Abstract
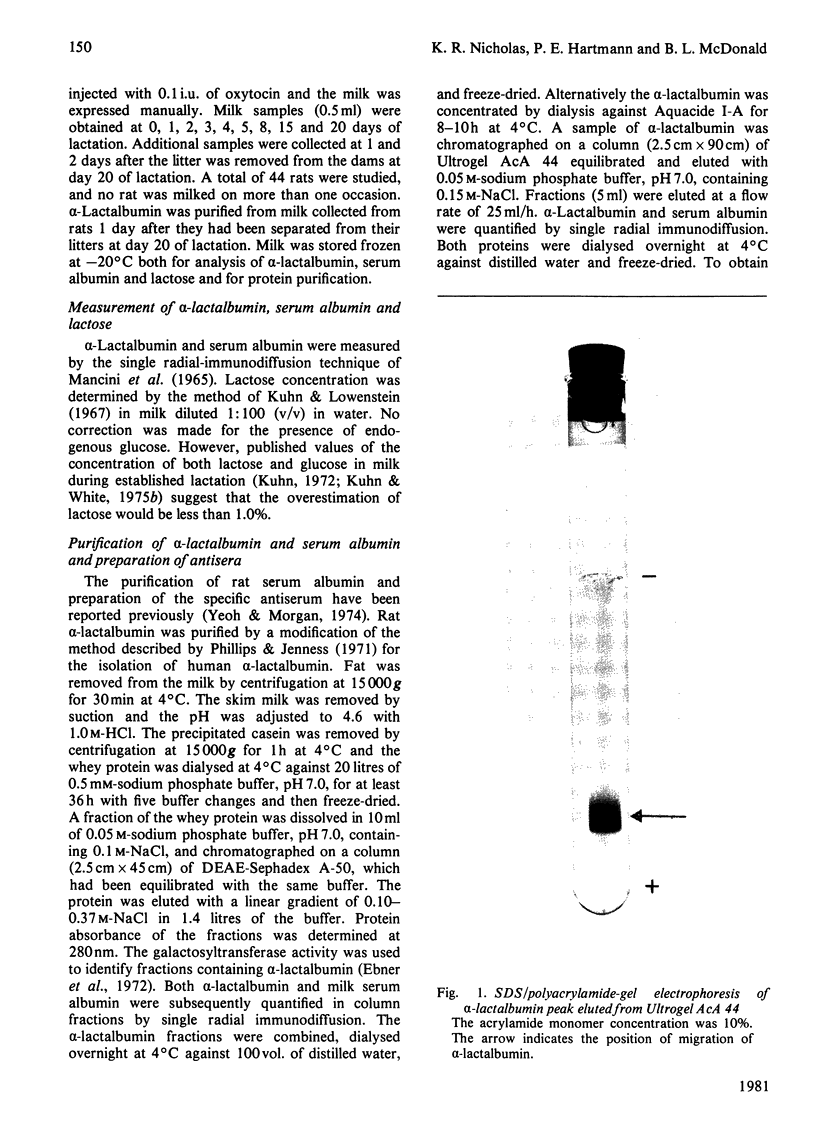
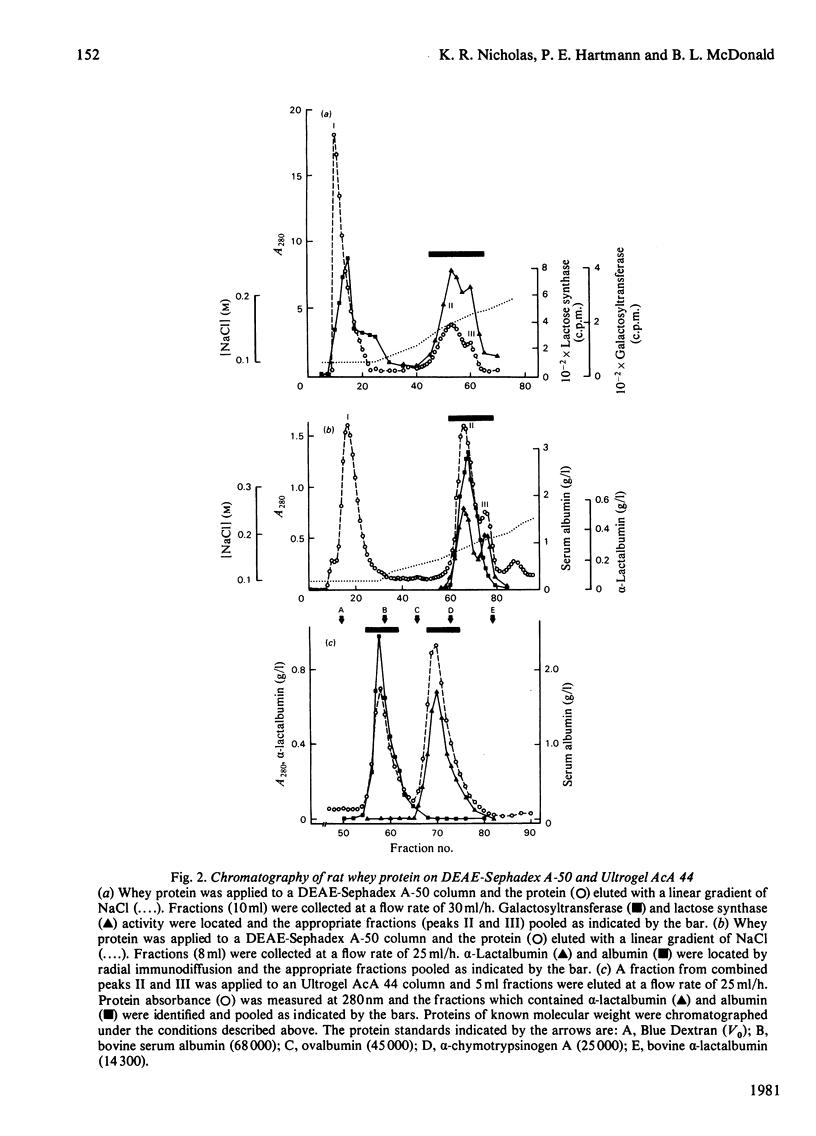
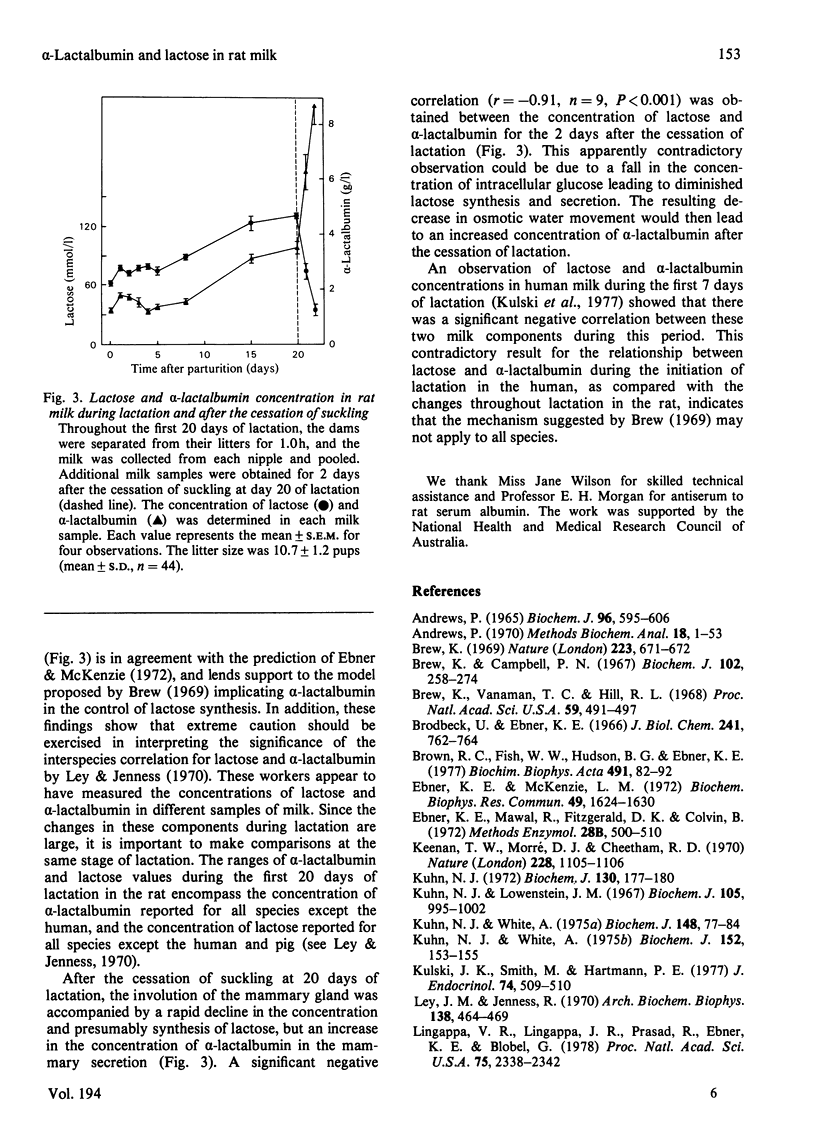
Homogeneous rat alpha-lactalbumin was prepared from whey by chromatography on DEAE-Sephadex A-50 and Ultrogel AcA 44. Two biologically active forms of alpha-lactalbumin were apparent after ion-exchange chromatography, but on gel filtration the combined forms were eluted as a single peak with a molecular weight of approx. 33000. The molecular weight when determined by sodium dodecyl sulphate/polyacrylamide-gel electrophoresis was 15100. Antiserum to alpha-lactalbumin was prepared from rabbits, and single radial immunodiffusion was used to measure the concentration of alpha-lactalbumin in milk expressed from rats during lactation and for 2 days after the cessation of lactation. A significant positive correlation (r = + 0.89) between the concentrations of alpha-lactalbumin and lactose was obtained for the first 20 days of lactation. This is consistent with the suggestion that alpha-lactalbumin may control the concentration of lactose in milk. However, a significant negative correlation (r = -0.91) between the concentration of alpha-lactalbumin and lactose was obtained for 2 days after the cessation of lactation on day 20.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews P. Estimation of molecular size and molecular weights of biological compounds by gel filtration. Methods Biochem Anal. 1970;18:1–53. [PubMed] [Google Scholar]
- Andrews P. The gel-filtration behaviour of proteins related to their molecular weights over a wide range. Biochem J. 1965 Sep;96(3):595–606. doi: 10.1042/bj0960595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brew K., Campbell P. N. Studies on the biosynthesis of protein