Abstract
BACKGROUND:
An interatrial shunt may provide an autoregulatory mechanism to decrease left atrial pressure and improve heart failure (HF) symptoms and prognosis.
METHODS:
Patients with symptomatic HF with any left ventricular ejection fraction (LVEF) were randomized 1:1 to transcatheter shunt implantation versus a placebo procedure, stratified by reduced (≤40%) versus preserved (>40%) LVEF. The primary safety outcome was a composite of device-related or procedure-related major adverse cardiovascular or neurological events at 30 days compared with a prespecified performance goal of 11%. The primary effectiveness outcome was the hierarchical composite ranking of all-cause death, cardiac transplantation or left ventricular assist device implantation, HF hospitalization, outpatient worsening HF events, and change in quality of life from baseline measured by the Kansas City Cardiomyopathy Questionnaire overall summary score through maximum 2-year follow-up, assessed when the last enrolled patient reached 1-year follow-up, expressed as the win ratio. Prespecified hypothesis-generating analyses were performed in patients with reduced and preserved LVEF.
RESULTS:
Between October 24, 2018, and October 19, 2022, 508 patients were randomized at 94 sites in 11 countries to interatrial shunt treatment (n=250) or a placebo procedure (n=258). Median (25th and 75th percentiles) age was 73.0 years (66.0, 79.0), and 189 patients (37.2%) were women. Median LVEF was reduced (≤40%) in 206 patients (40.6%) and preserved (>40%) in 302 patients (59.4%). No primary safety events occurred after shunt implantation (upper 97.5% confidence limit, 1.5%; P<0.0001). There was no difference in the 2-year primary effectiveness outcome between the shunt and placebo procedure groups (win ratio, 0.86 [95% CI, 0.61–1.22]; P=0.20). However, patients with reduced LVEF had fewer adverse cardiovascular events with shunt treatment versus placebo (annualized rate 49.0% versus 88.6%; relative risk, 0.55 [95% CI, 0.42–0.73]; P<0.0001), whereas patients with preserved LVEF had more cardiovascular events with shunt treatment (annualized rate 60.2% versus 35.9%; relative risk, 1.68 [95% CI, 1.29–2.19]; P=0.0001; Pinteraction<0.0001). There were no between-group differences in change in Kansas City Cardiomyopathy Questionnaire overall summary score during follow-up in all patients or in those with reduced or preserved LVEF.
CONCLUSIONS:
Transcatheter interatrial shunt implantation was safe but did not improve outcomes in patients with HF. However, the results from a prespecified exploratory analysis in stratified randomized groups suggest that shunt implantation is beneficial in patients with reduced LVEF and harmful in patients with preserved LVEF.
REGISTRATION:
URL: https://www.clinicaltrials.gov; Unique identifier: NCT03499236.
Keywords: atrial pressure, heart failure, prognosis
Clinical Perspective.
What Is New?
In patients with heart failure (HF) who remained symptomatic despite guideline-directed medical therapy, transcatheter implantation of a small interatrial shunt was safe but did not improve clinical outcomes during 2 years of follow-up.
The results varied strikingly according to baseline left ventricular ejection fraction (LVEF); adverse cardiovascular events were markedly reduced with shunt treatment in patients with reduced (≤40%) LVEF but increased in those with preserved (>40%) LVEF.
The difference in quality of life during follow-up between groups was not affected by shunt treatment either in all patients or in those with reduced or preserved LVEF.
What Are the Clinical Implications?
Transcatheter implantation of an interatrial shunt may be beneficial in patients with HF with reduced LVEF, markedly reducing adverse cardiovascular events (especially HF hospitalizations) in this high-risk cohort not responding to other therapies.
Shunt treatment may be harmful in patients with HF with preserved LVEF; in the current trial, all-cause mortality and HF hospitalization rates were increased after shunt implantation in this group.
• Additional studies in patients with HF with reduced LVEF are needed to substantiate the beneficial outcomes observed with shunt treatment and to understand why the quality-of-life measures assessed in the current placebo procedure–controlled study did not correlate with clinical prognosis.
Heart failure (HF) with either reduced or preserved left ventricular ejection fraction (LVEF) is characterized by increased left atrial pressure (LAP) and pulmonary venous congestion.1 LAP rises with exercise and fluid overload and may be difficult to regulate pharmacologically. Approximately 90% of HF hospitalizations (HFHs) manifest with symptoms of pulmonary venous congestion.2,3 In such patients, LAP is often elevated for days before hospitalization.1
A patent channel between the left and right atrium may provide an autoregulatory mechanism to decrease LAP and improve HF symptoms and prognosis.4,5 The presence of a congenital atrial septal defect may reduce symptoms from acquired mitral stenosis.6 Closure of a preexisting atrial septal defect or patent foramen ovale may provoke pulmonary edema in patients with left ventricular (LV) dysfunction.7,8 Atrial septostomy has been used to reduce intracardiac pressures and treat severe HF.9 In an ovine ischemic cardiomyopathy model, a percutaneously implanted interatrial shunt has been shown to decompress the left atrium and improve cardiac structure and function.10 In early human studies, this device has reduced filling pressures and provided symptomatic relief and functional improvement in patients with HF with both reduced and preserved LVEF.11,12
We therefore performed a randomized, double-blind, placebo procedure–controlled trial examining the safety and effectiveness of an interatrial device in symptomatic patients with HF with any LVEF.
Methods
Trial Design
RELIEVE-HF (Reducing Lung Congestion Symptoms in Advanced Heart Failure) was a randomized, double-blind, placebo procedure–controlled, multicenter trial that evaluated transcatheter implantation of the Ventura shunt in symptomatic patients with HF. The protocol and statistical analysis plan were designed by the principal investigators and sponsor and are provided in the Supplemental Material. The study organization and participating centers appear in Table S1. The study was approved by the investigational review board or ethics committee at each center, and all patients provided written informed consent. The trial was sponsored and funded by V-Wave Ltd. The sponsor participated in protocol design and site selection and management. The first author had unrestricted data access, prepared the article, and attests to the accuracy and completeness of the report. The report adheres to the CONSORT (Consolidated Standards of Reporting Trials) guidelines (Supplemental Material). The data from this study will not be made publicly available. The authors will consider requests for collaborative research. Any relevant inquiries should be emailed to the corresponding author.
Patients and Randomization
Patients were screened for enrollment at 113 sites in the United States, Canada, Israel, Germany, Spain, Switzerland, Belgium, Poland, the Netherlands, Australia, and New Zealand. Enrollment criteria are listed in Table S2. In brief, eligible patients had HF with either reduced (≤40%) or preserved (>40%) LVEF and remained symptomatic (New York Heart Association [NYHA] class II–IVa [ambulatory]) despite a stable maximally tolerated guideline-directed medical therapy regimen per societal guidelines.13,14 Exclusion criteria included marked LV dilatation, severe pulmonary hypertension, or moderate or greater right ventricular dysfunction. Sex and race and ethnicity data were self-reported. A central eligibility committee including HF specialists confirmed all entry criteria before enrollment.
