Abstract
Background
Previous studies have suggested that acupuncture could improve the clinical outcomes of women with premature ovarian insufficiency (POI) and polycystic ovary syndrome (PCOS). However recent meta-analyses have provided inconclusive findings. This umbrella meta-analysis aimed to explore the effect of acupuncture therapies on PCOS and POI outcomes.
Methods
A systematic literature search was carried out in in PubMed, Scopus, Web of Science, and Chinese databases, including Wan Fang Data Knowledge Service Platform, CBM, CNKI, and VIP up until April 2024 to gather relevant studies. Inclusion criteria were meta-analyses on the effect of acupuncture or combined therapies with standard medications or traditional Chinese medicine (TCM) on PCOS and POI. The outcomes were pregnancy rates, ovulation rates, hormone levels, glycemic indices, resumption of menstruation, endometrial thickness, live birth rates, abortion rates, and body mass index (BMI). Studies with irrelevant interventions, animal studies, reviews without quantitative analysis, and studies with insufficient data were excluded. Standardized mean difference (SMD) with a 95% confidence interval (CI) and relative risk (RR) with a 95% CI were used as effect sizes to pool the data using a random effects model.
Results
A total of 38 meta-analyses, 20 studies (sample size: 27,106 patients) for PCOS and 18 studies (sample size: 19,098 patients) for POI, were included. Overall, in women with PCOS, acupuncture therapies were significantly associated with a higher pregnancy rate, ovulation rate, and reduced serum levels of luteinizing hormone (LH), testosterone, LH/follicle-stimulating hormone (FSH), insulin resistance, and BMI. Moreover, FSH, fasting glucose, and fasting insulin levels were improved in subgroup analyses. For POI, acupuncture significantly improved serum levels of LH, FSH, LH/FSH ratio, and estradiol.
Conclusion
Acupuncture-related therapies improve pregnancy rate, and metabolic and hormonal imbalances in patients with POI and PCOS.
Systematic review registration
The protocol of the study was registered in PROSPERO (CRD42024572893). Available from: https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42024572893.
Keywords: acupuncture, polycystic ovary syndrome, premature ovarian insufficiency, premature ovarian failure, primary ovarian insufficiency, pregnancy rate, umbrella meta-analysis
Introduction
Polycystic ovary syndrome (PCOS) and premature ovarian insufficiency (POI) are two distinct complex endocrine disorders that potentially interfere with women’s reproductive health and fertility (1). PCOS and POI impact 10 and 1% of women in their reproductive years, respectively, and their prevalence is increasing globally (2). PCOS is the leading cause of hyperandrogenism, oligoanovulation, and infertility in women, featured by hormonal imbalances, irregular menstrual cycles, and the presence of polycystic ovaries (3). Women with PCOS may encounter insulin resistance, as well as issues like obesity, inflammation, acne, hair thinning, mood fluctuations, and excessive hair growth (4). The pathophysiology of PCOS involves elevated levels of gonadotropin-releasing hormone (GnRH), which disrupt the normal LH/FSH ratio, potentially causing early luteinization of granulosa cells and impairing follicular development (5). POI, on the other side, is featured by the cessation of ovarian function before the age of 40, leading to hypergonadotropic amenorrhea, elevated gonadotrophins, and low estradiol (E2) levels (6). Although PCOS and POI have different pathophysiology, autoimmune disorders, genetic factors, and environmental factors have all been implicated in their development (7, 8). The complications of both conditions extend beyond fertility challenges; they pose long-term health risks such as type 2 diabetes, cardiovascular diseases, and osteoporosis (9, 10).
Polycystic ovary syndrome can be managed by herbal formulations, following a healthy lifestyle, drugs, acupuncture, and bariatric surgery (4). To restore ovulation and improve fertility, current pharmacological treatments for PCOS include letrozole, an aromatase inhibitor, as a first-line therapy, with clomiphene citrate serving as an alternative when initial treatments fail (11). The combination of clomiphene citrate with metformin has shown promise for women who do not respond to clomiphene alone. This approach addresses both ovulation induction and insulin resistance, a common issue in PCOS (12). Newer medications targeting insulin sensitivity, such as incretin mimetics and Sodium/glucose cotransporter 2 (SGLT2) inhibitors, have emerged as effective options for managing metabolic dysfunction in PCOS, potentially reducing cardiovascular risks (13). Emerging evidence suggests that inositol and vitamin D supplementation may improve metabolic parameters in women with PCOS, although further research is needed to establish clear guidelines (14). Moreover, emphasis on lifestyle modifications, such as weight loss and increased physical activity, has been recommended as a critical component of managing PCOS. These changes can enhance metabolic health and improve reproductive outcomes (15). While these treatments are generally effective, concerns arise regarding adverse effects, including elevated risk of multifetal pregnancies, as well as patient adherence due to the extended duration of treatment necessary for individuals with PCOS (16).
For POI, HRT remains the cornerstone of POI management, focusing on estrogen repletion to alleviate symptoms and protect long-term health. Transdermal patches or transvaginal applications are preferred due to lower risks of venous thromboembolism compared to oral estrogen (17). However, HRT effectively addresses symptoms related to hormonal imbalances, it does not restore ovarian function or improve fertility outcomes. Additionally, a remarkable relapse rate of low estrogen symptoms is commonly observed following the cessation of the medication (18). Moreover, HRT has been linked to several serious adverse effects, such as an elevated risk of heart attack, breast cancer, and stroke (19). Oocyte donation continues to be the most effective assisted reproductive technology for women with POI, achieving pregnancy rates of 70–80%. However, ongoing research is exploring additional methods to enhance fertility in this population (20). Also, in vitro activation is an innovative technique that disrupts signaling pathways to activate dormant follicles in women with POI, showing potential for restoring ovarian function and improving fertility outcomes (21). Moreover, emerging biological therapies such as platelet-rich plasma (PRP) therapy, stem cell therapy, exosome therapy, and mitochondrial targeting therapies have offered new avenues for treating POI to activate oogenesis and improve the ovarian microenvironment (22).
Given these limitations in conventional treatments for PCOS and POI, alternative therapeutic approaches are being explored. Acupuncture a key component of traditional Chinese medicine, has gained attention as a potential alternative treatment for managing the symptoms of PCOS and POI because of its convenience and low side effects. Its use in treating reproductive disorders has shown promising results in improving menstrual regularity, hormone levels, metabolic factors, and overall fertility outcomes. Nevertheless, recent meta-analyses have provided contradictory results with positive (23, 24) or null (25) effects and the available evidence is insufficient to conclusively determine the effectiveness of acupuncture on critical reproductive outcomes in women with PCOS and POI. Differences in acupuncture methods, sample sizes, and treatment protocols may account for the heterogeneity reported in the findings of previous studies. This umbrella meta-analysis aimed to examine the effectiveness of acupuncture in addressing the symptoms of PCOS and POI by synthesizing data from multiple meta-analyses.
The term “premature ovarian insufficiency” (POI) has evolved significantly over time. In the 1940s, endocrinologist Fuller Albright first introduced the concept of “primary ovarian insufficiency (26).” Subsequently, the term “premature ovarian failure” (POF) became widely adopted. However, clinical practice has revealed that POF is a terminal stage of ovarian dysfunction, making its use as a universal diagnostic label inappropriate. In 2016, the European Society of Human Reproduction and Embryology (ESHRE) updated the terminology to “premature ovarian insufficiency” (POI) (27). This change underscored the early onset of ovarian function decline. In 2020, the International Menopause Society (IMS) released a white paper on POI, which continues to be referenced today (28). Therefore, this article will encompass all these terms as relevant to the discussion.
Methods
This umbrella meta-analysis adhered to the guidelines outlined in the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) (26). The study was registered in PROSPERO [CRD42024572893], available from https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42024572893.
Search strategy
A systematic literature search was conducted in PubMed, Scopus, Web of Science, Wan Fang Data Knowledge Service Platform, Chinese Biomedical Database (CBM), Chinese National Knowledge Infrastructure (CNKI), and Journal Integration Platform (VIP) databases up to April 2024 to obtain pertinent studies. The search strategy encompassed the following terms: (“Acupuncture”[Mesh] OR “Acupuncture Therapy”[Mesh] OR “Electric Stimulation”[Mesh] OR “Electric Stimulation Therapy”[Mesh] OR acupuncture OR electrical stimulation* OR electro stimulation* OR electric stimulation* OR electrostimulus OR needl* OR Electro acupuncture OR Electroacupuncture) AND (“Polycystic Ovary Syndrome”[Majr] OR “Primary Ovarian Insufficiency”[Majr] OR “polycystic ovary syndrome” OR “polycystic ovar* syndrom*” OR “polycystic ovar* disease” OR PCOS OR “primary ovarian insufficiency” OR early menopause OR “premature menopause” OR “premature ovarian insufficiency” OR “premature ovarian failure” OR amenorrhea OR POI OR POF) AND (meta-analysis OR meta analysis). No language restrictions were considered during the search process. In addition, the list of the references of the obtained reviews was screened manually to prevent overlooking any relevant studies. We did not include registered websites or gray literature in the meta-analysis.
Eligibility criteria
Two researchers individually assessed the identified publications, reviewed the titles/abstracts, and subsequently removed duplicate and irrelevant studies. Any disagreements were addressed through discussions and reaching a consensus. Eligible studies were then identified based on the predetermined inclusion and exclusion criteria. Meta-analyses of randomized clinical trials (RCT) meeting the following criteria were included: (1) participants: women diagnosed with PCOS or POI, (2) intervention: the intervention group used acupuncture or its combined therapy with standard medications or traditional Chinese medicine (TCM), (3) comparator: the control group received placebo/sham acupuncture or standard treatments, (4) outcomes: the primary outcome was pregnancy rate. Secondary outcomes included ovulation rates, hormone levels, such as luteinizing hormone (LH), estradiol (E2), follicle stimulating hormone (FSH), testosterone, LH/FSH ratio, glycemic indices, resumption of menstruation, endometrial thickness, live birth, abortion rate, and body mass index (BMI) after treatment for PCOS or POI. We included studies in which the mean and 95% confidence interval (CI) for continuous outcomes and relative risk (RR) with 95% CI for dichotomous outcomes were presented. Exclusion criteria were as follows: (1) subjects were treated with other interventions, (2) animal studies, (3) review studies with no quantitative analysis, and (4) studies that their data were not extractable.
Data extraction
Data extraction was conducted independently by two researchers and subsequently reviewed by another researcher to gather relevant information potentially linked to the research outcomes, as outlined below: First author, country, publication year, outcome measures, sample size, number of the included studies, risk of bias (ROB) assessment, type of intervention, type of control, and effect sizes for the outcomes. In cases of missing data, we contacted to the corresponding authors of the relevant studies to request the necessary information. This approach allowed us to obtain the required data directly from the original sources.
Quality evaluation
The quality of the included studies was evaluated using a critical appraisal tool for systematic reviews-2 (AMSTAR-2), which involves a structured approach to evaluate the reliability and rigor of systematic reviews, helping to determine the trustworthiness and validity of the study findings (27). The tool consists of 16 items divided into seven critical and nine non-critical domains that assesses various aspects of systematic reviews. The critical items evaluate aspects such as whether a priori protocols were established, the comprehensiveness of literature searches, justification for study exclusions, risk of bias assessments, appropriate meta-analysis methods, and consideration of publication bias. The non-critical items address additional factors like funding sources and potential conflicts of interest. The quality of studies were categorizes as very low, low, moderate, and high based on the AMSTAR-2 criteria. Two independent reviewers assessed each study using the AMSTAR-2 checklist. Any disagreements between reviewers were resolved through discussion to reach a consensus. We did not employ any automated tools in this process, as we aimed to ensure thorough and careful consideration of quality evaluation.