by lactating guinea-pig mammary gland. Characteristics of the synthesis of alpha-lactalbumin and total protein by slices and cell-free systems. Biochem J. 1967 Jan;102(1):265–274. doi: 10.1042/bj1020265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brew K. Secretion of alpha-lactalbumin into milk and its relevance to the organization and control of lactose synthetase. Nature. 1969 May 17;222(5194):671–672. doi: 10.1038/222671a0. [DOI] [PubMed] [Google Scholar]
- Brew K., Vanaman T. C., Hill R. L. The role of alpha-lactalbumin and the A protein in lactose synthetase: a unique mechanism for the control of a biological reaction. Proc Natl Acad Sci U S A. 1968 Feb;59(2):491–497. doi: 10.1073/pnas.59.2.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodbeck U., Ebner K. E. Resolution of a soluble lactose synthetase into two protein components and solubilization of microsomal lactose synthetase. J Biol Chem. 1966 Feb 10;241(3):762–764. [PubMed] [Google Scholar]
- Brown R. C., Fish W. W., Hudson B. G., Ebner K. E. Isolation and characterization of rat alpha-lactalbumin: a glycoprotein. Biochim Biophys Acta. 1977 Mar 28;491(1):82–92. doi: 10.1016/0005-2795(77)90043-5. [DOI] [PubMed] [Google Scholar]
- Ebner K. E., McKenzie L. M. -Lactalbumin and galactosyltransferase in rat serum and their relationship to milk secretion. Biochem Biophys Res Commun. 1972 Dec 18;49(6):1624–1630. doi: 10.1016/0006-291x(72)90528-1. [DOI] [PubMed] [Google Scholar]
- Keenan T. W., Morré D. J., Cheetham R. D. Lactose synthesis by a golgi apparatus fraction from rat mammary gland. Nature. 1970 Dec 12;228(5276):1105–1106. doi: 10.1038/2281105a0. [DOI] [PubMed] [Google Scholar]