After final screening with transesophageal or intracardiac echocardiography and right heart catheterization, qualifying patients were immediately randomized 1:1 in a blinded fashion to transcatheter implantation of the Ventura interatrial shunt (V-Wave) or a placebo procedure using random block sizes of 2 and 4 using an automated online system. Given uncertainty as to whether the response to a shunt would vary in patients with HF according to systolic function, randomization was stratified by reduced (≤40%) versus preserved (>40%) LVEF determined by the echocardiographic core laboratory. Randomization was also stratified by site.
Device, Procedures, Blinding, and Follow-Up
Description and images of the Ventura shunt and implant procedure appear in Table S3 and Figures S1 and S2. The shunt comprises an hourglass-shaped 12-mm-long nitinol frame with a 5.1-mm central orifice fully encapsulated with expanded polytetrafluoroethylene. The delivery catheter is introduced from the right femoral vein into the right atrium. After transseptal puncture, the shunt is implanted across the fossa ovalis. The ratio of pulmonary to systemic flow the shunt affords is ≈1.2:1.
Patients randomized to the placebo procedure had a mock transseptal catheterization and device placement performed following a script. To ensure blinding, all patients received deep sedation and wore masks and music-playing headphones. All health care providers, research personnel, and outcomes assessors were blinded during follow-up. Blinding effectiveness was assessed with a patient questionnaire (Table S4) before hospital discharge and at 1 year.
After the procedure, patients were treated with 75 to 100 mg of open-label oral aspirin per day and a masked platelet receptor P2Y12 inhibitor (75 mg of clopidogrel per day for shunt-treated patients or a matching placebo for control group patients) for 6 months if not otherwise taking oral anticoagulation, in which case antiplatelet medications were not administered. Clinical follow-up, quality of life (QOL) assessments, 6-minute walk testing (6MWT), and transthoracic echocardiography were performed at regular intervals through 2 years (Table S5). Patients were unblinded following the 2-year visit, after which shunt treatment was permitted in control group patients who still met all original enrollment criteria. All shunt-treated patients are followed for 5 years.
Outcome Measures
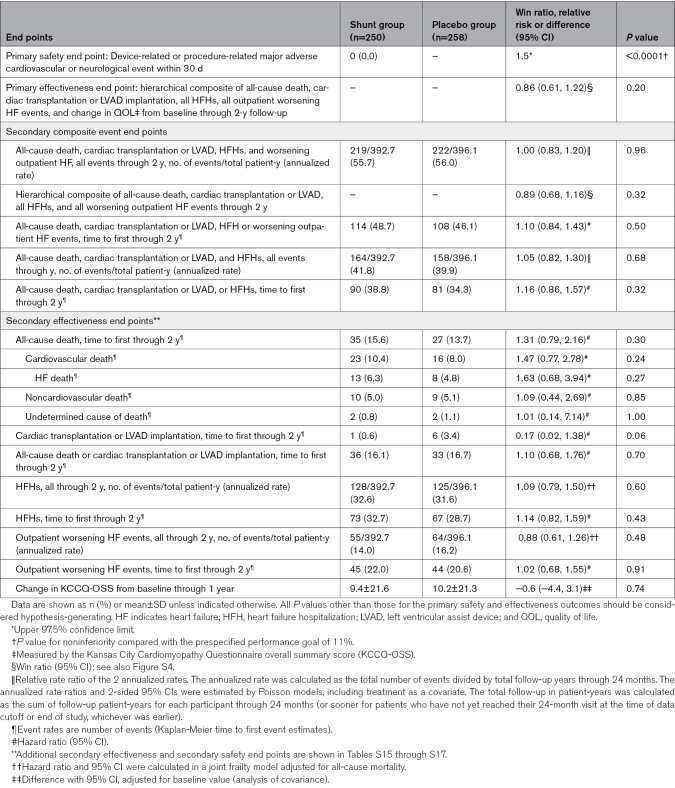
Detailed listings and definitions of the primary and secondary outcomes appear in Tables S6 and S7. The primary safety outcome was a composite of device-related or procedure-related major adverse cardiovascular or neurological events occurring in the shunt arm within 30 days after randomization. The primary effectiveness outcome was a hierarchical composite of cardiovascular events (all-cause death, cardiac transplantation or LV assist device [LVAD] implantation, HFHs, or outpatient worsening HF events) and change in QOL from baseline during follow-up measured by the Kansas City Cardiomyopathy Questionnaire overall summary score (KCCQ-OSS) with ≥5-point between-group difference through 2-year follow-up, assessed when the last enrolled patient reached 1-year follow-up. All outcomes were assessed in the total study population and separately in the stratified randomized reduced and preserved LVEF groups. Adverse outcomes were adjudicated by an independent clinical events committee blinded to randomization.
The original protocol included change in 6MWT from baseline to follow-up as the fifth component of the primary effectiveness outcome. Soon after the onset of the COVID-19 pandemic in March 2020, it became evident that many patients would not be able to return to the clinic for 6MWT evaluation. Thus, on May 19, 2020, the protocol was amended, substituting change in KCCQ-OSS for 6MWT in the primary effectiveness outcome.
Statistical Analysis
The trial was powered to examine outcomes in all randomized patients. The results in each LVEF strata, although prespecified, were not powered and are therefore hypothesis-generating.
For the primary 30-day safety outcome, 200 evaluable shunt group patients provided 87% power to detect a difference between the expected rate of 5% and a performance goal of 11%, a metric agreed upon with the US Food and Drug Administration, evaluated using an exact binomial test at a one-sided α of 0.025. The primary effectiveness outcome was evaluated with a sum of ranks test statistic using the method of Finkelstein and Schoenfeld,15 expressed using the unmatched win ratio, calculated as the total number of shunt group patient wins divided by the number of placebo procedure group wins and 95% CI after all pairwise comparisons (Table S8).16 A win ratio >1 indicates more positive results for the experimental treatment. Based on 10 000 simulated trials, 400 total patients (200 per arm) provided 90% power to detect a sum of ranks >1 in the shunt group, with a one-sided α of 0.025. Thus, 400 patients were planned for enrollment.
A single interim analysis of the primary effectiveness outcome with adaptive sample size re-estimation by an independent third party was planned when 200 enrolled patients completed 6-month follow-up. To prevent inflation of type 1 error, the final Finkelstein–Schoenfeld statistic is derived from data weighted differently before and after the interim analysis17 (Table S8). The result of the interim analysis was to leave the sample size unchanged, but the executive committee requested, and the Food and Drug Administration approved, that enrollment be increased to ≈500 patients to afford greater precision to assess outcomes in patients with preserved and reduced LVEF separately. This decision was made with the sponsor, executive committee, and investigators blinded to the interim results.
The primary safety end point was tested in all shunt-assigned patients in whom a device implant was attempted, regardless of whether the implantation was successful. All other analyses were performed in the intention-to-treat population, according to original group assignment regardless of treatment received. Sensitivity analyses were performed in the per-protocol population consisting of randomized participants who met all enrollment criteria, had no major protocol deviations, and were treated according to randomization.
