Abstract
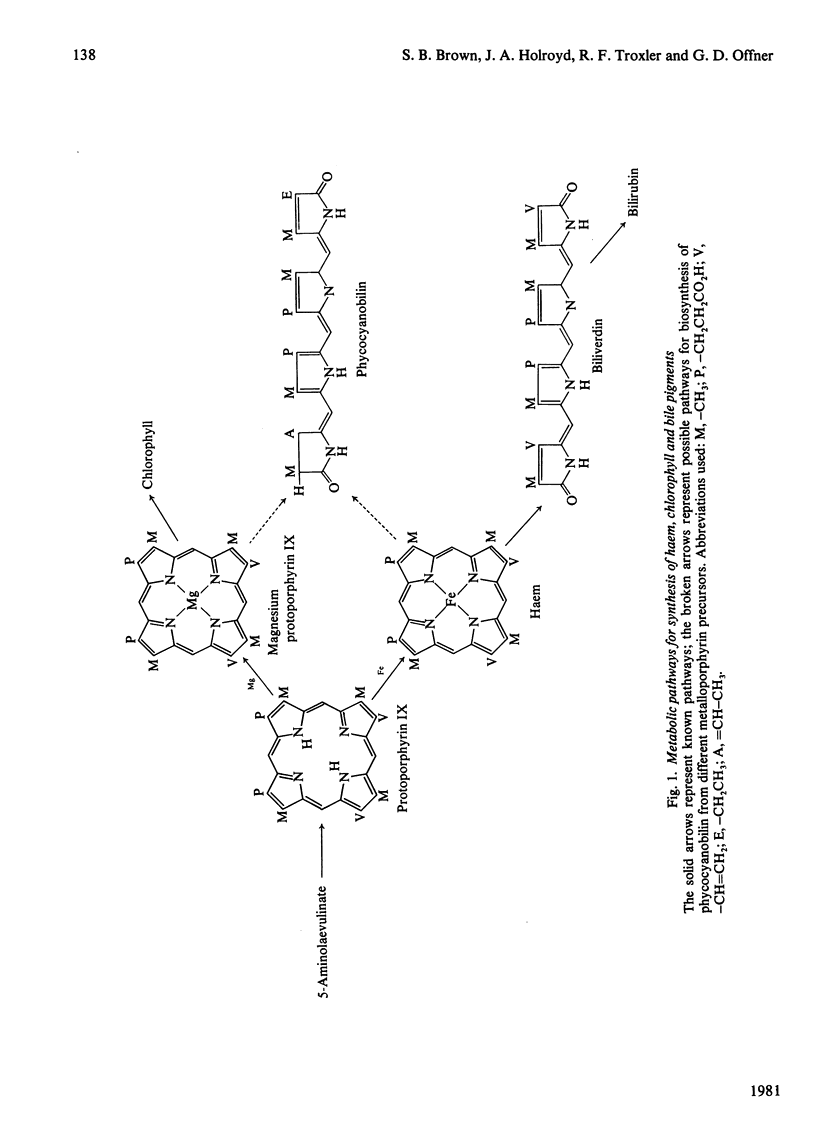
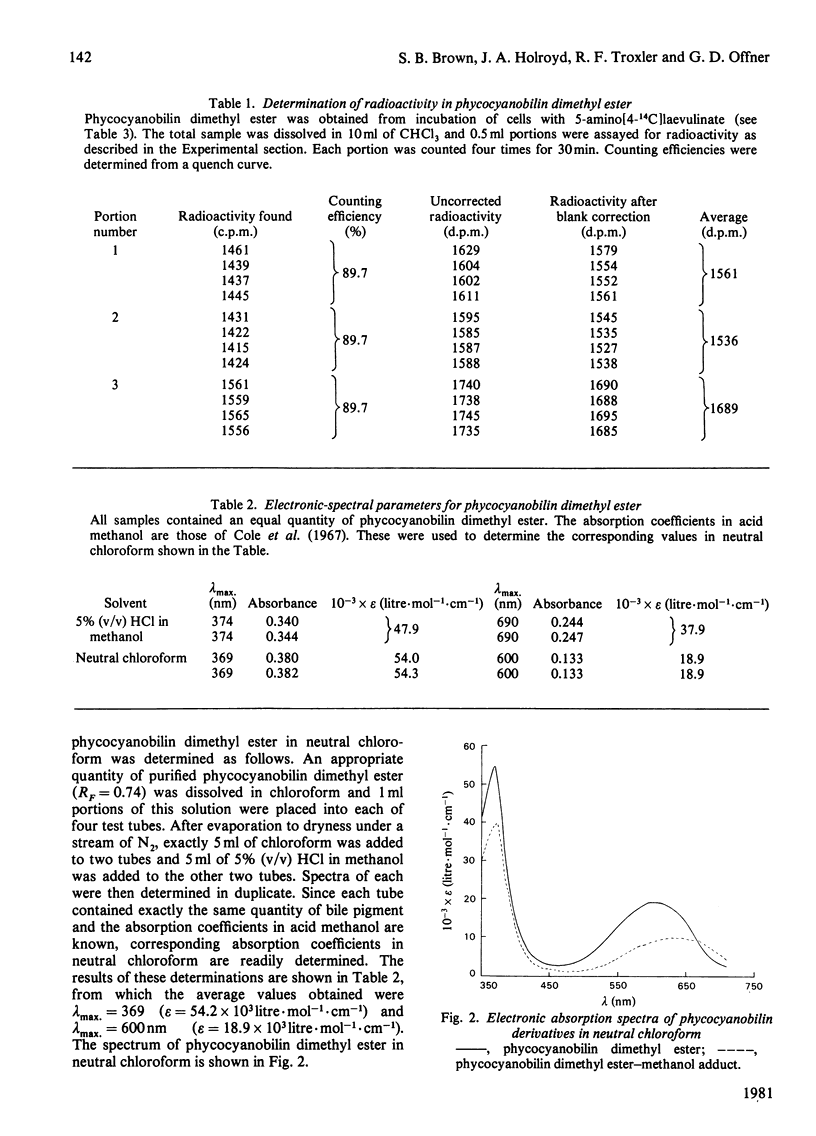
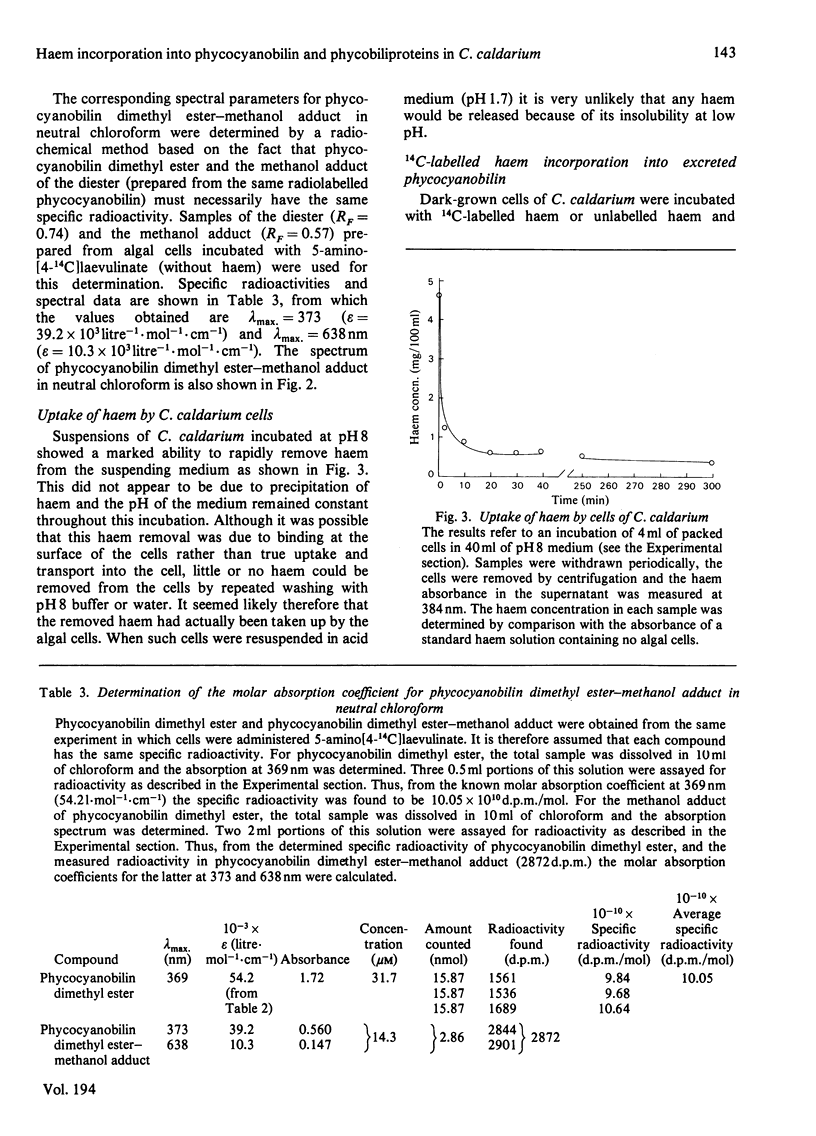
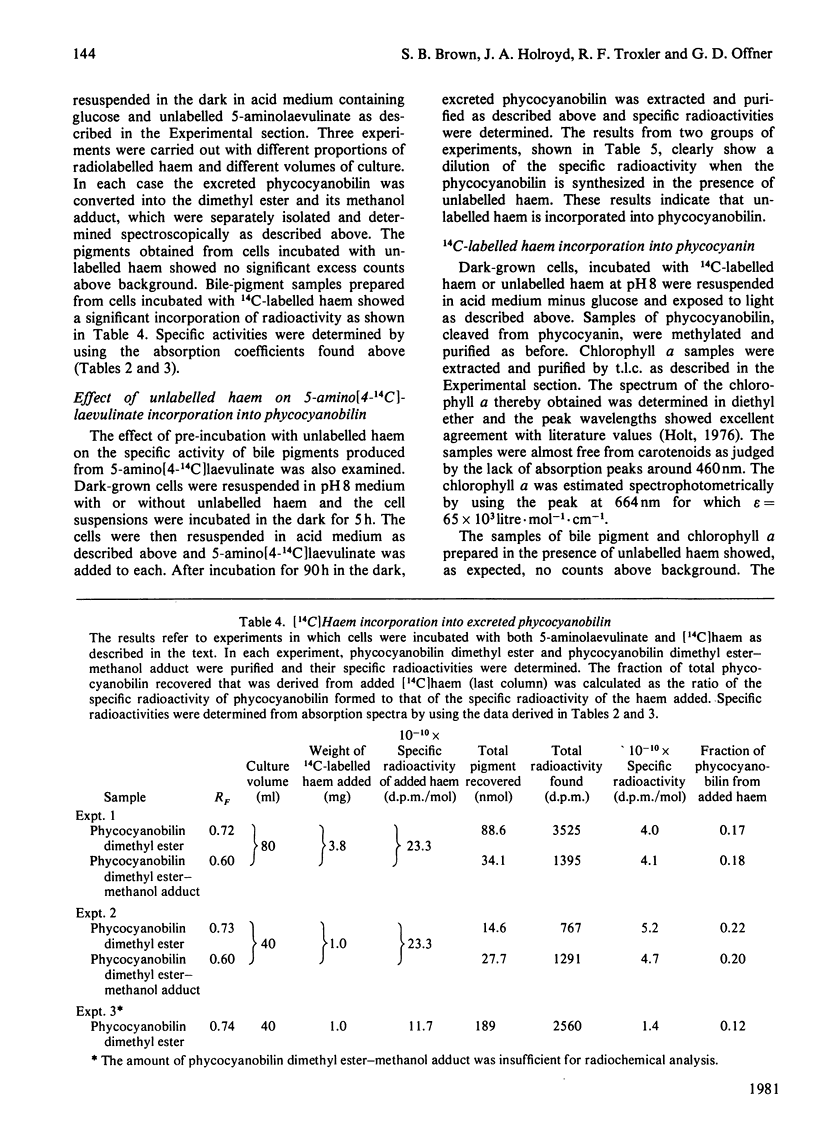
A procedure was developed whereby haem was taken up by dark-grown cells of the unicellular rhodophyte Cyanidium caldarium. These cells were subsequently incubated either in the dark with 5-aminolaevulinate, which results in excretion of phycocyanobilin into the suspending medium or incubated in the light, which results in synthesis and accumulation of phycocyanin and chlorophyll a within the cells. Phycocyanobilin was isolated from phycocyanin by cleavage from apoprotein in methanol. Phycocyanobilin prepared from phycocyanin or excreted from cells given 5-aminolaevulinate was methylated and purified by t.l.c. By using 14C labelling either in the haem or in 5-aminolaevulinate administered, haem incorporation into phycocyanobilin was demonstrated in both dark and light systems. Since chlorophyll a synthesized in the light in the presence of labelled haem contained no radioactivity, it was clear that haem was directly incorporated into phycocyanobilin and not first converted into protoporphyrin IX. These results clearly demonstrate phycocyanobilin synthesis via haem and not via magnesium protoporphyrin IX as has also been postulated.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALLEN M. B. Studies with Cyanidium caldarium, an anomalously pigmented chlorophyte. Arch Mikrobiol. 1959;32(3):270–277. doi: 10.1007/BF00409348. [DOI] [PubMed] [Google Scholar]
