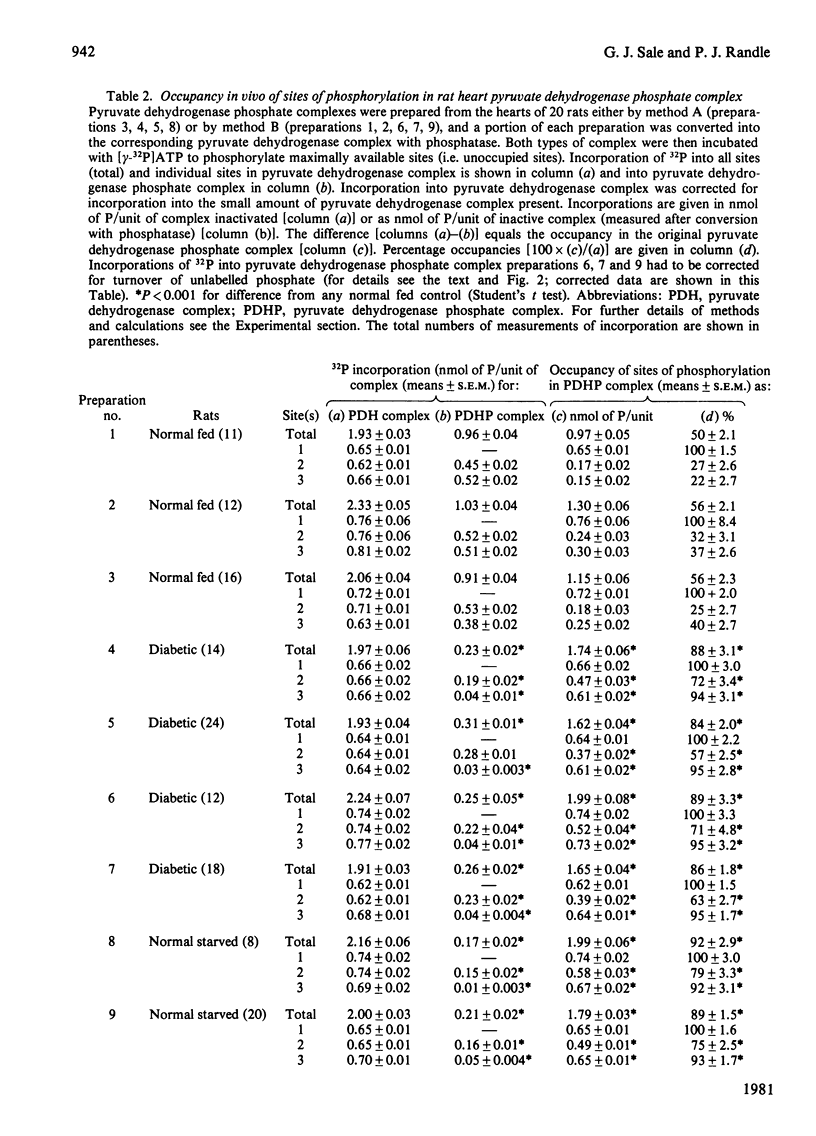
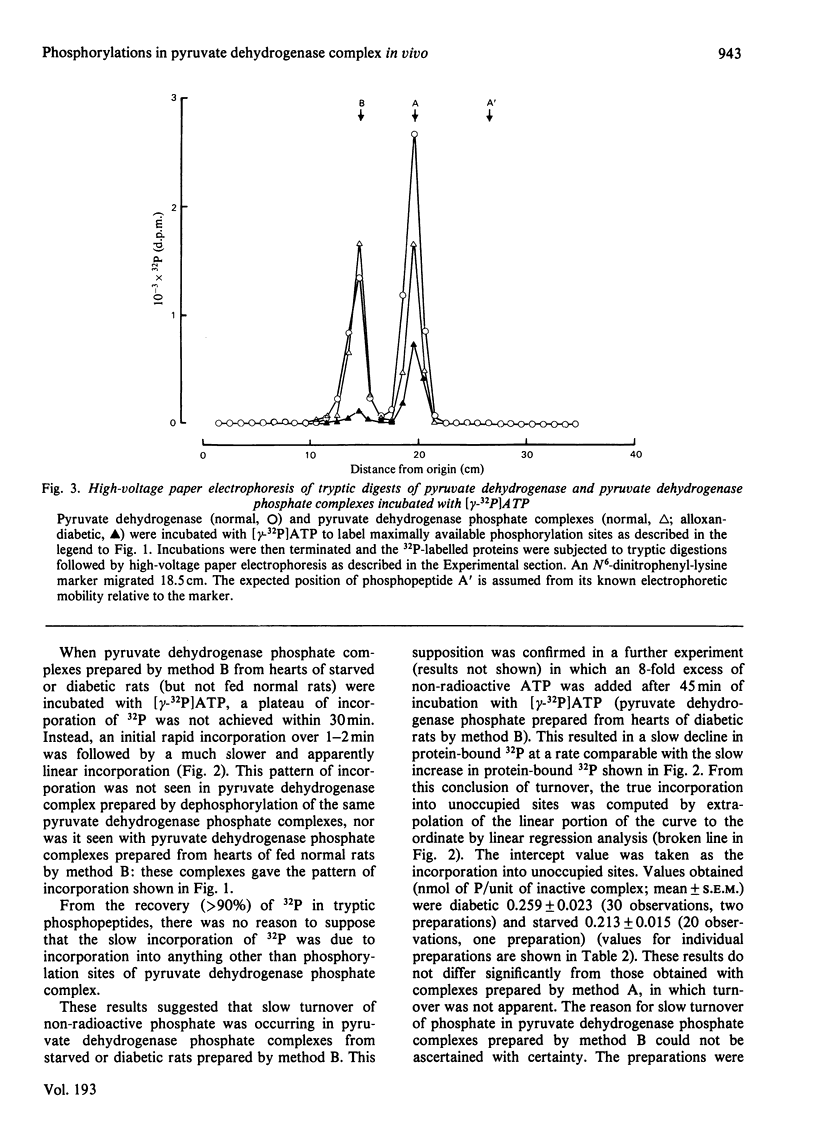
1. Inactive pyruvate dehydrogenase phosphate complexes were partially purified from hearts of fed, starved or alloxan-diabetic rats by using conditions that prevent phosphorylation or dephosphorylation. 2. Unoccupied sites of phosphorylation were assayed by incorporation of 32P from [gamma-32P]ATP into the complexes. Total sites of phosphorylation were assayed by the same method after complete reactivation, and thus dephosphorylation, of complexes by incubation with pyruvate dehydrogenase phosphate phosphatase. Occupancy is assumed from the difference (total sites--unoccupied sites). Percentage incorporation into individual sites was measured by high-voltage electrophoresis after tryptic digestion. 3. Values (means +/- S.E.M., in nmol of phosphate/unit of inactive complex) for total sites, occupied sites and percentage occupancies, with numbers of observations in parentheses were: fed, 2.1 +/- 0.04, 1.15 +/- 0.04, 54.8 +/- 1.6% (39); starved, 2.05 +/- 0.03, 1.85 +/- 0.03, 90.2 +/- 1.4% (28); alloxan-diabetic, 1.99 +/- 0.03, 1.72 +/- 0.03, 86.4 +/- 1.4% (68%). 4. Values (means +/- S.E.M. for percentage occupancy) for individual sites of phosphorylation in pyruvate dehydrogenase phosphate given in the order sites 1, 2 and 3 were : fed, 100 +/- 2.7, 27.8 +/- 1.6, 33.9 +/- .9; starved, 100 +/- 1.4, 76.2 +/- 2.0, 92.4 +/- 1.5; alloxan-diabetic, 100 +/- 1.2, 64.0 +/- 1.7, 94.6 +/- 1.4. 5. It is concluded that starvation or alloxan-diabetes leads to a 2--3-fold increase in the occupancy of phosphorylation sites 2 and 3 in pyruvate dehydrogenase phosphate in rat heart in vivo.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cooper R. H., Randle P. J., Denton R. M. Regulation of heart muscle pyruvate dehydrogenase kinase. Biochem J. 1974 Dec;143(3):625–641. doi: 10.1042/bj1430625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper R. H., Randle P. J., Denton R. M. Stimulation of phosphorylation and inactivation of pyruvate dehydrogenase by physiological inhibitors of the pyruvate dehydrogenase reaction. Nature. 1975 Oct 30;257(5529):808–809. doi: 10.1038/257808a0. [DOI] [PubMed] [Google Scholar]