Categorical variables were compared by χ2 test or Fisher exact tests. Continuous variables are presented as medians with 25th and 75th percentiles and were compared by the 2-sample t test for normally distributed data or otherwise by the Wilcoxon rank sum test. Follow-up time to first event rates were estimated using the Kaplan-Meier method and were compared by log-rank test. Hazard ratios (HRs) and 2-sided 95% CIs were estimated by Cox proportional hazards models, including treatment as a covariate. All HFHs occurring within 24 months were assessed in a joint frailty model adjusting for all-cause mortality. Total cardiovascular events over time were summarized as an annualized rate, calculated as the total number of events divided by total follow-up years through 24 months. The annualized rate ratios and 95% CIs were estimated by a Poisson distribution, including treatment as a covariate. All statistical tests are 2-sided and were performed at the 5% significance level, unless otherwise noted. All analyses were performed with SAS software, version 9.4 (SAS Institute).
Results
Patients and Procedures
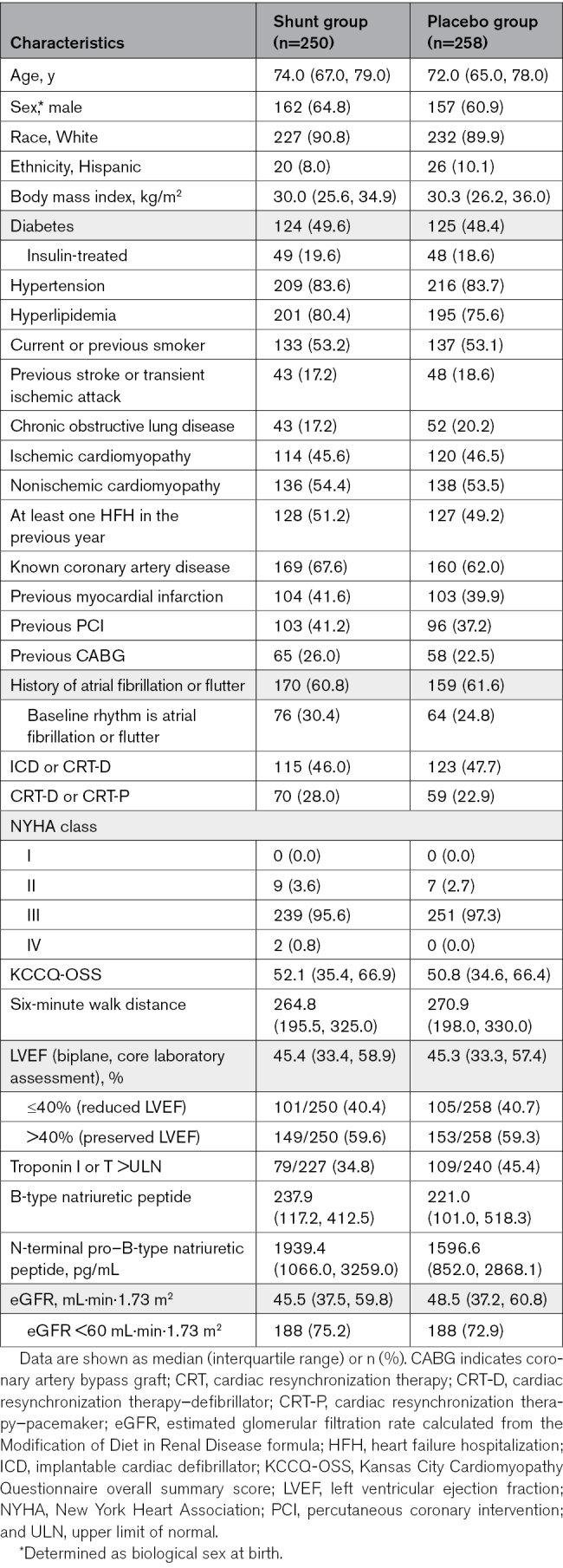
Between October 24, 2018, and October 19, 2022, 508 patients were randomized at 94 sites in 11 countries; 250 patients were assigned to receive the shunt, and 258 were assigned to a placebo procedure (Figure S3). Baseline characteristics are shown in Table 1. Median (25th and 75th percentiles) age was 73.0 years (66.0, 79.0), and 189 patients (37.2%) were women. Most patients (490 [96.5%]) were NYHA functional class III, and the median (25th and 75th percentiles) NT-proBNP (N-terminal pro–B-type natriuretic peptide) level was 1850 pg/mL (950.5, 3096.0). Baseline medication use, transthoracic echocardiography, and right heart catheterization data are shown in Tables S9 through S11. The median (25th and 75th percentiles) LVEF was 45.3% (33.4, 58.0) and was reduced (≤40%) in 206 patients (40.6%) and preserved (>40%) in 302 patients (59.4%).
Table 1.
Baseline Characteristics in the Randomized Groups, All Patients
The shunt was successfully implanted in all 250 patients assigned to active treatment and in 1 of 258 patients (0.4%) in the placebo procedure group because of site error (Table S12). Medication use at discharge and 1 year were similar between the groups (Table S13). The blinding procedures in-hospital and through 1 year were successful (Table S14). At both time periods, there were few episodes of actual patient-reported unblinding events; rather, most among the minority of patients who thought they knew their assigned treatment believed so because of changes in symptoms or other reasons.
Safety and Effectiveness, All Patients
One-year follow-up was completed for 505 of 508 patients (99.4%; Figure S3); median (25th and 75th percentiles) follow-up duration was 22.0 months (13.3, 23.9). The rate of the primary safety outcome of adjudicated device-related or procedure-related major adverse cardiovascular or neurological events occurring within 30 days in the 250 patients in the shunt group was 0 (upper 97.5% confidence limit, 1.5% [P<0.0001] for noninferiority compared with the 11% performance goal). No such events occurred in any shunt-treated patient through the 2-year follow-up. Other safety outcomes were infrequent (Table S15).