Statistical analysis
Continuous data were analyzed using the standardized mean difference (SMD) and 95% CI, whereas binary data were assessed using the RR and 95% CI. The I2 test was employed to evaluate statistical heterogeneity. An I2 value exceeding 50% suggests the presence of significant heterogeneity. Due to expected heterogeneity, the data were combined using a random effects model. The origin of heterogeneity was investigated through subgroup analyses based on the quality of studies, type of intervention, duration of follow-up, and sample size. For outcomes with more than 10 effect sizes, meta-regression analysis was also conducted to assess the effect of the percentage of primary studies with low ROB on the pooled effect sizes. Sensitivity analyses were performed to assess the reliability of the analyses. If more than 10 effect sizes were available, publication bias was evaluated with the use of the funnel plot and Egger’s test (28). When there was a significant publication bias, the trim-and-fill method (29) was applied to adjust the results for the observed publication bias. The analysis was conducted using the Stata software version 13.
Results
Characteristics of the studies
Through a systematic search of databases, 251 articles were identified. Among these, 65 duplicates were removed, 135 publications were excluded following title and abstract screening, and 13 studies were excluded after a full-text assessment based on the inclusion/exclusion criteria. Excluded studies were review studies, protocols, animal studies, or studies with irrelevant exposure/outcome. The process of study selection is reported in Figure 1. Ultimately, 38 studies (23–25, 30–64), published between 2014 and 2023, were included in the umbrella meta-analysis. There were 20 studies (sample size: 27,106 patients) on PCOS (24, 25, 30–33, 37, 38, 40, 42, 44, 48, 50, 52, 55, 56, 60–62, 64) and 18 studies (sample size: 19,098 patients) on POI (23, 34–36, 39, 41, 43, 45–47, 49, 51, 53, 54, 57–59, 63). The sample sizes of the studies varied from 368 to 3,231 participants. The mean age of patients was between 23.98 to 35.96 years. The interventions in the treatment group included various types of acupuncture (needle acupuncture, electroacupuncture, auricular points, acupoint catgut embedding, and warm acupuncture), acupuncture with other therapies (TCM or standard medications), acupuncture with moxibustion, moxibustion, and moxibustion with TCM. The control groups received shame acupuncture or standard medications plus TCM. The Follow-up duration of studies ranged from 2.5 to 5.7 months. The ROB in the studies was evaluated utilizing either the Cochrane tool (65) or Jadad score (66). The included meta-analyses exhibited significant diversity in the proportion of RCTs with low ROB, ranging from 0 to 75% of the included RCTs demonstrating low ROB. For PCOS outcomes, pregnancy rate was reported in 12 studies, ovulation rate in 7 studies, resumption of menstruation in 2 studies, rate of live birth in 3 studies, abortion rate in 2 studies, serum LH in 9 studies, serum FSH in 4 studies, LH/FSH ratio in 6 studies, serum testosterone in 7 studies, endometrial thickness in 2 studies, fasting plasma glucose (FPG) in 3 studies, oral glucose tolerance test (OGTT) in 2 studies, fasting insulin in 4 studies, homeostatic model assessment for insulin resistance (HOMA-IR) in 3 studies, and BMI in 6 studies. For POI outcomes, serum LH was presented in 15 studies, serum FSH in 17 studies, and serum estradiol (E2) in 15 studies. The characteristics of the included studies are presented in Table 1.
Figure 1.
Flow diagram of the study.
Table 1.
Characteristics of the included studies.
| Study | Year | Country | Type of disease | Included studies | Mean age | Sample size | Intervention type | Control | Risk of bias assessment, high quality/total studies | High quality studies (%) | Follow-up duration (month) | Outcomes | Quality |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lim (62) | 2016 | Australia | PCOS | 5 | NR | 413 | Acupuncture | Shame acupuncture/Medications | Cochrane tool, 1/5 | 20 | 2.5 | Pregnancy rate, resumption of menstruation | High |
| Lim (50) | 2019 | Australia | PCOS | 8 | NR | 1,546 | Acupuncture | Shame acupuncture/Medications | Cochrane tool, 1/8 | 12 | NR | Pregnancy rate, ovulation rate, live birth, abortion | High |
| Chen (24) | 2022 | China | PCOS | 9 | 27.44 | 1,159 | Acupuncture plus medications | Medications | Cochrane tool, 1/9 | 11 | 3.8 | Pregnancy rate, ovulation rate, HOMA-IR | Moderate |
| Ma (64) | 2014 | China | PCOS | 6 | NR | 587 | Acupuncture | Medications | Cochrane tool, 0/6 | 0 | 3 | ovulation rate, testosterone | Low |
| Jo (55) | 2017 | Republic of Korea | PCOS | 4 | 29.75 | 430 | Acupuncture | Medications | Cochrane tool, 3/4 | 75 | NR | Pregnancy rate, live birth | Moderate |
| Zheng (38) | 2021 | China | PCOS | 10 | 24.71 | 737 | Acupuncture | Medications | Cochrane tool, 5/10 | 50 | 4.62 | HOMA-IR, BMI, FPG, OGTT | Moderate |
| Wu (44) | 2020 | China | PCOS | 22 | 27.88 | 2,315 | Acupuncture Acupuncture plus medications |
Shame acupuncture/Medications | Cochrane tool, 14/22 | 63 | 3.9 | Pregnancy rate, ovulation rate, LH, testosterone, live birth, LH/FSH ratio | Moderate |
| Jo (56) | 2017 | Korea | PCOS | 28 | 23.98 | 2093 | Acupuncture Acupuncture plus medications |
Shame acupuncture/Medications | Cochrane tool, 6/28 | 21 | 3.2 | Pregnancy rate, LH, testosterone, LH/FSH ratio, fasting insulin | High |
| Li (37) | 2022 | China | PCOS | 25 | 35.38 | 1991 | Acupuncture plus Moxibustion | Shame acupuncture/Medications | Cochrane tool, 0/25 | 0 | 4.28 | Pregnancy rate, ovulation rate, LH, testosterone, LH/FSH ratio, FSH, abortion, fasting insulin, BMI | High |
| Liang (33) | 2023 | China | PCOS | 47 | NR | 3,537 | Acupuncture plus TCM | Shame acupuncture/Medications plus TCM | Cochrane tool, 19/47 | 40 | NR | Pregnancy rate, ovulation rate, LH, testosterone, LH/FSH ratio, BMI | Moderate |
| Yun (48) | 2019 | China | PCOS | 22 | 20–40 years | 2,591 | Acupuncture plus medications Acupuncture plus TCM and Medications |
Medications | Cochrane tool, 0/22 | 0 | NR | Pregnancy rate, ovulation rate, LH, LH/FSH ratio, endometrial thickness | Moderate |
| Qu (61) | 2016 | China | PCOS | 9 | NR | 531 | Acupuncture Acupuncture plus TCM |
Shame acupuncture/Medications plus TCM | Cochrane tool, 2/9 | 22 | NR | LH, LH/FSH ratio, FSH, fasting insulin, BMI, FPG | Moderate |
| Hu (40) | 2021 | China | PCOS | 13 | 29.5 | 1,297 | Acupuncture plus medications | Medications | Cochrane tool, 6/13 | 46 | NR | LH | Moderate |
| Liu (32) | 2022 | China | PCOS | 7 | 26.76 | 728 | Electroacupuncture Acupuncture plus Medications Acupuncture |
Shame acupuncture/Medications | Cochrane tool, 3/7 | 42 | 4.6 | HOMA-IR, fasting insulin, BMI, FPG, OGTT | Moderate |
| Chao-chao | 2017 | China | PCOS | 25 | NR | 1,636 | Acupoint catgut embedding | Medications | Cochrane tool, 0/32pcos | 0 | NR | Testosterone, resumption of menstruation | Moderate |
| Meizhu (31) | 2023 | China | PCOS | 15 | NR | 1,205 | Acupuncture | Medications | NR | 3.6 | LH, testosterone, FSH, BMI | Low | |
| Ruigen (60) | 2016 | China | PCOS | 14 | NR | 1,617 | Acupuncture | Medications | Cochrane tool, NR | NR | 3.78 | Pregnancy rate | Low |
| Ping | 2020 | China | PCOS | 7 | NR | 514 | Acupuncture | Medications | NR | NR | NR | Pregnancy rate | Low |
| Lina (52) | 2018 | China | PCOS | 9 | NR | 769 | Acupuncture plus TCM Acupuncture |
Medications | NR | NR | NR | Pregnancy rate, LH, FSH | Moderate |
| Yang (30) | 2023 | China | PCOS | 6 | 28.93 | 1,410 | Acupuncture plus Moxibustion | Medications | Cochrane tool, 3/6 | 50 | 3.16 | Endometrial thickness | Moderate |
| Li (34) | 2023 | China | POI | 10 | 33.35 | 594 | Electroacupuncture plus TCM Needle acupuncture plus TCM |
HRT | Cochrane tool, 0/10 | 0 | 3.6 | LH, FSH, estradiol | High |
| Jo (63) | 2015 | Republic of Korea | POI | 8 | 35.46 | 620 | Acupuncture | Shame acupuncture or HRT | Cochrane tool, 0/8 | 0 | 4.8 | LH, FSH, estradiol | High |
| Wang (23) | 2017 | China | POI | 8 | Under 40 | 368 | Acupuncture | HRT | Cochrane tool, 0/8 | 0 | NR | LH, FSH, estradiol | Moderate |
| Ya-Jian | 2020 | China | POI | 8 | NR | 496 | Acupuncture Acupuncture plus HRT |
HRT | Cochrane tool, 0/8 | 0 | 5.4 | LH, FSH, estradiol | Moderate |
| Li | 2020 | China | POI | 14 | 31.21 | 1,030 | Acupuncture | HRT | Cochrane tool, 0/14 | 0 | 5.7 | LH, FSH, estradiol | High |
| Yuanbo | 2019 | China | POI | 14 | NR | 879 | Acupuncture plus TCM | HRT | Cochrane tool, 7/14 | 50 | 3.42 | LH, FSH, estradiol | Low |
| Runzi (54) | 2016 | China | POI | 7 | Under 40 | 538 | Acupuncture | HRT | NR | NR | NR | LH, FSH, estradiol | Low |
| Tingting (45) | 2018 | China | POI | 16 | NR | 1,357 | Acupuncture | HRT | Cochrane tool, 0/16 | 0 | 5.43 | LH, FSH, estradiol | Moderate |
| Xiaoyang (39) | 2019 | China | POI | 14 | NR | 962 | Acupuncture plus TCM Electroacupuncture plus TCM Auricular points plus TCM Acupoint catgut embedding plus TCM Moxibustion plus TCM |
HRT | Cochrane tool, 0/14 | 0 | 3.85 | LH, FSH, estradiol | Moderate |
| Meiling (51) | 2018 | China | POI | 10 | 32.25 | 690 | Needle acupuncture | HRT | Jadad score, 3/12 | 25 | 5.16 | LH, FSH, estradiol | Low |
| Xiaojuan (35) | 2022 | China | POI | 11 | Under 40 | 775 | Acupuncture | Shame acupuncture or HRT | Cochrane tool, 0/11 | 0 | 4.57 | FSH, estradiol | Moderate |
| Jin-Huan (47) | 2020 | China | POI | 16 | 32 | 1,102 | Acupuncture and Moxibustion | HRT | Cochrane tool, 5/16 | 31 | NR | LH, FSH, estradiol | Low |
| Xi (59) | 2016 | China | POI | 12 | 34.2 | 863 | Acupuncture Acupuncture plus HRT |
HRT | NR | NR | NR | LH, FSH | Moderate |
| Yong (58) | 2016 | China | POI | 9 | NR | 719 | Acupuncture | HRT | NR | NR | NR | LH, FSH, estradiol | Low |
| Zhang (41) | 2020 | China | POI | 16 | 32.93 | 1,307 | Acupuncture Warm acupuncture Acupoint catgut embedding Acupuncture and Moxibustion |
HRT | Cochrane tool, NR | NR | 3.75 | LH, FSH, estradiol | Moderate |
| Xiao | 2017 | China | POI | 7 | NR | 512 | Acupuncture plus HRT | HRT | NR | NR | 5 | LH, FSH, estradiol | Moderate |
| Long (36) | 2022 | China | POI | 32 | NR | 3,231 | Acupuncture and Moxibustion Acupoint catgut embedding Acupuncture plus HRT Acupoint catgut embedding plus HRT Moxibustion Acupuncture Acupuncture plus TCM |
HRT | Cochrane tool, 6/32 | 18 | 4.06 | FSH | Moderate |
TCM, traditional Chinese medicine; NR, not reported; LH, luteinizing hormone; FSH, follicle stimulating hormone; OGTT, oral glucose tolerance test; HOMA-IR, homeostatic model assessment for insulin resistance; BMI, body mass index; HRT, hormone replacement therapy; PCOS, polycystic ovary syndrome; POI, primary ovarian insufficiency.