- Kuhn N. J., Lowenstein J. M. Lactogenesis in the rat. Changes in metabolic parameters at parturition. Biochem J. 1967 Dec;105(3):995–1002. doi: 10.1042/bj1050995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn N. J. The lactose and neuraminlactose content of rat milk and mammary tissue. Biochem J. 1972 Nov;130(1):177–180. doi: 10.1042/bj1300177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn N. J., White A. Milk glucose as an index of the intracellular glucose concentration of rat mammary gland. Biochem J. 1975 Oct;152(1):153–155. doi: 10.1042/bj1520153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn N. J., White A. The topography of lactose synthesis. Biochem J. 1975 Apr;148(1):77–84. doi: 10.1042/bj1480077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulski J. K., Smith M., Hartmann P. E. Perinatal concentrations of progesterone, lactose and alpha-lactalbumin in the mammary secretion of women. J Endocrinol. 1977 Sep;74(3):509–510. doi: 10.1677/joe.0.0740509. [DOI] [PubMed] [Google Scholar]
- Ley J. M., Jenness R. Lactose synthetase activity of alpha-lactalbumins from several species. Arch Biochem Biophys. 1970 Jun;138(2):464–469. doi: 10.1016/0003-9861(70)90370-x. [DOI] [PubMed] [Google Scholar]
- Lingappa V. R., Lingappa J. R., Prasad R., Ebner K. E., Blobel G. Coupled cell-free synthesis, segregation, and core glycosylation of a secretory protein. Proc Natl Acad Sci U S A. 1978 May;75(5):2338–2342. doi: 10.1073/pnas.75.5.2338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linzell J. L., Peaker M. Mechanism of milk secretion. Physiol Rev. 1971 Jul;51(3):564–597. doi: 10.1152/physrev.1971.51.3.564. [DOI] [PubMed] [Google Scholar]
- Mancini G., Carbonara A. O., Heremans J. F. Immunochemical quantitation of antigens by single radial immunodiffusion. Immunochemistry. 1965 Sep;2(3):235–254. doi: 10.1016/0019-2791(65)90004-2. [DOI] [PubMed] [Google Scholar]