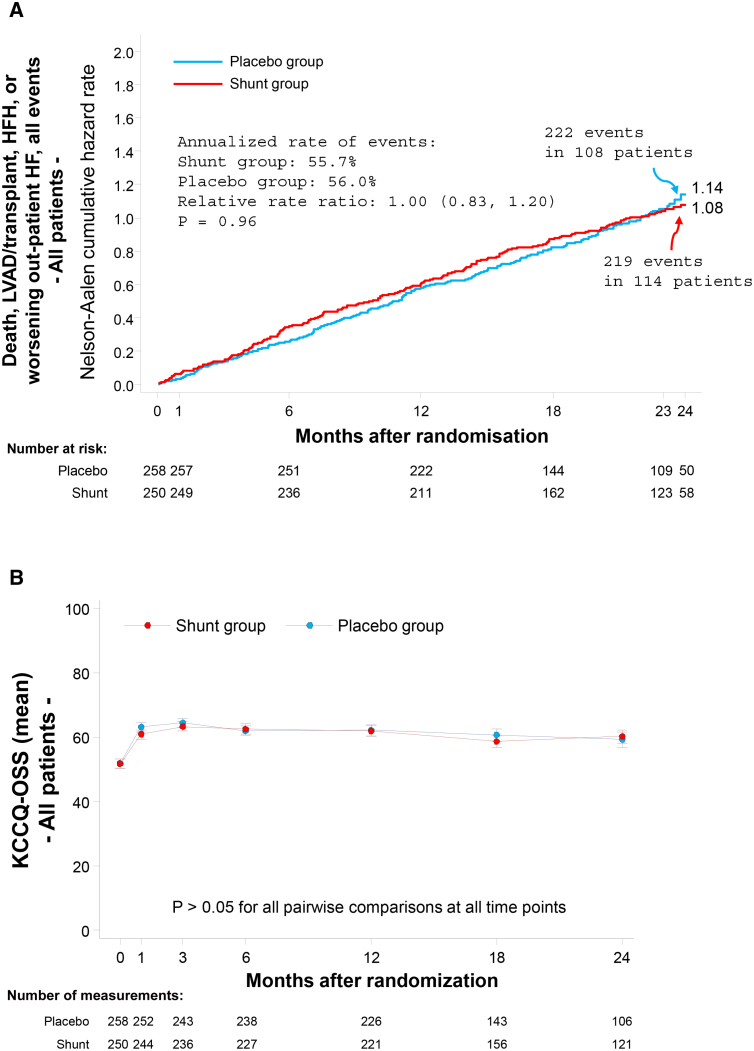
Among all randomized patients, the win ratio for the primary effectiveness outcome at 2 years in the shunt group compared with the placebo procedure group was 0.86 (0.61–1.22; P=0.20; Figure S4). Results were similar in the per-protocol population (0.88 [0.62–1.26]). Of note, 68.9% of win ratio decisions were based on cardiovascular events (the first 4 components of the hierarchy), whereas 31.1% of decisions were based on the KCCQ-OSS (Figure S4). The cumulative occurrence of the 4 cardiovascular event components of the primary effectiveness outcome and the change in KCCQ-OSS score during follow-up are shown graphically in Figure 1. There were no between-group differences in any of the secondary clinical effectiveness outcomes (Table 2; Tables S15 and S16). Core laboratory-assessed 1-year follow-up echocardiographic data are shown in Table S17.
Figure 1.
Graphical representation of the clinical events and quality of life components of the primary effectiveness outcome measure through 2-year follow-up in the entire intention-to-treat population. A, The clinical components of the hierarchical composite primary end point: the cumulative incidence of all events, including all-cause death, left ventricular assist device (LVAD) or heart transplant procedures, hospitalizations for heart failure (HFHs), or worsening heart failure (HF) outpatient events. The Nelson-Aalen cumulative hazard rate function describes the estimated rate at which events will have occurred, given that the individual has survived up to that time point (ie, at any given time, the Nelson-Aalen cumulative hazard rate denotes the expected number of events per patient followed for that length of time). The number at the end of each curve is the 2-year hazard rate. B, The quality-of-life component of the hierarchical composite primary end point: change in Kansas City Cardiomyopathy Questionnaire overall summary score (KCCQ-OSS) from baseline during 2-year follow-up.
Table 2.
Primary and Secondary Outcomes in the Randomized Groups, All Patients
Safety and Effectiveness, Randomized LVEF Strata
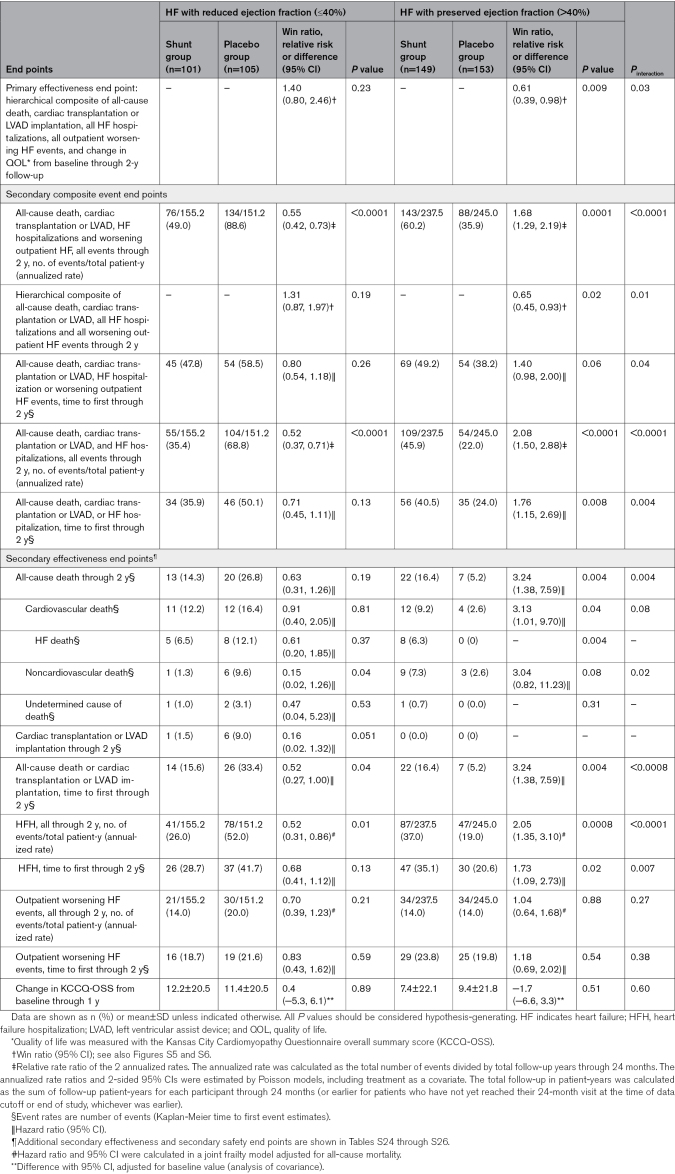
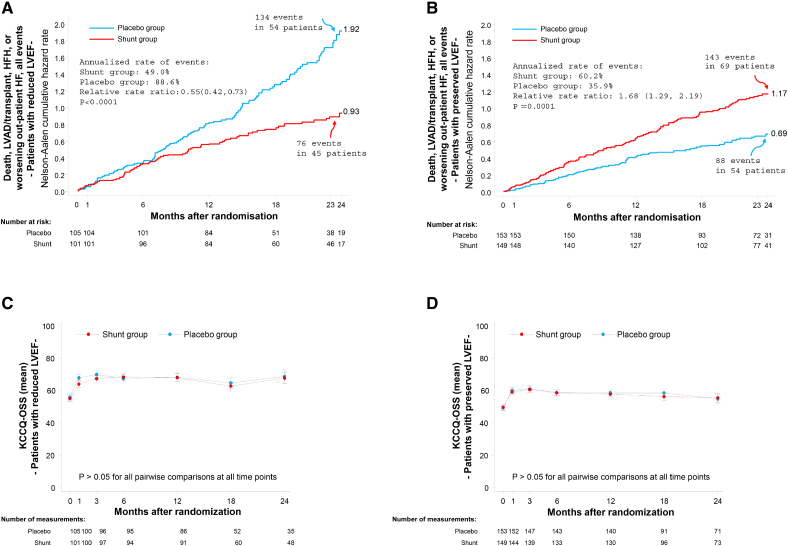
Baseline characteristics, medication use, and outcomes in patients with HF stratified and randomized by reduced LVEF (≤40%) and preserved LVEF (>40%) are shown in Table 3 and Tables S18 through S27. Compared with patients with reduced LVEF, those with preserved LVEF were older, were more often women, and more commonly had hypertension and higher body mass index, but were less likely to smoke or have known coronary artery disease. Despite lower natriuretic peptide levels, patients with preserved LVEF also had lower baseline KCCQ and 6MWT scores and were less likely to be treated with medications for heart failure, other than diuretics. The primary effectiveness outcome with shunt treatment compared with a placebo procedure was better in patients with a reduced LVEF compared with a preserved LVEF (win ratio 1.40 [0.80–2.46] versus 0.61 [0.39–0.98], respectively; Pinteraction=0.03; Figures S5 and S6). The occurrence of the cardiovascular event components of the primary effectiveness outcome and change in KCCQ-OSS during follow-up in patients with reduced and preserved LVEF are shown in Figure 2. Fewer adverse cardiovascular events (death, cardiac transplantation or LVAD implantation, HFHs, or outpatient worsening HF events) were observed with shunt treatment compared with a placebo procedure in patients with reduced LVEF (event rate per year, 49.0% versus 88.6%; relative risk, 0.55 [95% CI, 0.42–0.73]; P<0.0001), whereas more cardiovascular events occurred with shunt treatment in patients with preserved LVEF (event rate per year, 60.2% versus 35.9%; relative risk, 1.68 [95% CI, 1.29–2.19]; P=0.0001; Pinteraction<0.0001; Table 3; Figure 2, top). The cardiovascular benefits with shunt treatment in reduced LVEF were driven by fewer total HFHs (rate per year, 26.0% versus 52.0%; HR, 0.52 [95% CI, 0.31–0.86]; P=0.01), whereas the worse cardiovascular outcomes with shunt treatment in preserved LVEF were driven by increased all-cause death (2-year rate, 16.4% versus 5.2%; HR, 3.24 [95% CI, 1.38–7.59]; P=0.004) and greater total HFHs (rate per year, 37.0% versus 19.0%; HR, 2.05 [95% CI, 1.35–3.10]; P=0.0008; Table 3). When clinical outcomes were assessed according to baseline LVEF as a continuous measure, shunt effectiveness progressively increased as LVEF decreased, with a cutoff below ≈40% representing the threshold at which outcomes with shunt treatment transitioned from relative harm to benefit (Figures S7 and S8). In contrast to the differential cardiovascular outcomes with shunt treatment compared with placebo according to LVEF, there were no between-group differences in change in KCCQ-OSS during follow-up in patients with reduced or preserved LVEF (Table 3; Figure 2, bottom).