Quality of the studies
Among the 20 meta-analyses on PCOS, the quality of studies was high in 4 studies, moderate in 12 studies, and low in 4 studies. Of 18 meta-analyses on POI, the study quality was rated as high in 3 studies, moderate in 10 studies, and low in 5 studies (Supplementary Table S1).
Umbrella meta-analysis for PCOS
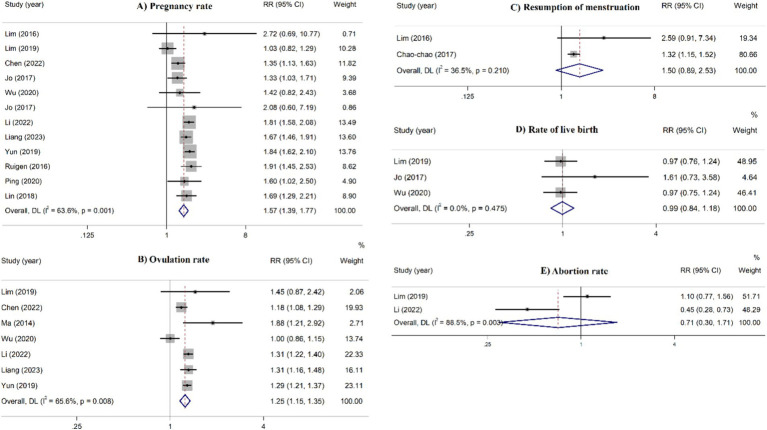
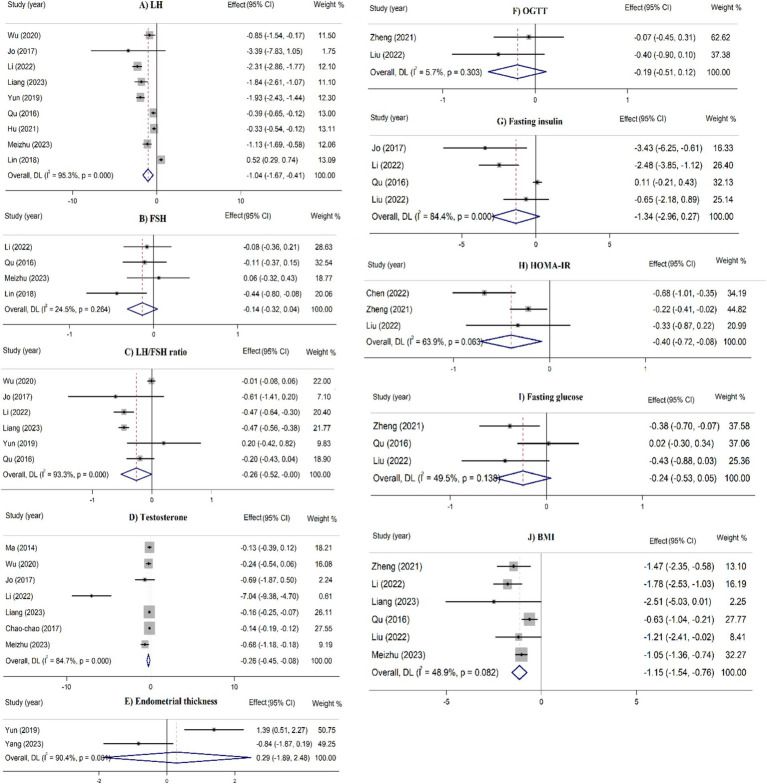
In the overall analyses, acupuncture therapies were significantly 439 associated with a higher pregnancy rate (RR = 1.57, 95% CI: 1.39– 440 1.77), and ovulation rate (RR = 1.25, 95% CI: 1.15–1.35) (Figure 2). Moreover, acupuncture therapies significantly reduced serum levels of LH (SMD = −1.04, 95% CI: −1.67 to −0.41), LH/FSH ratio (SMD = −0.26, 95% CI: −0.52 to −0.003), testosterone levels (SMD = −0.26, 95% CI: −0.45 to −0.08), HOMA-IR (SMD = −0.40, 445, 95% CI: −0.72 to −0.08), and BMI (SMD = −1.15, 95% CI: −1.54 to 446 −0.76) (Figure 3). These effects were supported by the majority of subgroups. However, for ovulation rate and LH/FSH ratio, the treatment was effective when administered for a longer duration (≥4 months) (Table 2).
Figure 2.
Umbrella meta-analysis for the effect of acupuncture on pregnancy-related dichotomous outcomes in patients with PCOS.
Figure 3.
Umbrella meta-analysis for the effect of acupuncture on continuous outcomes (metabolic factors and serum hormones) in patients with PCOS FSH: follicle stimulating hormone, LH: luteinizing hormone, OGGT: oral glucose tolerance test, HOMA-IR: homeostatic model assessment for insulin resistance, BMI: body mass index.
Table 2.
Subgroup analyses for the effect of acupuncture on PCOS.
| Dichotomous outcome | Test of effect | Test of heterogeneity | ||||
|---|---|---|---|---|---|---|
| Subgroups | Studies | RR (95%CI) | I2 (%) | P | ||
| Pregnancy rate | Overall | 12 | 1.57 (1.39–1.77) | 63.6 | 0.001 | |
| Type of intervention | Acupuncture | 7 | 1.70 (1.42–2.02) | 20.0 | 0.27 | |
| Acupuncture plus medications | 4 | 1.52 (1.23–1.88) | 60.6 | 0.05 | ||
| Acupuncture plus moxibustion | 1 | 1.81 (1.58–2.08) | – | – | ||
| Acupuncture plus TCM | 2 | 1.79 (1.51–2.11) | 45.5 | 0.17 | ||
| Acupuncture plus medications and TCM | 1 | 1.52 (1.25–1.85) | – | – | ||
| Quality of studies | Low | 2 | 1.82 (1.44–2.30) | 0.0 | 0.51 | |
| Moderate | 6 | 1.58 (1.40–1.78) | 51.6 | 0.06 | ||
| High | 4 | 1.53 (0.96–2.43) | 83.5 | 0.001 | ||
| Sample size | ≥1,000 participants | 8 | 1.58 (1.36–1.83) | 74.2 | 0.001 | |
| <1,000 participants | 4 | 1.52 (1.28–1.80) | 0.0 | 0.49 | ||
| Duration of treatment | NR | 5 | 1.55 (1.27–1.89) | 79.3 | 0.001 | |
| ≥4 months | 1 | 1.81 (1.58–2.08) | – | – | ||
| <4 months | 5 | 1.56 (1.28–1.91) | 22.8 | 0.26 | ||
| Ovulation rate | Overall | 7 | 1.25 (1.15–1.35) | 65.6 | 0.008 | |
| Type of intervention | Acupuncture | 3 | 1.33 (0.84–2.09) | 77.9 | 0.01 | |
| Acupuncture plus medications | 2 | 1.18 (1.11–1.27) | 0.0 | 0.90 | ||
| Acupuncture plus moxibustion | – | 1.31 (1.22–1.40) | – | – | ||
| Acupuncture plus TCM | – | 1.31 (1.16–1.48) | – | – | ||
| Quality of studies | Low | 1 | 1.88 (1.21–2.92) | – | – | |
| Moderate | 4 | 1.20 (1.09–1.33) | 74.8 | 0.008 | ||
| High | 2 | 1.31 (1.23–1.40) | 0.0 | 0.70 | ||
| Sample size | ≥1,000 participants | 6 | 1.23 (1.15–1.33) | 64.9 | 0.01 | |
| <1,000 participants | 1 | 1.88 (1.21–2.92) | – | – | ||
| Duration of treatment | NR | 3 | 1.30 (1.23–1.37) | 0.0 | 0.88 | |
| ≥4 months | 1 | 1.31 (1.22–1.40) | – | – | ||
| <4 months | 3 | 1.19 (0.96–1.46) | 76.9 | 0.01 | ||
| Resumption of menstruation | Overall | 2 | 1.50 (0.89–2.53) | 36.5 | 0.21 | |
| Rate of live birth | Overall | 3 | 0.99 (0.84–1.18) | 0.0 | 0.47 | |
| Type of intervention | Acupuncture | 3 | 0.99 (0.84–1.18) | 0.0 | 0.47 | |
| Quality of studies | Moderate | 2 | 1.08 (0.72–1.62) | 29.5 | 0.23 | |
| High | 1 | 0.97 (0.76–1.24) | – | – | ||
| Sample size | ≥1,000 participants | 2 | 0.97 (0.83–1.16) | 0.0 | 0.99 | |
| <1,000 participants | 1 | 1.61 (0.73–3.57) | – | – | ||
| Abortion rate | Overall | 2 | 0.71 (0.30–1.71) | 88.5 | 0.003 | |
| Continuous outcomes | Subgroups | Studies | SMD (95%CI) | I2 (%) | P | |
|---|---|---|---|---|---|---|
| LH | Overall | 9 | −1.04 (−1.67 to −0.41) | 95.5 | 0.001 | |
| Type of intervention | Acupuncture | 3 | −1.05 (−1.50 to −0.61) | 0.0 | 0.49 | |
| Acupuncture plus medications | 3 | −0.79 (−1.52 to −0.06) | 62.8 | 0.06 | ||
| Acupuncture plus moxibustion | 1 | −2.31 (−2.86 to −1.76) | – | – | ||
| Acupuncture plus TCM | 1 | −1.84 (−2.61 to −1.07) | – | – | ||
| Acupuncture plus medications and TCM | 1 | −0.39 (−0.65 to −0.39) | – | – | ||
| Quality of studies | Low | 1 | −1.13 (−1.69 to −0.57) | – | – | |
| Moderate | 6 | −0.75 (−1.39 to −0.11) | 95.5 | 0.001 | ||
| High | 2 | −2.33 (−2.87 to −1.79) | 0.0 | 0.63 | ||
| Sample size | ≥1,000 participants | 7 | −1.44 (−2.20 to −0.67) | 92.3 | 0.001 | |
| <1,000 participants | 2 | 0.07 (−0.82 to 0.96) | 96.2 | 0.001 | ||
| Duration of treatment | NR | 5 | −0.74 (−1.44 to −0.03) | 96.3 | 0.001 | |
| ≥4 months | 1 | −2.33 (−2.86 to −1.76) | - | - | ||
| <4 months | 3 | −1.07 (−1.47 to −0.61) | 0.0 | 0.47 | ||
| FSH | Overall | 4 | −0.14 (−0.32 to 0.04) | 24.5 | 0.26 | |
| Type of intervention | Acupuncture | 2 | −0.09 (−0.46 to −0.18) | 41.8 | 0.29 | |
| Acupuncture plus moxibustion | 1 | −0.08 (−0.37 to 0.21) | 1 | 1 | ||
| Acupuncture plus medications and TCM | 1 | −0.11 (−0.37 to −0.15) | 1 | 1 | ||
| Quality of studies | Low | 1 | 0.06 (−0.31 to 0.43) | – | – | |
| Moderate | 2 | −0.25 (−0.57 to 0.07) | 52.9 | 0.14 | ||
| High | 1 | −0.08 (−0.37 to 0.21) | – | – | ||
| Sample size | ≥1,000 participants | 2 | −0.03 (−0.26 to 0.20) | 0.0 | 0.56 | |
| <1,000 participants | 2 | −0.25 (−0.57 to 0.07) | 52.9 | 0.14 | ||
| Duration of treatment | NR | 2 | −0.25 (−0.57 to 0.07) | 52.9 | 0.14 | |
| ≥4 months | 1 | −0.08 (−0.37 to 0.21) | – | – | ||
| <4 months | 1 | 0.06 (−0.31 to 0.43) | – | – | ||
| LH/FSH ratio | Overall | 6 | −0.26 (−0.52 to −0.003) | 93.3 | 0.001 | |