- BACON M. F. SEPARATION OF CHLOROPHYLLS A AND B AND RELATED COMPOUNDS BY THIN-LAYER CHROMATOGRAPHY ON CELLULOSE. J Chromatogr. 1965 Feb;17:322–326. doi: 10.1016/s0021-9673(00)99875-3. [DOI] [PubMed] [Google Scholar]
- Beuhler R. J., Pierce R. C., Friedman L., Siegelman H. W. Cleavage of phycocyanobilin from C-phycocyanin. Separation and mass spectral identification of the products. J Biol Chem. 1976 Apr 25;251(8):2405–2411. [PubMed] [Google Scholar]
- Bissell D. M., Liem H. H., Muller-Eberhard U. Secretion of haem by hepatic parenchymal cells. Biochem J. 1979 Dec 15;184(3):689–694. doi: 10.1042/bj1840689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown S. B., Holroyd A. J., Troxler R. F. Mechanism of bile-pigment synthesis in algae. 18O incorporation into phycocyanobilin in the unicellular rhodophyte, Cyanidium caldarium. Biochem J. 1980 Aug 15;190(2):445–449. doi: 10.1042/bj1900445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown S. B., King R. F. The mechanism of haem catabolism. Bilirubin formation in living rats by [18O]oxygen labelling. Biochem J. 1978 Feb 15;170(2):297–311. doi: 10.1042/bj1700297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaney B. D., Brown S. B. The mechanism of coupled oxidation of octaethylhaem to octaethylbiliverdin [proceedings]. Biochem Soc Trans. 1978;6(2):419–421. doi: 10.1042/bst0060419. [DOI] [PubMed] [Google Scholar]