Table 3.
Primary and Secondary Outcomes in Patients With Reduced or Preserved Ejection Fraction by Randomized Treatment
Figure 2.
Graphical representation of the clinical events and quality of life components of the primary effectiveness outcome measure through 2-year follow-up in the stratified randomized groups of patients with reduced ejection fraction (≤40%) or preserved ejection fraction (>40%). A and C, Reduced left ventricular ejection fraction (LVEF). B and D, Preserved LVEF. A and B, The clinical components of the hierarchical composite primary end point: the cumulative incidence of all events, including all-cause death, left ventricular assist device (LVAD) or heart transplant procedures, hospitalizations for heart failure (HFHs), or worsening heart failure (HF) outpatient events. The Nelson-Aalen cumulative hazard rate function describes the estimated rate at which events will have occurred, given that the individual has survived up to that time point (ie, at any given time, the Nelson-Aalen cumulative hazard rate denotes the expected number of events per patient followed for that length of time). The number at the end of each curve is the 2-year hazard rate. C and D, The quality-of-life component of the hierarchical composite primary end point: the change in Kansas City Cardiomyopathy Questionnaire overall summary score (KCCQ-OSS) from baseline during 2-year follow-up. All P values should be considered hypothesis-generating.
Discussion
The principal results from this double-blind, placebo procedure–controlled, randomized trial are that among symptomatic patients with HF with any LVEF, transcatheter implantation of the Ventura interatrial shunt was safe, but did not reduce clinical events or improve QOL during 2-year follow-up. However, the occurrence of adverse cardiovascular outcomes appeared to be sensitive to the baseline LVEF on which randomization was stratified. Fewer total cardiovascular events (in particular HFHs) were observed with shunt treatment in the randomized strata of patients with reduced LVEF (≤40%), whereas cardiovascular events were increased (including mortality and HFHs) with shunt treatment in the randomized strata of patients with preserved LVEF (>40%). Change in QOL from baseline during follow-up as assessed by the KCCQ-OSS (which constituted 31.1% of all wins and losses in the win ratio) was not different between patients treated with the shunt or a placebo procedure, either in all patients or in those with reduced or preserved LVEF.
These results may be considered in the context of the single other completed large-scale, blinded, randomized trial of shunt treatment in patients with symptomatic HF: the REDUCE LAP-HF II trial (Reduce Elevated Left Atrial Pressure in Patients With Heart Failure II).18 All patients in that trial had preserved LVEF (≥40%), but in contrast to RELIEVE-HF, enrollment in REDUCE LAP-HF II required an elevated pulmonary capillary wedge pressure during exercise in the cardiac catheterization laboratory. In REDUCE LAP-HF II, the frequency of HF events during 2-year follow-up was similar in patients who received the interatrial shunt device (Corvia Medical) and a placebo procedure. In contrast, shunt-treated patients in the current study in the randomized strata with preserved LVEF had a worse prognosis during 2-year follow-up, including a 3-fold increased rate of mortality (P=0.004) and a 2-fold increase in HFHs (P=0.0008). Whereas these analyses were prespecified, they were not powered, and should thus be considered exploratory. Nonetheless, the strength of statistical evidence is strong, raising concerns for shunt treatment in patients with LVEF >40%. The worse prognosis of patients with HF with preserved LVEF in RELIEVE-HF compared with REDUCE LAP-HF II may be attributable to recruitment of higher-risk patients with more comorbidities, with higher resting pulmonary vascular resistance, higher natriuretic peptide levels, lower 6MWD, and higher rates of cardiovascular death in the control group. Notably, among patients with preserved LVEF in both studies, the KCCQ-OSS substantially increased from baseline to follow-up in both the shunt group and control group, signifying that patients perceived improved QOL with both treatments. However, there were no differences in change in KCCQ-OSS at any time point between the shunt group and placebo procedure group in either trial, and this measure improved in both randomized groups despite the absence of clinical benefit with shunt treatment in REDUCE LAP-HF II and the worsened clinical prognosis (including HFHs and mortality) with shunt treatment in RELIEVE-HF.