- Corbin J. D., Reimann E. M. Assay of cyclic AMP-dependent protein kinases. Methods Enzymol. 1974;38:287–290. doi: 10.1016/0076-6879(74)38044-5. [DOI] [PubMed] [Google Scholar]
- Davis P. F., Pettit F. H., Reed L. J. Peptides derived from pyruvate dehydrogenase as substrates for pyruvate dehydrogenase kinase and phosphatase. Biochem Biophys Res Commun. 1977 Apr 11;75(3):541–549. doi: 10.1016/0006-291x(77)91506-6. [DOI] [PubMed] [Google Scholar]
- Garland P. B., Randle P. J. Regulation of glucose uptake by muscles. 10. Effects of alloxan-diabetes, starvation, hypophysectomy and adrenalectomy, and of fatty acids, ketone bodies and pyruvate, on the glycerol output and concentrations of free fatty acids, long-chain fatty acyl-coenzyme A, glycerol phosphate and citrate-cycle intermediates in rat heart and diaphragm muscles. Biochem J. 1964 Dec;93(3):678–687. doi: 10.1042/bj0930678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutson N. J., Kerbey A. L., Randle P. J., Sugden P. H. Conversion of inactive (phosphorylated) pyruvate dehydrogenase complex into active complex by the phosphate reaction in heart mitochondria is inhibited by alloxan-diabetes or starvation in the rat. Biochem J. 1978 Aug 1;173(2):669–680. doi: 10.1042/bj1730669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutson N. J., Randle P. J. Enhanced activity of pyruvate dehydrogenase kinase in rat heart mitochondria in alloxan-diabetes or starvation. FEBS Lett. 1978 Aug 1;92(1):73–76. doi: 10.1016/0014-5793(78)80724-8. [DOI] [PubMed] [Google Scholar]
- Kerbey A. L., Radcliffe P. M., Randle P. J. Diabetes and the control of pyruvate dehydrogenase in rat heart mitochondria by concentration ratios of adenosine triphosphate/adenosine diphosphate, of reduced/oxidized nicotinamide-adenine dinucleotide and of acetyl-coenzyme A/coenzyme A. Biochem J. 1977 Jun 15;164(3):509–519. doi: 10.1042/bj1640509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerbey A. L., Radcliffe P. M., Randle P. J., Sugden P. H. Regulation of kinase reactions in pig heart pyruvate dehydrogenase complex. Biochem J. 1979 Aug 1;181(2):427–433. doi: 10.1042/bj1810427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerbey A. L., Randle P. J., Cooper R. H., Whitehouse S., Pask H. T., Denton R. M. Regulation of pyruvate dehydrogenase in rat heart. Mechanism of regulation of proportions of dephosphorylated and phosphorylated enzyme by oxidation of fatty acids and ketone bodies and of effects of diabetes: role of coenzyme A, acetyl-coenzyme A and reduced and oxidized nicotinamide-adenine dinucleotide. Biochem J. 1976 Feb 15;154(2):327–348. doi: 10.1042/bj1540327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerbey A. L., Randle P. J. Role of multi-site phosphorylation in regulation of pig heart pyruvate dehydrogenase phosphatase. FEBS Lett. 1979 Dec 15;108(2):485–488. doi: 10.1016/0014-5793(79)80594-3. [DOI] [PubMed] [Google Scholar]
- Linn T. C., Pettit F. H., Hucho F., Reed L. J. Alpha-keto acid dehydrogenase complexes. XI. Comparative studies of regulatory properties of the pyruvate dehydrogenase complexes from kidney, heart, and liver mitochondria. Proc Natl Acad Sci U S A. 1969 Sep;64(1):227–234. doi: 10.1073/pnas.64.1.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linn T. C., Pettit F. H., Reed L. J. Alpha-keto acid dehydrogenase complexes. X. Regulation of the activity of the pyruvate dehydrogenase complex from beef kidney mitochondria by phosphorylation and dephosphorylation. Proc Natl Acad Sci U S A. 1969 Jan;62(1):234–241. doi: 10.1073/pnas.62.1.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettit F. H., Pelley J. W., Reed L. J. Regulation of pyruvate dehydrogenase kinase and phosphatase by acetyl-CoA/CoA and NADH/NAD ratios. Biochem Biophys Res Commun. 1975 Jul 22;65(2):575–582. doi: 10.1016/s0006-291x(75)80185-9. [DOI] [PubMed] [Google Scholar]
- Radcliffe P. M., Kerbey A. L., Randle P. J. Inactivation of pig heart pyruvate dehydrogenase complex by adenosine-5'-O(3-thiotriphosphate). FEBS Lett. 1980 Feb 25;111(1):47–50. doi: 10.1016/0014-5793(80)80758-7. [DOI] [PubMed] [Google Scholar]