| Type of intervention | Acupuncture | 2 | −0.17 (−0.69 to 0.35) | 52.5 | 0.14 | |
| Acupuncture plus medications | 1 | −0.58 (−0.81 to −0.35) | – | – | ||
| Acupuncture plus moxibustion | 1 | −0.47 (−0.64 to −0.30) | – | – | ||
| Acupuncture plus TCM | 1 | −0.47 (−0.56 to −0.38) | – | – | ||
| Acupuncture plus medications and TCM | 1 | −0.20 (−0.43 to 0.03) | – | – | ||
| Quality of studies | Moderate | 4 | −0.17 (−0.48 to 0.15) | 95.3 | 0.001 | |
| High | 2 | −0.48 (−0.64 to −0.31) | 0.0 | 0.73 | ||
| Sample size | ≥1,000 participants | 5 | −0.28 (−0.58 to 0.03) | 94.7 | 0.001 | |
| <1,000 participants | 1 | −0.20 (−0.43 to 0.03) | – | – | ||
| Duration of treatment | NR | 3 | −0.27 (−0.56 to 0.03) | 76.1 | 0.02 | |
| ≥4 months | 1 | −0.47 (−0.64 to −0.30) | – | – | ||
| <4 months | 2 | −0.17 (−0.69 to 0.35) | 52.8 | 0.14 | ||
| Testosterone | Overall | 7 | −0.26 (−0.45 to −0.08) | 84.7 | 0.001 | |
| Type of intervention | Acupuncture | 4 | −0.27 (−0.51 to −0.04) | 30.5 | 0.23 | |
| Acupuncture plus medications | 2 | −0.50 (−1.16 to 0.15) | 88.9 | 0.003 | ||
| Acupuncture plus moxibustion | 1 | −7.04 (−9.38 to −4.70) | – | – | ||
| Acupuncture plus TCM | 1 | −0.16 (−0.25 to −0.07) | – | – | ||
| Acupoint catgut embedding | 1 | −0.14 (−0.17 to −0.11) | – | – | ||
| Quality of studies | Low | 2 | −0.36 (−0.89 to 0.17) | 72.9 | 0.05 | |
| Moderate | 3 | −0.14 (−0.18 to −0.11) | 0.0 | 0.75 | ||
| High | 2 | −3.78 (−0.10 to 2.44) | 95.6 | 0.001 | ||
| Sample size | ≥1,000 participants | 6 | −0.30 (−0.52 to −0.09) | 87.2 | 0.001 | |
| <1,000 participants | 1 | −0.13 (−0.38 to 0.12) | – | – | ||
| Duration of treatment | NR | 2 | −0.14 (−0.18 to −0.11) | 0.0 | 0.68 | |
| ≥4 months | 1 | −7.04 (−9.38 to −4.70) | – | – | ||
| <4 months | 4 | −0.29 (−0.52 to −0.05) | 0.29 | 0.23 | ||
| Endometrial thickness | Overall | 2 | 0.29 (−1.89 to 2.48) | 90.4 | 0.001 | |
| Fasting plasma glucose | Overall | 3 | −0.24 (−0.53 to 0.05) | 49.5 | 0.13 | |
| Acupuncture | 2 | −0.24 (−0.44 to −0.05) | 11.5 | 0.28 | ||
| Acupuncture plus medications | 1 | −0.40 (−1.03 to 0.23) | – | – | ||
| Electroacupuncture | 1 | −0.79 (−2.18 to 0.60) | – | – | ||
| Acupuncture plus medications and TCM | 1 | 0.02 (−0.30 to 0.34) | – | – | ||
| OGTT | Overall | 2 | −0.19 (−0.51 to 0.12) | 5.7 | 0.30 | |
| Fasting insulin | Overall | 4 | −1.34 (−2.96 to 0.27) | 84.4 | 0.001 | |
| Type of intervention | Acupuncture | 2 | −1.09 (−5.50 to 3.33) | 83.5 | 0.01 | |
| Acupuncture plus medications | 2 | −2.50 (−2.76 to −2.24) | 0.0 | 0.64 | ||
| Acupuncture plus moxibustion | 1 | −2.48 (−3.85 to −1.11) | – | – | ||
| Electroacupuncture | 1 | −1.36 (−5.47 to 2.75) | ||||
| Acupuncture plus medications and TCM | 1 | 0.11 (−0.21 to 0.43) | – | – | ||
| Quality of studies | Moderate | 2 | 0.08 (−0.23 to 0.39) | 0.0 | 0.34 | |
| High | 2 | −2.66 (−3.89 to −1.43) | 0.0 | 0.55 | ||
| Sample size | ≥1,000 participants | 2 | −2.66 (−3.89 to −1.43) | 0.0 | 0.55 | |
| <1,000 participants | 2 | 0.08 (−0.23 to 0.39) | 0.0 | 0.34 | ||
| Duration of treatment | NR | 1 | 0.11 (−0.21 to 0.43) | – | – | |
| ≥4 months | 2 | −3.43 (−6.25 to −0.61) | 67.2 | 0.08 | ||
| <4 months | 1 | −1.60 (−3.39 to 0.19) | – | – | ||
| HOMA-IR | Overall | 3 | −0.40 (−0.72 to −0.08) | 63.9 | 0.06 | |
| Type of intervention | Acupuncture | 2 | −0.20 (−0.39 to −0.01) | 0.0 | 0.50 | |
| Acupuncture plus medications | 2 | −0.68 (−1.01 to −0.35) | 0.0 | 0.64 | ||
| Electroacupuncture | 1 | −0.64 (−2.20 to 0.92) | – | – | ||
| Quality of studies | Moderate | 3 | −0.40 (−0.72 to −0.08) | 63.9 | 0.06 | |
| Sample size | ≥1,000 participants | 1 | −0.68 (−1.01 to −0.35) | – | – | |
| <1,000 participants | 2 | −0.23 (−0.42 to −0.05) | 0.0 | 0.71 | ||
| Duration of treatment | ≥4 months | 2 | −0.23 (−0.42 to −0.05) | 0.0 | 0.71 | |
| <4 months | 1 | −0.68 (−1.01 to −0.35) | – | – | ||
| BMI | Overall | 6 | −1.15 (−1.54 to −0.76) | 48.9 | 0.08 | |
| Type of intervention | Acupuncture | 3 | −1.09 (−1.38 to −0.80) | 0.0 | 0.64 | |
| Acupuncture plus medications | 1 | −1.36 (−2.06 to −0.66) | 1 | 1 | ||
| Acupuncture plus moxibustion | 1 | −1.78 (−2.53 to −1.03) | – | – | ||
| Electroacupuncture | 1 | −1.78 (−4.03 to 0.47) | – | – | ||
| Acupuncture plus TCM | 1 | −2.51 (−5.03 to 0.01) | – | – | ||
| Acupuncture plus medications and TCM | 1 | −0.63 (−1.04 to −0.22) | – | – | ||
| Quality of studies | Low | 1 | −1.05 (−1.36 to −0.74) | – | – | |
| Moderate | 4 | −1.06 (−1.67 to −0.46) | 39.8 | 0.17 | ||
| High | 1 | −1.78 (−2.53 to −1.03) | – | – | ||
| Sample size | ≥1,000 participants | 3 | −1.40 (−2.07 to −0.74) | 52.4 | 0.12 | |
| <1,000 participants | 3 | −0.96 (−1.52 to −0.39) | 39.1 | 0.19 | ||
| Duration of treatment | NR | 2 | −1.14 (−2.78 to 0.50) | 52.0 | 0.14 | |
| ≥4 months | 3 | −1.57 (−2.08 to −1.05) | 0.0 | 0.70 | ||
| <4 months | 1 | −1.05 (−1.36 to −0.74) | – | – |
RR, relative risk; SMD, standardized mean difference; TCM, traditional Chinese medicine; NR, not reported; LH, luteinizing hormone; FSH, follicle stimulating hormone; OGTT, oral glucose tolerance test; HOMA-IR, homeostatic model assessment for insulin resistance; BMI, body mass index.
No significant effect was identified on the resumption of 451 menstruation, rate of live birth, abortion rate, FSH levels, endometrial 452 thickness, FPG, OGTT, and fasting insulin in the overall analyses 453 (Figures 2, 3). However, in the stratified analyses, patients who received acupuncture only or acupuncture plus other therapies had a lower FSH levels. Treatment with acupuncture was also associated with a lower FPG levels. Furthermore, acupuncture plus medications as well as acupuncture plus moxibustion were linked to a lower fasting insulin in high quality studies and studies with ≥1,000 participants.
Umbrella meta-analysis for POI
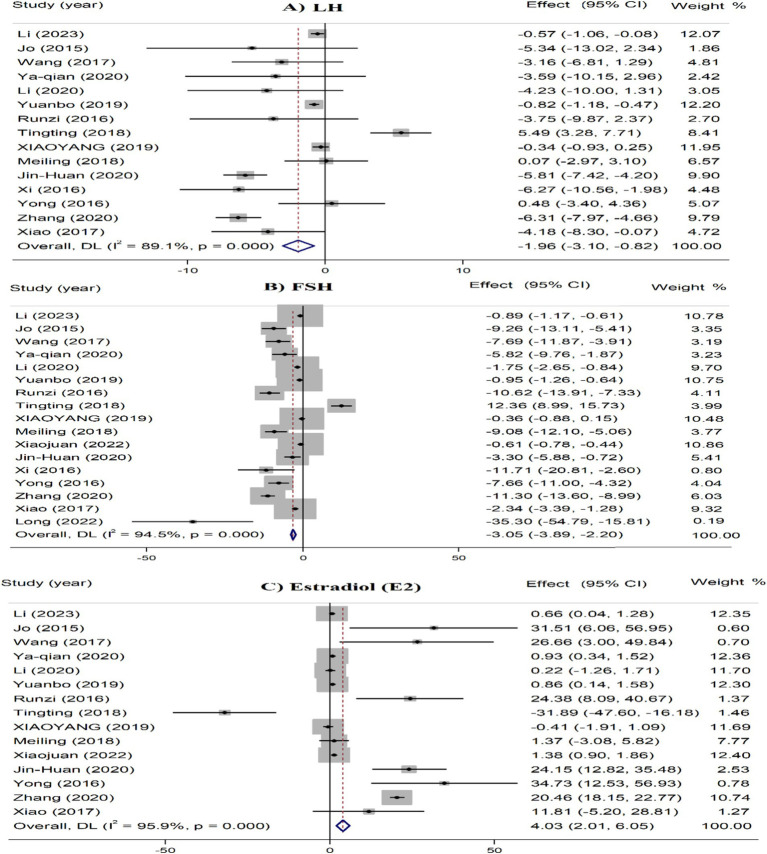
In the overall analyses, acupuncture interventions were significantly linked to the reduced serum levels of LH (SMD = −1.96, 95% CI: −3.10 to −0.82) and FSH (SMD = −3.05, 95% CI: −3.89 to −2.20), and increased levels of estradiol (SMD = 4.03, 95% CI: 2.01 to 6.05) (Figure 4).