In contrast to the deleterious outcomes in patients with preserved LVEF, the cardiovascular prognosis appeared to be improved in shunt-treated patients with reduced LVEF in the current study; the annualized rate of all cardiovascular events was reduced by 45% in patients treated with the shunt versus a placebo procedure (49.0% versus 88.6%; P<0.0001). The point estimates favored shunt treatment for all 4 cardiovascular event components of the primary end point, all-cause death (HR, 0.63), heart transplantation or LVAD (HR, 0.16), all HFHs (HR, 0.52), and all outpatient worsening HF events (HR 0.70), and the event curves favoring shunt treatment were continuing to diverge at 2 years. Subgroup outcomes according to LVEF were not powered and are thus hypothesis-generating, but LVEF was the principal prespecified subgroup of interest, with randomization stratified by this metric given uncertain effects according to baseline systolic function. Assessment of the continuous relationship between LVEF and total cardiovascular events demonstrated an increasingly better prognosis after shunt treatment with progressively lower LVEF (and worsening prognosis with increasing LVEF); an ≈40% cutoff signified the inflection point at which shunt treatment shifted from harm to benefit.
Differential changes in echocardiographic measures during 1-year follow-up may provide a mechanistic basis for the varying clinical outcomes according to LVEF. After shunt treatment in patients with LVEF ≤40%, estimated left atrial and ventricular filling pressures and LV end-diastolic volumes were reduced with no change in cardiac index. These effects shift the Starling relationship up and to the left, indicating improved systolic function (Figures S9 and S10). The left and right heart remained compliant after shunt treatment; there were no changes in pulmonary artery systolic pressure, right ventricular end-diastolic volume, or inferior vena cava diameter despite increased blood flow from the left to right atrium. These findings suggest that greater compliance of the right heart allowed adaptation to increased shunt flow. In contrast, after shunt treatment in patients with LVEF >40%, estimated left atrial and ventricular filling pressures and LV end-diastolic volumes were only slightly decreased, but cardiac index was substantially reduced. These effects shift the Starling relationship downward, indicating reduced systolic function (Figures S9 and S10). The left and right heart, already noncompliant at baseline, remained noncompliant after shunt treatment; there were substantial increases in pulmonary artery systolic pressure, right ventricular end-diastolic volume, and inferior vena cava diameter consistent with increased right ventricular preload and afterload, in part because a less compliant heart could not adapt to or tolerate the increased flow across the shunt from the left atrium to the right atrium.
Limitations
The current results apply only to the profile of the patients enrolled and treated with the Ventura shunt. Other investigational shunts have a larger orifice that may enable even greater left-to-right blood flow.19 Second, the reduced and preserved LVEF groups were not powered for effectiveness within each randomized strata. Nonetheless, the effects of the shunt were sufficiently strong to demonstrate a marked reduction in all cardiovascular events (especially HFHs) in patients with LVEF ≤40% and a marked increase in all cardiovascular events (especially HFHs and mortality) in patients with LVEF >40%, with a P value for interaction of <0.0001 indicating that this difference in relative effect is unlikely to be spurious (and that in retrospect, these groups are not poolable). However, the modest number of patients in each of the LVEF groups may have prevented appreciating other important differences in shunt-related outcome effects (eg, significant mortality reduction with shunt treatment in patients with LVEF ≤40%; HR, 0.63 [95% CI, 0.31–1.26]) or reduction in composite outcomes in time to first event analyses. Third, we have not yet completed detailed assessments of the effect of changes in medication use and dose over time, serial changes in paired echocardiographic measures over time, or detailed cost-effectiveness analyses. Nor have we completed subgroup analyses to determine whether there are other specific patient phenotypes (beyond reduced versus preserved LVEF) that may benefit (or be harmed) with shunt treatment. Fourth, the large and similar increase in KCCQ-OSS in both the shunt group and control group, despite prerandomization eligibility committee confirmation of maximal HF medication use, emphasizes the relevance of the placebo effect and necessity for blinded trials. Moreover, the similar magnitude of KCCQ-OSS improvement and the lack of between-group differences in this metric despite a large increase in cardiovascular events in shunt-treated patients with preserved LVEF and a large decrease in cardiovascular events in shunt-treated patients with reduced LVEF confounds its interpretation in blinded (and open-label) trials. Fifth, in the current trial, HF with preserved ejection fraction was defined with an LVEF >40%, similar to the cutoff used in the recent trials REDUCE LAP-HF II and EMPEROR-Preserved (Empagliflozin Outcome Trial in Patients With Chronic Heart Failure With Preserved Ejection Fraction).18,20 More recently, societal guidelines in the United States and Europe have defined HF with preserved ejection fraction with an LVEF ≥50%, categorizing patients with an LVEF >40% but <50% as having HF with mildly reduced LVEF, in part reflecting that patients with HF with mildly reduced LVEF may respond favorably to pharmacological interventions that also benefit those with LVEF ≤40%.13,14 However, spline analysis from the current study suggests that an LVEF cutoff of ≈40% is the value at which shunt treatment shifts from benefit (≤40%) to harm (>40%), justifying the definition of HF with preserved ejection fraction used in RELIEVE-HF. However, the actual LVEF value for this transition might be somewhat higher or lower. Sixth, several prespecified and post hoc predictors of adverse outcomes after shunt treatment in HF with preserved ejection fraction have been identified from the REDUCE LAP-HF II trial.18,21 A comprehensive analysis of the predictors of response to the Ventura shunt in HF with preserved as well as reduced ejection fraction from the current study is underway. Seventh, additional studies on patients with HF with reduced LVEF are needed to substantiate the beneficial outcomes that were observed with shunt treatment in the current study and to determine whether a select cohort of patients with preserved LVEF might benefit. Nonetheless, the observation of differential cardiovascular outcomes in shunt-treated patients with reduced or preserved LVEF are especially important, as 3 shunt devices have received CE Mark approval in Europe.
In the randomized, double-blind, placebo procedure–controlled RELIEVE-HF trial, transcatheter implantation of the Ventura interatrial shunt was safe but did not reduce symptoms or improve prognosis in patients with HF through 2-year follow-up. However, a prespecified analysis in stratified randomized groups suggests that interatrial shunt implantation is beneficial in patients with reduced LVEF and harmful in patients with preserved LVEF.
Article Information
Acknowledgments
The authors thank Deborah Deutsch, BSN, and Beverly L. Walker, MSN, ANP, for their expertise and contributions to the V-Wave clinical program.
Sources of Funding
The trial was sponsored and funded by V-Wave Ltd, Caesarea, Israel.