- Craig R. K., Brown P. A., Harrison O. S., McIlreavy D., Campbell P. N. Guinea-pig milk-protein synthesis. Isolation and characterization of messenger ribonucleic acids from lactating mammary gland and identification of caseins and pre-alpha-lactalbumin as translation products in heterologous cell-free systems. Biochem J. 1976 Oct 15;160(1):57–74. doi: 10.1042/bj1600057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killilea S. D., O'Carra P., Murphy R. F. Structures and apoprotein linkages of phycoerythrobilin and phycocyanobilin. Biochem J. 1980 May 1;187(2):311–320. doi: 10.1042/bj1870311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King R. F., Brown S. B. The mechanism of haem catabolism. A study of haem breakdown in spleen microsomal fraction and in a model system by 18O labelling and metal substitution. Biochem J. 1978 Jul 15;174(1):103–109. doi: 10.1042/bj1740103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LABBE R. F., NISHIDA G. A new method of hemin isolation. Biochim Biophys Acta. 1957 Nov;26(2):437–437. doi: 10.1016/0006-3002(57)90033-1. [DOI] [PubMed] [Google Scholar]
- O'Carra P., Murphy R. F., Killilea S. D. The native forms of the phycobilin chromophores of algal biliproteins. A clarification. Biochem J. 1980 May 1;187(2):303–309. doi: 10.1042/bj1870303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid R., McDonagh A. F. The enzymatic formation of bilirubin. Ann N Y Acad Sci. 1975 Apr 15;244:533–552. doi: 10.1111/j.1749-6632.1975.tb41553.x. [DOI] [PubMed] [Google Scholar]