Figure 4.
Umbrella meta-analysis for the effect of acupuncture on various outcomes in patients with POF. FSH, follicle stimulating hormone; LH, luteinizing hormone.
In the subgroup analysis, acupuncture plus TCM, electroacupuncture plus TCM, and acupuncture plus moxibustion remarkably reduced serum LH levels. For FSH, acupuncture, acupuncture plus TCM, acupuncture plus HRT, needle acupuncture, needle acupuncture plus TCM, electroacupuncture plus TCM, and auricular points plus TCM were linked to reduced serum FSH. Regarding estradiol, acupuncture only, needle acupuncture plus TCM, auricular points plus TCM, acupoint catgut embedding plus TCM, and moxibustion plus TCM were linked to increased serum estradiol. For all outcomes, the significant effects were observed in studies with smaller sample sizes (<1,000 participants) and shorter treatment durations (<5 months) (Table 3).
Table 3.
Subgroup analyses for the effect of acupuncture on premature ovary inefficiency.
| Continuous outcomes | Subgroups | Studies | SMD (95%CI) | I2 (%) | P | |
|---|---|---|---|---|---|---|
| LH | Overall | 15 | −1.96 (−3.10 to −0.82) | 89.1 | 0.001 | |
| Type of intervention | Acupuncture | 8 | −2.71 (−6.86 to 1.44) | 90.9 | 0.001 | |
| Acupuncture plus TCM | 2 | −0.64 (−1.10 to −0.18) | 46.4 | 0.17 | ||
| Acupuncture plus HRT | 2 | −6.80 (−13.84 to 0.23) | 54.2 | 0.14 | ||
| Needle acupuncture | 1 | 0.07 (−2.97 to 3.11) | – | – | ||
| Needle acupuncture plus TCM | 1 | −0.22 (−0.48 to 0.04) | – | – | ||
| Electroacupuncture plus TCM | 2 | −1.29 (−1.60 to −0.98) | 0.0 | 0.99 | ||
| Auricular points plus TCM | 1 | −1.46 (−3.26 to 0.34) | – | – | ||
| Acupoint catgut embedding plus TCM | 1 | −0.40 (−2.84 to 2.06) | – | – | ||
| Warm acupuncture | 1 | 0.32 (−5.64 to 6.28) | – | – | ||
| Acupuncture and moxibustion | 1 | −5.81 (−7.42 to −4.20) | – | – | ||
| Moxibustion plus TCM | 1 | 0.84 (−0.47 to 1.23) | – | – | ||
| Quality of studies | Low | 5 | −1.97 (−4.77 to 0.83) | 89.2 | 0.001 | |
| Moderate | 7 | −2.41 (−5.65 to 0.84) | 93.1 | 0.001 | ||
| High | 3 | −1.78 (−4.60 to 1.01) | 34.6 | 0.21 | ||
| Sample size | ≥1,000 participants | 4 | −2.68 (−8.38 to 3.03) | 96.4 | 0.001 | |
| <1,000 participants | 11 | −0.80 (−1.31 to −0.28) | 39.3 | 0.08 | ||
| Duration of treatment | NR | 5 | −3.91 (−6.46 to −1.36) | 59.3 | 0.04 | |
| ≥5 months | 5 | −0.88 (−5.32 to 3.55) | 85.1 | 0.001 | ||
| <5 months | 5 | −1.68 (−2.81 to −0.55) | 91.5 | 0.001 | ||
| FSH | Overall | 17 | −3.05 (−3.89 to −2.20) | 94.5 | 0.001 | |
| Type of intervention | Acupuncture | 11 | −4.84 (−7.60 to −2.08) | 96.2 | 0.001 | |
| Acupuncture plus TCM | 3 | −0.70 (−1.31 to −0.09) | 61.1 | 0.07 | ||
| Acupuncture plus HRT | 3 | −5.79 (−11.19 to −0.40) | 67.1 | 0.05 | ||
| Needle acupuncture | 1 | −9.08 (−12.60 to −5.56) | – | – | ||
| Needle acupuncture plus TCM | 1 | −0.62 (−1.02 to −0.22) | – | – | ||
| Electroacupuncture plus TCM | 2 | −1.17 (−1.38 to −0.97) | 0.0 | 0.88 | ||
| Auricular points plus TCM | 1 | −2.19 (−2.97 to −1.41) | – | – | ||
| Acupoint catgut embedding | 2 | −0.77 (−37.14 to 35.60) | 95.5 | 0.001 | ||
| Acupoint catgut embedding plus TCM | 1 | −0.72 (−2.13 to 0.69) | – | – | ||
| Acupoint catgut embedding plus HRT | 1 | −10.05 (−22.09 to 1.97) | – | – | ||
| Warm acupuncture | 1 | 2.50 (−5.67 to 10.67) | – | – | ||
| Acupuncture and moxibustion | 3 | 3.15 (−4.52 to 10.83) | 87.0 | 0.002 | ||
| Moxibustion | 1 | 2.32 (−5.84 to 10.48) | – | – | ||
| Moxibustion plus TCM | 1 | −0.02 (−0.38 to 0.34) | – | – | ||
| Quality of studies | Low | 5 | −6.15 (−10.39 to −1.90) | 94.3 | 0.001 | |
| Moderate | 9 | −2.75 (−4.61 to −0.90) | 95.5 | 0.001 | ||
| High | 3 | −2.55 (−4.44 to −0.65) | 90.4 | 0.001 | ||
| Sample size | ≥1,000 participants | 5 | −3.87 (−10.72 to 2.98) | 97.2 | 0.001 | |
| <1,000 participants | 12 | −2.50 (−3.23 to −1.77) | 91.6 | 0.001 | ||
| Duration of treatment | NR | 5 | −7.59 (−10.74 to −4.44) | 71.0 | 0.008 | |
| ≥5 months | 5 | −1.30 (−5.05 to 2.44) | 95.5 | 0.001 | ||
| <5 months | 7 | −1.96 (−2.79 to −1.14) | 94.9 | 0.001 | ||
| Estradiol (E2) | Overall | 15 | 4.03 (2.01 to 6.05) | 95.5 | 0.001 | |
| Type of intervention | Acupuncture | 9 | 6.59 (2.38 to 10.80) | 97.4 | 0.001 | |
| Acupuncture plus TCM | 2 | 0.40 (−0.79 to 1.60) | 55.3 | 0.13 | ||
| Acupuncture plus HRT | 2 | 3.00 (−4.29 to 10.28) | 30.1 | 0.23 | ||
| Needle acupuncture | 1 | 1.37 (−3.08 to 5.82) | – | – | ||
| Needle acupuncture plus TCM | 1 | 0.60 (0.14 to 1.06) | – | – | ||
| Electroacupuncture plus TCM | 2 | 0.63 (−0.40 to 1.66) | 0.0 | 0.87 | ||
| Auricular points plus TCM | 1 | 1.63 (1.21 to 2.05) | – | – | ||
| Acupoint catgut embedding | 1 | 8.06 (−10.88 to −5.24) | – | – | ||
| Acupoint catgut embedding plus TCM | 1 | 1.32 (0.69 to 1.95) | – | – | ||
| Warm acupuncture | 1 | 4.60 (−24.55 to 15.35) | – | – | ||
| Acupuncture and moxibustion | 2 | 13.35 (−7.32 to 34.02) | 87.4 | 0.05 | ||
| Moxibustion plus TCM | 1 | 0.64 (0.27 to 1.01) | – | – | ||
| Quality of studies | Low | 5 | 11.99 (3.67 to 20.32) | 87.9 | 0.001 | |
| Moderate | 7 | 4.37 (0.51 to 8.31) | 97.9 | 0.001 | ||
| High | 3 | 0.62 (−1.15 to 2.39) | 66.4 | 0.05 | ||
| Sample size | ≥1,000 participants | 4 | 4.64 (−10.55 to 19.84) | 98.8 | 0.001 | |
| <1,000 participants | 11 | 0.98 (0.17 to 1.80) | 71.9 | 0.0.001 | ||
| Duration of treatment | NR | 4 | 25.90 (17.84 to 33.95) | 0.0 | 0.86 | |
| ≥5 months | 5 | 0.14 (−2.50 to 2.78) | 79.0 | 0.001 | ||
| <5 months | 6 | 4.65 (1.68 to 7.62) | 98.2 | 0.001 |
SMD, standardized mean difference; TCM, traditional Chinese medicine; NR, not reported; HRT, hormone replacement therapy; LH, luteinizing hormone; FSH, follicle stimulating hormone.
Heterogeneity, meta-regression, sensitivity analysis, and publication bias
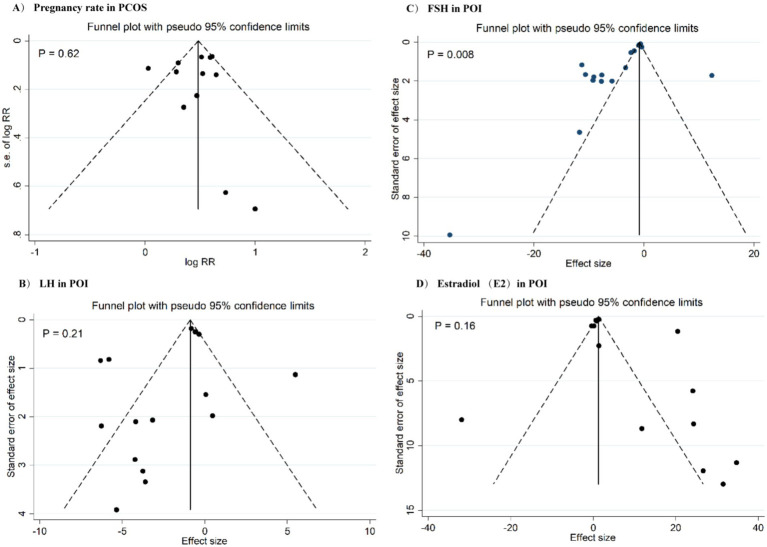
There was a significant heterogeneity across the studies for most outcomes (Tables 2, 3). Meta-regression analysis indicated that the results were not significantly affected by the proportion of RCTs with low ROB in each meta-analysis, publication year, and the number of effect sizes for each outcomes. In the sensitivity analyses, the pooled effect sizes for HOMA-IR, LH, fasting insulin, and FPG were significantly affected by the individual studies. A significant publication bias was detected for FSH levels in POI patients (Figure 5).
Figure 5.
Funnel plots for publication bias for pregnancy rate in PCOS (A), LH in POF (B), FSH in POF (C), and estradiol in POF (D).