Disclosures
Dr Stone has received speaker honoraria from Medtronic, Pulnovo, Abiomed, Amgen, and Boehringer Ingelheim; has served as a consultant to Abbott, Daiichi Sankyo, Ablative Solutions, CorFlow, CardioMech, Robocath, Miracor, Vectorious, Apollo Therapeutics, Elucid Bio, Cardiac Success, Valfix, TherOx, HeartFlow, Neovasc, Ancora, Occlutech, Impulse Dynamics, Adona Medical, Millennia Biopharma, Oxitope, HighLife, Elixir, Remote Cardiac Enablement, and Aria; and has equity or options from Cardiac Success, Ancora, Cagent, Applied Therapeutics, Biostar family of funds, SpectraWave, Orchestra Biomed, Aria, Valfix, and Xenter. Dr Stone’s employer, Mount Sinai Hospital, receives research grants from Shockwave, Abbott, Abiomed, BioVentrix, Cardiovascular Systems Inc, Phillips, Biosense-Webster, Vascular Dynamics, Pulnovo, V-wave, and PCORI (via Weill Cornell Medical Center). Dr Lindenfeld has received consulting fees from Abbott, Alleviant, Axon, AstraZeneca, Boston Scientific, CVRx, Merck, Medtronic, Edwards Lifesciences, V-Wave Medical, WhiteSwell, Vascular Dynamics, and Bayer. Dr Rodés-Cabau has received grants from V-Wave, Edwards Lifesciences, Medtronic, and Boston Scientific; received consulting fees from V-Wave Medical, Edwards Lifesciences, and Medtronic; and has received payment for presentations from Edwards Lifesciences and Medtronic. Dr Rodés-Cabau’s employer receives funding for enrollment in clinical trials for V-Wave Medical. Dr Anker serves as a consultant to Abbott Vascular, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Brahms GmbH, Cardiac Dimensions, Cardior, Cordia, CVRx, Inc, Cytokinetics, Edwards Lifesciences, Faraday Pharmaceuticals, GlaxoSmithKline, HeartKinetics, Impulse Dynamics (USA) Inc, Novartis, Occlutech, Pfizer, Regeneron, Repairon, Sensible Medical Innovations Ltd, Servier, V-Wave Medical, Vectorius, and Vifor; and has 2 patent applications regarding MR-proANP (midregional pro-atrial natriuretic peptide) through Brahms GmbH. Dr Anker’s employer Charité University Medicine Berlin has received grants from Vifor (International) Ltd. Dr Zile has consulting contracts for advisory committees with Abbott, Adona Medical, Aria CV, Avery Therapeutics, Inc, Boehringer-Ingelheim, Boston Scientific, Cardiovascular Research Foundation (CRF) Clinical Trials Center, CVRx, Diasol Therapeutics, LLC, EBR, Edwards, Eli Lilly, Endotronix, Medtronic, Merck, Morphic Therapeutics, Novartis, Salubris Biotherapeutics, Sonata, Srnalytics, Inc, V-Wave Medical, and Vectorious. Dr Kar has received grants and contracts from Abbott, Boston Scientific, Medtronic, Edwards Lifesciences, Laminar, and Picardia; has received consulting fees from Abbott, Medtronic, Boston Scientific, and V-Wave Medical; is a member of the executive committee for V-Wave Medical and advisory boards for Abbott, Boston Scientific, Medtronic, and InterShunt; has stock or stock options in PiCardia and Laminar; serves as the co–principal investigator for REAPIR-MR, CHAMPION, and Laminar Pivotal Trial. Dr Holcomb has received support for preparation of this article and has received consulting fees from V-Wave Medical and has received support for meeting attendance. Dr Pfeiffer has received honoraria from Abbott Cardiovascular and AncoraHeart. Dr Pfeiffer’s employer, The Penn State University and The Milton S. Hershey Medical Center, receives funding from V-Wave Medical for serving as the echo core laboratory. Dr Bayes-Genis has received honoraria for presentations from Abbott, AstraZeneca, Bayer, Boehringer Ingelheim, Medtronic, Novartis, Novo Nordisk, Roche Diagnostics, and Vifor. Dr Bax has served on the advisory board for V-Wave and receives reimbursement for serving on the eligibility committee for V-Wave and receives speaker fees from Abbott Vascular and Edwards Lifesciences. Dr Bax’s employer, Leiden University Medical Center, receives research grants from Abbot Vascular, Alnylam, Bayer, Bioventrix, Biotronik, Boston Scientific, Edwards Lifesciences, GE Healthcare, Medtronic, Medis, Novartis, Pfizer, and Pie Medical. Dr Bank has received consulting fees for serving on the advisory board and eligibility committee for V-Wave. Dr Costanzo has received grants or contracts from V-Wave, Bayer, and Alleviant as a site PI; has received consulting fees from Boehringer-Ingelheim, Nuwellis, V-Wave, and Alleviant; participated on DSMB/advisory boards for Merck and Boehringer-Ingelheim; and serves on the board of directors of Nuwellis. Dr Verheye has received consulting fees from Shockwave and Elixir Medical and stock or options for V-Wave and serves on the advisory board and eligibility committee for V-Wave. Dr Roguin serves on the advisory board for V-Wave and receives money for serving on the eligibility committee for V-Wave. Dr Filippatos has received grants from the European Commission; has received honoraria from Bayer, Boehringer Ingelheim, Servier, and Novartis; serves in a fiduciary role for the Heart Failure Association and JACC Heart Failure; serves on trial committees for Medtronic, Bayer, Boehringer Ingelheim, Vifor, Amgen, Servier, Impulse Dynamics, Cardior, and Novo Nordisk; and has received consulting fees from Cardio and Novo Nordisk. Dr Núñez has received consulting fees from Novartis, Boehringer Ingelheim, Bayer, and Novo Nordisk; and receives fees for lecture presentations from Alleviant, AstraZeneca, Boehringer Ingelheim, Bayer, Novartis, Novo Nordisk, Pfizer, Rovi, and Vifor Pharma. Dr Laufer-Perl has received consulting fees from Alleviant Medical and Unipharm; receives payment or honoraria from AbbVie Biopharmaceuticals, Alleviant Medical, AstraZeneca, Boehringer Ingelheim, Novartis, Medison, Novo Nordisk, Bayer, Unipharm, and CTS; received support for attending meetings from Alleviant Medical; is a participant on DSMB or advisory boards for Boehringer Ingelheim, AstraZeneca, and Bayer; and has received institutional grants from Boehringer Ingelheim, AstraZeneca, Pfizer, Novartis, Bayer, and the Israel Cancer Association. Dr Litwin has received grants for patient selection committees for a clinical trial for a different atrial shunt study for Corvia and served in a fiduciary role as associate editor for the Journal of the American Society for Echocardiography. Dr Litwin’s employer, MUSC, receives funding for enrollment in trials for V-Wave Medical and Corvia. Dr Prihadi has received consulting fees from Novartis, Boehringer Ingelheim, Bayer, Abbott, and Astra Zeneca; has received honoraria for presentations from Novartis, Boehringer Ingelheim, AstraZeneca, Menarinin, Vifor, and Novo Nordisk; has received support for attendance at meetings from Pfizer and Bayer; and has participated on advisory boards for AstraZeneca and Novartis. Dr Chung has received consulting fees from Intershunt, Corvia, Medtronic, and Abbott. Dr Price has received consulting fees from Abbott, Boston Scientific, Medtronic, Shockwave, Philips Medical, Alleviant Medical, and Innovheart; has received honoraria for presentations from WL Gore, Abbott, Shockwave, and Philips Medical; has served on DSMB or advisory boards for Axio Research (Portico NG and Vantage Trials); has served on the American College of Cardiology NCDR LAAO Research and Publication Committee; and holds stock or stock options from InterShunt, Indian Welss, Bolt Medical, Atraverse, and Advanced Bifurcation systems. Dr Thohan has received honoraria from Pfizer and Boehringer Ingelheim. Dr Schewel has received consulting fees from V-Wave Medical for proctoring intervention procedures. Dr Eigler has patents issued and planned for V-Wave Ltd and V-Wave Inc; is a shareholder and stock option holder as an employee of V-Wave Medical; is the chief executive officer of V-Wave Medical; and receives compensation in the form of salary, bonus, and stock option grants. Dr Abraham has received honoraria from Impulse Dynamics, Edwards Lifesciences, and Abbott; has received consulting fees from Zoll Respicardia; has stock in V-Wave Ltd; has a patent for mobile ultrawideband radar for monitoring thoracic fluid levels and cardiorespiratory function (publication No. 20210290074); has participated on advisory boards or data safety and monitoring boards for Sensible Medical, WhiteSwell, AquaPass, Cordio Medical, and Boehringer-Ingelheim; and his institution has received a grant from the National Institutes of Health (1 UG3/UH3 HL140144-01; LOFT-HF [Impact of Low Flow Nocturnal Oxygen Therapy on Hospital Readmission/Mortality in Patients with Heart Failure and Central Sleep Apnea]). The other authors report no disclosures.