- Sale G. J., Randle P. J. Incorporation of [32P]phosphate into the pyruvate dehydrogenase complex in rat heart mitochondria. Biochem J. 1980 May 15;188(2):409–421. doi: 10.1042/bj1880409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Severson D. L., Denton R. M., Pask H. T., Randle P. J. Calcium and magnesium ions as effectors of adipose-tissue pyruvate dehydrogenase phosphate phosphatase. Biochem J. 1974 May;140(2):225–237. doi: 10.1042/bj1400225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugden P. H., Hutson N. J., Kerbey A. L., Randle P. J. Phosphorylation of additional sites on pyruvate dehydrogenase inhibits its re-activation by pyruvate dehydrogenase phosphate phosphatase. Biochem J. 1978 Feb 1;169(2):433–435. doi: 10.1042/bj1690433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugden P. H., Kerbey A. L., Randle P. J., Waller C. A., Reid K. B. Amino acid sequences around the sites of phosphorylation in the pig heart pyruvate dehydrogenase complex. Biochem J. 1979 Aug 1;181(2):419–426. doi: 10.1042/bj1810419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugden P. H., Simister N. E. Role of multisite phosphorylation in the regulation of ox kidney pyruvate dehydrogenase complex. FEBS Lett. 1980 Mar 10;111(2):299–302. doi: 10.1016/0014-5793(80)80814-3. [DOI] [PubMed] [Google Scholar]
- Teague W. M., Pettit F. H., Yeaman S. J., Reed L. J. Function of phosphorylation sites on pyruvate dehydrogenase. Biochem Biophys Res Commun. 1979 Mar 15;87(1):244–252. doi: 10.1016/0006-291x(79)91672-3. [DOI] [PubMed] [Google Scholar]
- Whitehouse S., Cooper R. H., Randle P. J. Mechanism of activation of pyruvate dehydrogenase by dichloroacetate and other halogenated carboxylic acids. Biochem J. 1974 Sep;141(3):761–774. doi: 10.1042/bj1410761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieland O., Siess E., Schulze-Wethmar F. H., von Funcke H. G., Winton B. Active and inactive forms of pyruvate dehydrogenase in rat heart and kidney: effect of diabetes, fasting, and refeeding on pyruvate dehydrogenase interconversion. Arch Biochem Biophys. 1971 Apr;143(2):593–601. doi: 10.1016/0003-9861(71)90244-x. [DOI] [PubMed] [Google Scholar]
- Yeaman S. J., Hutcheson E. T., Roche T. E., Pettit F. H., Brown J. R., Reed L. J., Watson D. C., Dixon G. H. Sites of phosphorylation on pyruvate dehydrogenase from bovine kidney and heart. Biochemistry. 1978 Jun 13;17(12):2364–2370. doi: 10.1021/bi00605a017. [DOI] [PubMed] [Google Scholar]