Discussion
This umbrella meta-analysis aimed to explore the impact of acupuncture on the clinical outcomes of patients with PCOS and POI. The analysis revealed that acupuncture significantly improved pregnancy rate and ovulation rate, and favorably affected serum levels of LH, testosterone, LH/FSH ratio, HOMA-IR, and BMI in PCOS patients. Moreover, there was weak evidence for the positive effects of acupuncture on other hormonal and metabolic parameters including, FSH, FPG, and fasting insulin. Additionally, we found that acupuncture, when compared to standard care (HRT) resulted in a reduction in LH and FSH levels, alongside an increase in serum estradiol levels.
Acupoint stimulation and TCM are frequently used in several Asian countries as complementary and alternative therapies for POI and PCOS. Although acupuncture has shown potential in treating both POI and PCOS, the lack of high-quality evidence-based studies hinders drawing any firm conclusion on whether acupuncture affects the clinical outcomes of women with POI and PCOS. Recent meta-analyses have yielded contradictory results. While Chen et al. (24) and Liang et al. (33) showed an improvement in the pregnancy rate, other meta-analyses found no significant change (44, 50). The results for hormonal and metabolic changes are also inconsistent (23, 37). The heterogeneity in the results of the previous studies could be due to the differences in the treatment protocol, sample size, duration of the intervention, and disease phenotype. Our analysis revealed that acupuncture increases pregnancy rate and improves metabolic and hormonal imbalances in these patients. Currently, there is no sufficient data to determine the optimal acupuncture treatment in patients with POI and PCOS due to the limited number of available studies. In addition, several contributing factors, including acupoint specificity and selection, stimulation methods, the number of sessions, and the practitioner’s expertise could affect the results (55). Additional studies are recommended to investigate the optimal acupuncture protocols for POI/PCOS.
The present study found that the duration of acupuncture treatment significantly impacted outcome. For some PCOS outcomes and for all outcomes of POI, acupuncture improved the clinical outcomes when administered for a longer duration, but not when used for a shorter period. The explanation for this finding is that the longer duration of acupuncture treatment allows for a more sustained and profound impact on the neuro-endocrine system and the ovarian environment, especially in cases of POI where the ovarian function is compromised. In contrast, shorter durations of acupuncture treatment may not be sufficient to achieve the same level of improvement in ovarian function. This could be due to the limited time for acupuncture to stimulate the neuro-endocrine system and promote follicular development.
The clinical utility of our findings is that acupuncture can be a valuable adjunctive therapy for managing POI and PCOS, particularly in cases where conventional treatments are ineffective or have significant side effects. Furthermore, the improvement in hormonal and metabolic imbalances, such as insulin resistance, obesity, and hyperandrogenism, indicates that acupuncture can have a positive impact on the underlying pathophysiology of PCOS (67). This can lead to better management of symptoms, enhanced life quality, and a decrease in the odds of long-term complications, such as cardiovascular disease and diabetes (68).
Acupuncture could improve PCOS through several key mechanisms, including neuroendocrine regulation, hormone regulation, stem cell regulation, follicular development and maturation, gene expression, and improving the hemodynamics of ovary and uterus (69, 70). Acupuncture regulates the neuroendocrine system, which plays an essential role in the progression of PCOS. This includes the hypothalamus-pituitary-ovary axis, affecting the secretion of ovarian-related hormones, such as estradiol, FSH, and LH, which are essential for normal ovarian function and menstruation cycles (71). Studies have shown that acupuncture decreases cortisol levels and triggers the production of pituitary beta-endorphins, which exert a tonic inhibitory influence on the gonadotropin-releasing hormone pulse generator and pituitary LH secretion, which is a potential for acupuncture to alleviate ovulatory dysfunction in PCOS (44, 46). Acupuncture may also regulate stem cell populations in the ovaries, which are important for follicular development and maturation (72). Furthermore, animal studies have indicated a significant increase in ovarian tissue and endometrium thickness, an increase in normal glands and blood vessels, a rise in the number of antral follicles, and a decrease in interstitial cells after treatment with acupuncture (46).
Acupuncture has been shown to affect specific genes that play an important role in treating PCOS. Acupuncture improves follicular development and maturation by inhibiting granulosa cell apoptosis and promoting the proliferation of granulosa cells. This is achieved through the downregulation of the MEG3 gene in ovarian granulosa cells, which leads to decreased expression of miR-21-3p. This pathway contributes to the development of PCOS and is related to ovulatory dysfunction and abnormal follicular development (72). Acupuncture also promotes Bcl-2 gene expression and inhibits Bax gene expression (71), which are involved in the pathogenesis of PCOS. Acupuncture also upregulates PLA2G4A and downregulates miR-32-3p, potentially enhancing ovulation and improving endocrine function, especially in PCOS patients with diabetes (73). A study found that electroacupuncture altered the expression of metabolic-related genes, particularly FOSB and LOC100128899. It also enhanced the expression of LXR/RX while reversing PPARγ and ADIPOR2 expressions (74). Acupuncture has been found to improve insulin sensitivity by increasing the expression of insulin receptor substrate 1 (IRS1) and IRS2 in the endometrial tissue of PCOS cases. This may contribute to better insulin sensitivity in the endometrium (75). Gene expression analysis revealed that electroacupuncture upregulated the IRS-1/PI3K/GLUT4 signaling pathway in PCOS patients, which may enhance oocyte quality and embryonic development potential (76). Overall, these findings suggest that acupuncture alters gene expression in ways that may alleviate PCOS symptoms, indicating its potential as an effective therapeutic option.
In the present study, acupuncture decreased FSH and LH levels but increased estradiol levels in POI patients. Acupuncture may improve hormone imbalance in patients with POI through improvement of ovarian function, modulation of the hypothalamic–pituitary-ovarian (HPO) Axis, and reduction of stress (77, 78). The treatment may enhance ovarian blood flow and function, which can lead to improved ovarian response and hormone production (79). Acupuncture can influence the HPO axis, potentially enhancing the secretion of gonadotropin-releasing hormone (GnRH), which regulates FSH and LH release (80). By alleviating stress and anxiety, acupuncture may help reduce cortisol levels, which can negatively impact reproductive hormones and overall hormonal balance (81).
This study is the first umbrella meta-analysis examining the clinical efficacy of acupuncture on patients with PCOS and POI. The strengths of the study includes a high number of analyzed studies with a large sample size, examination of various important clinical outcomes, and subgroup and meta-regression analyses to assess the origins of heterogeneity. Moreover, the efficacy of various acupuncture therapies were analyzed compared to their parallel controls to investigate their specific impacts. Some limitation of this study should be acknowledged. First, a remarkable heterogeneity was revealed in most analyses, limiting the generalizability of the results. Subgroup analyses found that differences in treatment protocol, follow-up duration, sample size, and quality of studies contributed to the observed heterogeneity across the studies. The heterogeneity was not originated from the variability in the age of participants and the proportion of RCTs with low ROB in included studies. We also applied a random effects approach for analyses to decrease the effect of the heterogeneity on the pooled effect sizes. Second, publication bias was significant for some outcomes. Despite conducting searches without language restrictions across various databases, some small studies might be ignored, resulting to the significant small-study effect. Nevertheless, the trim-and-fill analysis indicated a minimal impact of publication bias on the combined estimates, suggesting the reliability of the results. Third, among the studies, different acupuncture strategies with different combinations with other therapies were used, which can influence the findings. To evaluate the distinct effects of different acupuncture treatments on the outcomes, we conducted subgroup analyses based on the type of treatment. The heterogeneity in acupoint selection and acupuncture techniques, may limit the reliability of our conclusions. Therefore, we recommend the need for standardized protocols in future studies to improve comparability and reliability in acupuncture research for both PCOS and POI. Moreover, the presence of overlapping studies among systematic reviews is an inherent limitation of umbrella meta-analyses that can affect the reliability of our findings. This represents another limitation of our study. Lastly, the sensitivity analysis identified that the pooled estimates for HOMA-IR, LH/FSH ratio, fasting insulin, and fasting glucose in PCOS patients were influenced by individual studies, highlighting that these results should be interpreted with caution. Further studies are required to validate our findings. Furthermore, given the limited number of studies for some subgroups, the results of should be interpreted with caution as there is a likely risk of false negative results.
In conclusion, this umbrella meta-analysis suggests that acupuncture-related interventions are potential alternative therapies for patients with PCOS and POI. Acupuncture not only increased the pregnancy rate and ovulation rate in PCOS patients, but also showed favorable effects on hormonal and metabolic parameters. Acupuncture therapy also improved hormonal changes in women with POI. Given the high risk of bias in existing RCTs and a notable heterogeneity across the meta-analyses of RCTs, large-scale RCTs with robust methodological standards are required to gain a better understanding of the role of acupuncture in these patients.
Funding Statement
This study was supported by Shandong Province Natural Science Foundation Joint Fund Project (No. ZR2022LZY008), Shandong Province Chinese Medicine High-level Talent Cultivation Project (Lu Wei Talent No. [2021] 6), Shandong Province Chinese Medicine Science and Technology Project (No. B-2022002), Jinan Science and Technology Bureau Clinical Medicine Science and Technology Innovation Programme Project (No. 202225059), Qilu Health and Health Outstanding Young Talent Cultivation Project (Lu Wei Talent No. [2020] 3).