- Shapiro A., Hutner S. H., Katz L., Bacchi C. J., Tamburro K. O., Baker H. Dense Crithidia growth and heme sparing: relation to Fe, Cu, Mo chelation. J Protozool. 1978 Nov;25(4):530–534. doi: 10.1111/j.1550-7408.1978.tb04180.x. [DOI] [PubMed] [Google Scholar]
- Tenhunen R., Marver H. S., Schmid R. The enzymatic conversion of heme to bilirubin by microsomal heme oxygenase. Proc Natl Acad Sci U S A. 1968 Oct;61(2):748–755. doi: 10.1073/pnas.61.2.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troxler R. F., Bogorad L. Studies on the formation of phycocyanin, porphyrins, and a blue phycobilin by wild-type and mutant strains of Cyanidium caldarium. Plant Physiol. 1966 Mar;41(3):491–499. doi: 10.1104/pp.41.3.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troxler R. F., Brown A. S., Brown S. B. Bile pigment synthesis in plants. Mechanism of 18O incorporation into phycocyanobilin in the unicellular rhodophyte. Cyanidium caldarium. J Biol Chem. 1979 May 10;254(9):3411–3418. [PubMed] [Google Scholar]
- Troxler R. F., Kelly P., Brown S. B. Phycocyanobilin synthesis in the unicellular rhodophyte Cyanidium caldarium. Biochem J. 1978 Jun 15;172(3):569–576. doi: 10.1042/bj1720569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troxler R. F. Synthesis of bile pigments in plants. Formation of carbon monoxide and phycocyanobilin in wild-type and mutant strains of the alga, Cyanidium caldarium. Biochemistry. 1972 Nov 7;11(23):4235–4242. doi: 10.1021/bi00773a007. [DOI] [PubMed] [Google Scholar]