- McKenzie R. M., Larson B. L. Purification and characterization of rat alpha-lactalbumins: apparent genetic variants. J Dairy Sci. 1978 Jun;61(6):714–722. doi: 10.3168/jds.S0022-0302(78)83638-8. [DOI] [PubMed] [Google Scholar]
- Morrison J. F., Ebner K. E. Studies on galactosyltransferase. Kinetic effects of -lactalbumin with N-acetylglucosamine and glucose as galactosyl group acceptors. J Biol Chem. 1971 Jun 25;246(12):3992–3998. [PubMed] [Google Scholar]
- Morrison J. F., Ebner K. E. Studies on galactosyltransferase. Kinetic investigations with N-acetylglucosamine as the galactosyl group acceptor. J Biol Chem. 1971 Jun 25;246(12):3977–3984. [PubMed] [Google Scholar]
- Morrison J. F., Ebner K. E. Studies on galactosyltransferase. Kinetic investigations with glucose as the galactosyl group acceptor. J Biol Chem. 1971 Jun 25;246(12):3985–3991. [PubMed] [Google Scholar]
- Phillips N. I., Jenness R. Isolation and properties of human alpha-lactalbumin. Biochim Biophys Acta. 1971 Feb 16;229(2):407–410. doi: 10.1016/0005-2795(71)90199-1. [DOI] [PubMed] [Google Scholar]
- Qasba P. K., Chakrabartty P. K. Purification and properties of two forms of rat alpha-lactalbumin. J Biol Chem. 1978 Feb 25;253(4):1167–1173. [PubMed] [Google Scholar]
- Schmidt D. V., Ebner K. E. Isolation and properties of -lactalbumin from various sources. Biochim Biophys Acta. 1971 Aug 27;243(2):273–283. doi: 10.1016/0005-2795(71)90084-5. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Yeoh G. C., Morgan E. H. Albumin and transferrin synthesis during development in the rat. Biochem J. 1974 Nov;144(2):215–224. doi: 10.1042/bj1440215. [DOI] [PMC free article] [PubMed] [Google Scholar]