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
TB: Formal analysis, Investigation, Supervision, Writing – original draft, Writing – review & editing. XD: Conceptualization, Data curation, Funding acquisition, Software, Validation, Visualization, Writing – original draft, Writing – review & editing. JB: Funding acquisition, Project administration, Resources, Validation, Visualization, Writing – original draft, Writing – review & editing. LN: Data curation, Formal analysis, Methodology, Project administration, Validation, Writing – original draft, Writing – review & editing. ZL: Conceptualization, Data curation, Investigation, Methodology, Project administration, Software, Writing – original draft, Writing – review & editing. XZ: Data curation, Formal analysis, Methodology, Resources, Software, Validation, Writing – original draft, Writing – review & editing.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2024.1471243/full#supplementary-material
References
- 1.Petríková J, Lazúrová I. Ovarian failure and polycystic ovary syndrome. Autoimmun Rev. (2012) 11:A471–8. doi: 10.1016/j.autrev.2011.11.010 [DOI] [PubMed] [Google Scholar]
- 2.Du J, Zhang W, Guo L, Zhang Z, Shi H, Wang J, et al. Two FSHR variants, haplotypes and meta-analysis in Chinese women with premature ovarian failure and polycystic ovary syndrome. Mol Genet Metab. (2010) 100:292–5. doi: 10.1016/j.ymgme.2010.03.018, PMID: [DOI] [PubMed] [Google Scholar]
- 3.Pérez Rojas JM, Maroto Fernandez KE. Polycystic ovary syndrome (PCOS). Med Leg Costa Rica. (2018) 35:94–101. Available at: https://www.scielo.sa.cr/scielo.php?script=sci_arttext&pid=S1409-00152018000100094&lng=en&tlng=. [Google Scholar]
- 4.Chaudhuri A. Polycystic ovary syndrome: causes, symptoms, pathophysiology, and remedies. Obesity Med. (2023) 39:100480. doi: 10.1016/j.obmed.2023.100480 [DOI] [Google Scholar]
- 5.Balen A. The pathophysiology of polycystic ovary syndrome: trying to understand PCOS and its endocrinology. Best Pract Res Clin Obstet Gynaecol. (2004) 18:685–706. doi: 10.1016/j.bpobgyn.2004.05.004, PMID: [DOI] [PubMed] [Google Scholar]
- 6.Fenton AJ. Premature ovarian insufficiency: pathogenesis and management. J Midlife Health. (2015) 6:147–53. doi: 10.4103/0976-7800.172292, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Siddiqui S, Mateen S, Ahmad R, Moin S. A brief insight into the etiology, genetics, and immunology of polycystic ovarian syndrome (PCOS). J Assist Reprod Genet. (2022) 39:2439–73. doi: 10.1007/s10815-022-02625-7, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vujovic S. Aetiology of premature ovarian failure. Menopause Int. (2009) 15:72–5. doi: 10.1258/mi.2009.009020 [DOI] [PubMed] [Google Scholar]
- 9.Davies MJ, Marino JL, Willson KJ, March WA, Moore VM. Intergenerational associations of chronic disease and polycystic ovary syndrome. PLoS One. (2011) 6:e25947. doi: 10.1371/journal.pone.0025947, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rafique S, Sterling EW, Nelson LM. A new approach to primary ovarian insufficiency. Obstet Gynecol Clin. (2012) 39:567–86. doi: 10.1016/j.ogc.2012.09.007, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Legro RS, Arslanian SA, Ehrmann DA, Hoeger KM, Murad MH, Pasquali R, et al. Diagnosis and treatment of polycystic ovary syndrome: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metabol. (2013) 98:4565–92. doi: 10.1210/jc.2013-2350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Costello M, Misso M, Balen A, Boyle J, Devoto L, Garad R, et al. Evidence summaries and recommendations from the international evidence-based guideline for the assessment and management of polycystic ovary syndrome: assessment and treatment of infertility. Hum Reprod Open. (2019) 2019:hoy021. doi: 10.1093/hropen/hoy021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Akre S, Sharma K, Chakole S, Wanjari MB. Recent advances in the management of polycystic ovary syndrome: a review article. Cureus. (2022) 14:7689. doi: 10.7759/cureus.27689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang J, Xing C, Zhao H, He B. The effectiveness of coenzyme Q10, vitamin E, inositols, and vitamin D in improving the endocrine and metabolic profiles in women with polycystic ovary syndrome: a network meta-analysis. Gynecol Endocrinol. (2021) 37:1063–71. doi: 10.1080/09513590.2021.1926975, PMID: [DOI] [PubMed] [Google Scholar]
- 15.Domecq JP, Prutsky G, Mullan RJ, Hazem A, Sundaresh V, Elamin MB, et al. Lifestyle modification programs in polycystic ovary syndrome: systematic review and meta-analysis. J Clin Endocrinol Metabol. (2013) 98:4655–63. doi: 10.1210/jc.2013-2385, PMID: [DOI] [PubMed] [Google Scholar]
- 16.Hoeger KM, Dokras A, Piltonen T. Update on PCOS: consequences, challenges, and guiding treatment. J Clin Endocrinol Metabol. (2021) 106:e1071–83. doi: 10.1210/clinem/dgaa839 [DOI] [PubMed] [Google Scholar]
- 17.POI EGGo. Webber L, Davies M, Anderson R, Bartlett J, Braat D, et al. ESHRE guideline: management of women with premature ovarian insufficiency. Hum Reprod. (2016) 31:926–37. doi: 10.1093/humrep/dew027 [DOI] [PubMed] [Google Scholar]
- 18.Pacello P, Yela D, Rabelo S, Giraldo P, Benetti-Pinto C. Dyspareunia and lubrication in premature ovarian failure using hormonal therapy and vaginal health. Climacteric. (2014) 17:342–7. doi: 10.3109/13697137.2013.860116, PMID: [DOI] [PubMed] [Google Scholar]
- 19.Biscup P. Risks and benefits of long-term hormone replacement therapy. Am J Health Syst Pharm. (2003) 60:1419–25. doi: 10.1093/ajhp/60.14.1419 [DOI] [PubMed] [Google Scholar]
- 20.Chon SJ, Umair Z, Yoon M-S. Premature ovarian insufficiency: past, present, and future. Front Cell Dev Biol. (2021) 9:672890. doi: 10.3389/fcell.2021.672890, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chang CL. Facilitation of ovarian response by mechanical force—latest insight on fertility improvement in women with poor ovarian response or primary ovarian insufficiency. Int J Mol Sci. (2023) 24:14751. doi: 10.3390/ijms241914751, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moustaki M, Kontogeorgi A, Tsangkalova G, Tzoupis H, Makrigiannakis A, Vryonidou A, et al. Biological therapies for premature ovarian insufficiency: what is the evidence? Front Reproduct Health. (2023) 5:1194575. doi: 10.3389/frph.2023.1194575, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang S, Wan X, Zhao J, Mao X, La X. Comparative effectiveness of acupuncture and HRT interventions for premature ovarian failure: a bayesian meta-analysis. Int J Clin Exp Med. (2017) 10:15040–50. [Google Scholar]
- 24.Chen X, Lan Y, Yang L, Liu Y, Li H, Zhu X, et al. Acupuncture combined with metformin versus metformin alone to improve pregnancy rate in polycystic ovary syndrome: a systematic review and meta-analysis. Front Endocrinol. (2022) 13:978280. doi: 10.3389/fendo.2022.978280, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yu CC, Ma CY, Xiong Y, Wu M, Shen F, Zhou YL, et al. Effectiveness of acupoint catgut embedding therapy for polycystic ovary syndrome: a systematic review and meta-analysis. World J Acupunc Moxibust. (2017) 27:41–51. doi: 10.1016/S1003-5257(18)30010-2 [DOI] [Google Scholar]
- 26.Welt CK. Primary ovarian in sufficiency: a more accurate term for premature ovarian failure. Clin Endocrinol. (2008) 68:499–509. [DOI] [PubMed] [Google Scholar]
- 27.European Society for Human Reproduction and Embryology (ESHRE) . Guideline Group on POI, Webber L, Davies M, et al. ESHRE Guideline: management of women with premature ovarian insufficiency. Hum Reprod (2016) 31:926–937. [DOI] [PubMed] [Google Scholar]
- 28.Panay N, Anderson RA, Nappi RE, Vincent AJ, Vujovic S, Webber L, et al. Premature ovarian insufficiency: an International Menopause Society White Pape. Climacteric. (2020) 23:426–446. [DOI] [PubMed] [Google Scholar]
- 29.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. Statement: an updated guideline for reporting systematic reviews. BMJ. (2020) 2021:372. doi: 10.1136/bmj.n71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shea BJ, Reeves BC, Wells G, Thuku M, Hamel C, Moran J, et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ. (2017):j4008. doi: 10.1136/bmj.j4008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lin L, Chu H. Quantifying publication bias in meta-analysis. Biometrics. (2018) 74:785–94. doi: 10.1111/biom.12817, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Duval S. The trim and fill method In: Publication bias in meta-analysis: Prevention, assessment and adjustments Wiley; (2005). 127–44. [Google Scholar]
- 33.Yang L, Yang W, Sun M, Luo L, Li HR, Miao R, et al. Meta analysis of ovulation induction effect and pregnancy outcome of acupuncture and moxibustion combined with clomiphene in patients with polycystic ovary syndrome. Front Endocrinol. (2023) 14:14. doi: 10.3389/fendo.2023.1261016, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Meizhu C, Surong L, Hao W. Meta-analysis of the combination of acupuncture and medicine in the treatment of obese polycystic ovary syndrome. China Med Pharm. (2023) 13:133–45. doi: 10.20116/j.issn2095-0616.2023.21.31 [DOI] [Google Scholar]
- 35.Liu Y, Fan HY, Hu JQ, Wu TY, Chen J. Effectiveness and safety of acupuncture for insulin resistance in women with polycystic ovary syndrome: a systematic review and meta-analysis. Heliyon. (2023) 9:e13991. doi: 10.1016/j.heliyon.2023.e13991, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liang XY, Lu CL, Su Y, Jin XY, Wu ML, Lu Y, et al. Effectiveness and safety of the combination of Chinese herbal medicine and acupuncture for women with polycystic ovarian syndrome: a systematic review and meta-analysis of randomized controlled trials. Eur J Integr Med. (2023) 63:102291. doi: 10.1016/j.eujim.2023.102291 [DOI] [Google Scholar]
- 37.Li HF, Zhang JX, Chen WJ. Dissecting the efficacy of the use of acupuncture and Chinese herbal medicine for the treatment of premature ovarian insufficiency (POI): a systematic review and meta-analysis. Heliyon. (2023) 9:e20498. doi: 10.1016/j.heliyon.2023.e20498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xiaojuan L, Wenying S, Zhigao T, Chuang F, Ziqing N, Wei Z. Clinical effects of acupuncture in the treatment of premature ovarian failure: a meta analysis Western. J Tradit Chin Med. (2022) 35:73–8. doi: 10.12174/j.issn.2096-9600.2022.09.14 [DOI] [Google Scholar]
- 39.Long W, Peng L, Bingrong L, Wenhui W, Zixiang G, Kaiyong Z, et al. Efficacy of acupuncture-related therapy in the treatment of premature ovarian failure: a network meta-analysis. Shanghai J Tradit Chin Med. (2022) 56:19–27. doi: 10.16305/j.1007-1334.2022.2007182 [DOI] [Google Scholar]
- 40.Li P, Peng J, Ding Z, Zhou X, Liang R. Effects of acupuncture combined with Moxibustion on reproductive and metabolic outcomes in patients with polycystic ovary syndrome: a systematic review and Meta-analysis. Evid Based Complement Alternat Med. (2022) 2022:1–15. doi: 10.1155/2022/3616036, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zheng R, Qing P, Han M, Song J, Hu M, Ma H, et al. The effect of acupuncture on glucose metabolism and lipid profiles in patients with PCOS: a systematic review and meta-analysis of randomized controlled trials. Evid Based Complement Alternat Med. (2021) 2021:5028. doi: 10.1155/2021/5555028, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xiaoyang L, Yanjie C, Pu Y, Tongsheng S. Meta-analysis of acupuncture combined with Chinese medicinal for premature ovarian insufficiency. World Chin Med. (2021) 16:1982–97. [Google Scholar]
- 43.Hu J, Shi W, Xu J, Liu S, Hu S, Fu W, et al. Complementary and alternative medicine for the treatment of abnormal endometrial conditions in women with PCOS: a systematic review and Meta-analysis of randomized controlled trials. Evid Based Complement Alternat Med. (2021) 2021:1–17. doi: 10.1155/2021/5536849, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang J, Huang X, Liu Y, He Y, Yu H. A comparison of the effects of Chinese non-pharmaceutical therapies for premature ovarian failure: a PRISMA-compliant systematic review and network meta-analysis. Medicine. (2020) 99:E20958. doi: 10.1097/MD.0000000000020958, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yin P, Li L, Lu L, Chen Y, Li K, Zhang W. Effects of acupuncture with assisted reproductive technology on clinical outcome in polycystic ovary syndrome patients: a meta-analysis and GRADE classification. China J Tradit Chin Med Pharm. (2020) 35:854–8. [Google Scholar]
- 46.Xu Y, Li M, An H, Huang Z, Li M, Gao Y, et al. Meta-analysis of acupuncture combined with traditional Chinese medicine in the treatment of premature ovarian failure. Modern Chin Med Clin. (2020) 27:49–54. [Google Scholar]
- 47.Wu J, Chen D, Liu N. Effectiveness of acupuncture in polycystic ovary syndrome: a systematic review and meta-analysis of randomized controlled trials. Medicine. (2020) 99:e20441. doi: 10.1097/md.0000000000020441 [DOI] [PubMed] [Google Scholar]
- 48.Tingting Z, Lixia P, Rongkui H, Hao C, Lu C, Jing G, et al. Meta-analysis of acupuncture treatment for premature ovarian insufficiency. Liaoning J Tradit Chin Med. (2020) 47:10–6. doi: 10.13192/j.issn.1000-1719.2020.03.004 [DOI] [Google Scholar]
- 49.Li Y, Xia G, Tan Y, Shuai J. Acupoint stimulation and Chinese herbal medicines for the treatment of premature ovarian insufficiency: a systematic review and meta-analysis. Complement Ther Clin Pract. (2020) 41:101244. doi: 10.1016/j.ctcp.2020.101244, PMID: [DOI] [PubMed] [Google Scholar]
- 50.Jin-Huan Z, Yong-Feng L, Xing-Xian H, Hai-Bo Y. Systematic evaluation and Meta-analysis of acupuncture-Moxibustion alone in treating premature ovarian failure. J Guangzhou Univ Tradit Chin Med. (2020) 37:1189–97. doi: 10.13359/j.cnki.gzxbtcm.2020.06.037 [DOI] [Google Scholar]
- 51.Yun L, Liqun W, Shuqi Y, Chunxiao W, Liming L, Wei Y. Acupuncture for infertile women without undergoing assisted reproductive techniques (ART) a systematic review and meta-analysis. Medicine. (2019) 98:e16463. doi: 10.1097/MD.0000000000016463, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Song Y-J, Liang F-X, Wu S, Yang H-S, Chen L, Huang Q, et al. Network meta-analysis on the effects of the acupuncture-related therapy on ovulation rate and pregnancy rate in patients with polycystic ovary syndrome. Zhongguo Zhen Jiu. (2019) 39:792–8. doi: 10.13703/j.0255-2930.2019.07.029, PMID: [DOI] [PubMed] [Google Scholar]
- 53.Lim CED, Ng RWC, Cheng NCL, Zhang GS, Chen H. Acupuncture for polycystic ovarian syndrome. Cochrane Database Syst Rev. (2019) 7:CD007689. doi: 10.1002/14651858.CD007689.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Meiling F, Pang Y, Xiaoyan W, Lu L, Xiaoping C. Meta-analysis of the therapeutic effect of acupuncture on premature ovarian failure. J Yunnan Univ Tradit Chin Med. (2018) 41:63–9. doi: 10.19288/j.cnki.issn.1000-2723.2018.06.013 [DOI] [Google Scholar]
- 55.Lina S, Lixia L, Yijiao C, Sida C, Buping L. Clinical effect of acupuncture combined with clomiphene in the treatment of infertility caused by polycystic ovary syndrome. World Tradit Chin Med. (2018) 13:759–66. [Google Scholar]
- 56.Yang HS, Fang YG, Xu HF, Li XT, Shang J, Yin YQ. Systematic evaluation on the clinical efficacy of acupoint stimulation therapy for treatment of premature ovarian insufficiency on the basis of network meta-analysis. World J Acupunct Moxibustion. (2017) 27:26–39. doi: 10.1016/S1003-5257(17)30137-X [DOI] [Google Scholar]
- 57.Runzi J, Tiantian H, Qiongdan Z, Mengyun K, Jiangbin L, Dongmei H. Meta-analysis of the efficacy of acupuncture and western medicine in the treatment of premature ovarian failure. Sci Technol Vision. (2017) 1:123–4. doi: 10.19694/j.cnki.issn2095-2457.2017.01.079 [DOI] [Google Scholar]
- 58.Jo J, Lee YJ, Lee H. Acupuncture for polycystic ovarian syndrome. Medicine. (2017) 96:e7066. doi: 10.1097/MD.0000000000007066, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jo J, Lee YJ. Effectiveness of acupuncture in women with polycystic ovarian syndrome undergoing in vitro fertilisation or intracytoplasmic sperm injection: a systematic review and meta-analysis. Acupunct Med. (2017) 35:162–70. doi: 10.1136/acupmed-2016-011163, PMID: [DOI] [PubMed] [Google Scholar]
- 60.Geng X, Cong P, Ni F, Cao R, Wu Z. Clinical meta-analysis of treating premature ovarian failure by medicine and acupuncture. Chin Arch Tradit Chin Med. (2017) 35:1295–8. doi: 10.13193/j.issn.1673-7717.2017.05.067 [DOI] [Google Scholar]
- 61.Yong P, Yanyan H. Meta analysis the effect of acupuncture in treating premature ovarian failure. Clin J Tradit Chin Med. (2016) 28:108–12. doi: 10.16448/j.cjtcm.2016.0042 [DOI] [Google Scholar]
- 62.Xi L, Qian L, Jie C, Qixin H, Zhengyun X, Pengyan Y, et al. Systematic review and meta analysis of efficacy of acupuncture in the treatment of premature ovarian failure. J Tradit Chin Med. (2016) 57:1027–32. doi: 10.13288/j.11-2166/r.2016.12.010 [DOI] [Google Scholar]
- 63.Ruigen L, Wei W, Xiaobei X, Weijing B, Zhaoli W. A systematic review and meta-analysis of the efficacy of acupuncture combined with medicine in the treatment of polycystic ovary syndrome. J Pract Chin Inter Med. (2016) 30:1–5. doi: 10.13729/j.issn.1671-7813.2016.01.01 [DOI] [Google Scholar]
- 64.Qu F, Wu Y, Hu XY, Barry JA, Zhou J, Wang FF, et al. The effects of acupuncture on polycystic ovary syndrome: a systematic review and meta-analysis. Eur J Integr Med. (2016) 8:12–8. doi: 10.1016/j.eujim.2016.02.001 [DOI] [Google Scholar]
- 65.Lim CED, Ng RW, Xu K, Cheng NCL, Xue CC, Liu JP, et al. Acupuncture for polycystic ovarian syndrome. Cochrane Database Syst Rev. (2016) 2016:CD007689. doi: 10.1002/14651858.CD007689.pub3 [DOI] [PubMed] [Google Scholar]
- 66.Jo J, Lee YJ, Lee H. Effectiveness of acupuncture for primary ovarian insufficiency: a systematic review and Meta-analysis. Evid Based Complement Alternat Med. (2015) 2015:1–12. doi: 10.1155/2015/842180, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ma H, Quan X, Li J, Chen X. (editors). Acupuncture for polycystic ovary syndrome: a systematic review and meta-analysis In: 2014 IEEE workshop on electronics, computer and applications: IEEE; (2014). doi: 10.1109/IWECA.2014.6845743 [DOI] [Google Scholar]
- 68.Jørgensen L, Paludan-Müller AS, Laursen DR, Savović J, Boutron I, Sterne JA, et al. Evaluation of the Cochrane tool for assessing risk of bias in randomized clinical trials: overview of published comments and analysis of user practice in Cochrane and non-Cochrane reviews. Syst Rev. (2016) 5:1–13. doi: 10.1186/s13643-016-0259-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jie M, Ying L, Lai-ping Z, Chen-ping Z, Zhi-yuan Z. Comparison between Jadad scale and Cochrane collaboration's tool for assessing risk of bias on the quality and risk of bias evaluation in randomized controlled trials. J Oral Maxillofacial Surg. (2012) 10. [Google Scholar]
- 70.Acién P, Quereda F, Matallín P, Villarroya E, López-Fernández JA, Acién M, et al. Insulin, androgens, and obesity in women with and without polycystic ovary syndrome: a heterogeneous group of disorders. Fertil Steril. (1999) 72:32–40. doi: 10.1016/S0015-0282(99)00184-3, PMID: [DOI] [PubMed] [Google Scholar]
- 71.Teede HJ, Hutchison S, Zoungas S, Meyer C. Insulin resistance, the metabolic syndrome, diabetes, and cardiovascular disease risk in women with PCOS. Endocrine. (2006) 30:45–54. doi: 10.1385/ENDO:30:1:45 [DOI] [PubMed] [Google Scholar]
- 72.Johansson J, Stener-Victorin E. Polycystic ovary syndrome: effect and mechanisms of acupuncture for ovulation induction. Evid Based Complement Alternat Med. (2013) 2013:762615: 1–16. doi: 10.1155/2013/762615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Xia L, Xia Y. Clinical research and the effect mechanism on premature ovarian failure treated with acupuncture in recent 20 years. Zhongguo Zhen Jiu. (2018) 38:5653–70. doi: 10.13703/j.0255-2930.2018.05.031, PMID: [DOI] [PubMed] [Google Scholar]
- 74.Yuan Y, Xia Q, Cui W, Cao W, Zhou Z, Peng J, et al. Study on the mechanism of action of different acupuncture regimens on premature ovarian failure model rats. Comput Math Methods Med. (2022) 2022:1–10. doi: 10.1155/2022/5254628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chen X, He H, Long B, Wei B, Yang P, Huang X, et al. Acupuncture regulates the apoptosis of ovarian granulosa cells in polycystic ovarian syndrome-related abnormal follicular development through LncMEG3-mediated inhibition of miR-21-3p. Biol Res. (2023) 56:31. doi: 10.1186/s40659-023-00441-6, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wu J, Chen X. Acupuncture therapy protects PCOS patients with diabetes by regulating miR-32-3p/PLA2G4A pathway. Am J Transl Res. (2021) 13:8819., PMID: [PMC free article] [PubMed] [Google Scholar]
- 77.Kokosar M, Benrick A, Perfilyev A, Nilsson E, Källman T, Ohlsson C, et al. A single bout of electroacupuncture remodels epigenetic and transcriptional changes in adipose tissue in polycystic ovary syndrome. Sci Rep. (2018) 8:1878. doi: 10.1038/s41598-017-17919-5, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lai M-H, Ma H-X, Li J, Song X-H, Liu H. Effects of electroacupuncture on mRNA expressions of insulin-receptor substrates 1 and 2 in the endometrium of PCOS rats and insulin sensitivity. Chin J Integr Tradit Western Med. (2016) 36:1082–6. PMID: [PubMed] [Google Scholar]
- 79.Xiang S, Xia M-f, Song J-y, Liu D-q, Lian F. Effect of electro-acupuncture on expression of IRS-1/PI3K/GLUT4 pathway in ovarian granulosa cells of infertile patients with polycystic ovary syndrome-insulin resistance of phlegm-dampness syndrome. Chin J Integr Med. (2021) 27:330–5. doi: 10.1007/s11655-020-3219-z, PMID: [DOI] [PubMed] [Google Scholar]
- 80.Zhou K, Jiang J, Wu J, Liu Z. Electroacupuncture modulates reproductive hormone levels in patients with primary ovarian insufficiency: results from a prospective observational study. Evid Based Complement Alternat Med. (2013) 2013:657234: 1–7. doi: 10.1155/2013/657234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Fu Y, Ding D-N, Shen Y, Jia L-Y, Yan M-Y, Wei W, et al. Complementary and alternative medicine for premature ovarian insufficiency: a review of utilization and mechanisms. Evid Based Complement Alternat Med. (2022) 2022:1–15. doi: 10.1155/2022/9053930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ko JH, Kim S-N. A literature review of women’s sex hormone changes by acupuncture treatment: analysis of human and animal studies. Evid Based Complement Alternat Med. (2018) 2018:3752723. doi: 10.1155/2018/3752723, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Cao H, Li H, Lin G, Li X, Liu S, Li P, et al. The clinical value of acupuncture for women with premature ovarian insufficiency: a systematic review and meta-analysis of randomized controlled trials. Front Endocrinol. (2024) 15:1361573. doi: 10.3389/fendo.2024.1361573, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wang Z, Dong H, Wang Q, Zhang L, Wu X, Zhou Z, et al. Effects of electroacupuncture on anxiety and depression in unmarried patients with polycystic ovarian syndrome: secondary analysis of a pilot randomised controlled trial. Acupunct Med. (2019) 37:40–6. doi: 10.1136/acupmed-2017-011615, PMID: [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.