Supplemental Material
Methods
Tables S1–S27
Figures S1–S10
Supplementary Material
Nonstandard Abbreviations and Acronyms
- 6MWT
- 6-minute walk test
- CONSORT
- Consolidated Standards of Reporting Trials
- EMPEROR-
- Empagliflozin Outcome Trial Preserved in Patients With Chronic Heart Failure With Preserved Ejection Fraction
- HF
- heart failure
- HFH
- heart failure hospitalization
- HR
- hazard ratio
- KCCQ-OSS
- Kansas City Cardiomyopathy Questionnaire overall summary score
- LAP
- left atrial pressure
- LV
- left ventricular
- LVAD
- left ventricular assist device
- LVEF
- left ventricular ejection fraction
- NT-proBNP
- N-terminal pro–B-type natriuretic peptide
- NYHA
- New York Heart Association
- QOL
- quality of life
- REDUCE LAP-HF II
- Reduce Elevated Left Atrial Pressure in Patients With Heart Failure II
- RELIEVE-HF
- Reducing Lung Congestion Symptoms in Advanced Heart Failure
Continuing medical education (CME) credit is available for this article. Go to http://cme.ahajournals.org to take the quiz.
Supplemental Material, the podcast, and transcript are available with this article at https://www.ahajournals.org/doi/suppl/10.1161/CIRCULATIONAHA.124.070870.
The investigators, institutions, and research organizations participating in RELIEVE-HF (Reducing Lung Congestion Symptoms Using the V-Wave Shunt in Advanced Heart Failure) are listed in the Appendix in the Supplemental Material.
For Sources of Funding and Disclosures, see page 1941.
Circulation is available at www.ahajournals.org/journal/circ
Contributor Information
JoAnn Lindenfeld, Email: joann.lindenfeld@vumc.org.
Josep Rodés-Cabau, Email: Josep.Rodes@criucpq.ulaval.ca.
Stefan D. Anker, Email: s.anker@cachexia.de.
Michael R. Zile, Email: zilem@musc.edu.
Saibal Kar, Email: saibalkar60@gmail.com.
Richard Holcomb, Email: rgholcomb2@gmail.com.
Jeroen J. Bax, Email: j.j.bax@lumc.nl.
Alan J. Bank, Email: alan.bank@allina.com.
Maria Rosa Costanzo, Email: maria_rosa_costanzo@msn.com.
Stefan Verheye, Email: stefan.verheye@gmail.com.
Ariel Roguin, Email: ARIELR@hymc.gov.il.
Gerasimos Filippatos, Email: gfilippatos@gmail.com.
Julio Núñez, Email: elizabeth.lee2@rochesterregional.org.
Elizabeth C. Lee, Email: elizabeth.lee2@rochesterregional.org.
Michal Laufer-Perl, Email: michallp@tlvmc.gov.il.
Gil Moravsky, Email: gilmorav@gmail.com.
Sheldon E. Litwin, Email: litwins@musc.edu.
Edgard Prihadi, Email: edgard.prihadi@zna.be.
Hemal Gada, Email: hgada@pinnaclehealth.org.
Matthew J. Price, Email: price.matthew@scrippshealth.org.
Vinay Thohan, Email: Vinay.Thohan@hcahealthcare.com.
Dimitry Schewel, Email: d.schewel@marienkrankenhaus.org.
Stephan Kische, Email: stephan.kische@vivantes.de.
Kevin S. Shah, Email: kevin.shah@hsc.utah.edu.
Daniel J. Donovan, Email: donodj@aol.com.
Yiran Zhang, Email: ezhang@crf.org.
Neal L. Eigler, Email: neal@vwavemedical.com.
William T. Abraham, Email: william.abraham@osumc.edu.
Marrick Kukin, BioClever 2005 SL, Barcelona, Spain; Creative Consulting Solutions, Nashville, TN.
Kunjan Bhatt, Keck Medical Center of University of Southern California, Michael, Fong.
David Shavelle, Los Robles Regional Medical Center, Thousand Oaks, CA.
Liviu Klein, Kaiser Permanente – San Francisco Hospital, San Francisco, CA.
Alicia Romero, Mission Hospital, Ashville, NC.
Garrie Haas, Arizona Heart Rhythm Center, Phoenix, AZ.
Vijay Swarup, Minneapolis Heart Institute Foundation, Minneapolis, MN.
Elizabeth Volz, Rush University Medical Center, Chicago, IL.
Fareed Collado, Sentara Norfolk General Hospital, Norfolk, VA.
Amit Badiye, University of Miami, Miami, FL.
Luanda Grazette, Mauricio; University of Utah Hospital, Salt Lake City, UT.
Michelle Hamilton, Centennial Medical Center, Nashville, TN.
Tom McRae, Memorial Jacksonville Hospital, Jacksonville, FL.
Sumant Lamba, Methodist Healthcare System of San Antonio; San Antonio, TX.
Steven Krueger, Abrazo Arizona Heart Hospital, Phoenix, AZ.
Timothy Byrne, Morton Plant Mease Health Care, Clearwater, FL.
Leslie Miller, First Coast Cardiovascular Institute, Jacksonville, FL.
Youssef Al-Saghir, Lundquist Institute (Harbor-UCLA) Medical Center, Torrance, CA.
Robin Chand, Memorial Health Services, Long Beach, CA.
David Shavelle, St. Elizabeth Healthcare, Edgewood KY.
Saeb Khoury, South Denver Cardiology, Littleton, CO.
Ira Dauber, University of Virginia, Charlottesville, VA.
Sula Mazimba, Valley Health, Ridgewood, NJ.
Suneet Mittal, Advocate Illinois Masonic Medical Center, Chicago, IL.
Steven Driver, CHRISTUS Trinity Mother Frances Health System, Tyler, TX.
Stanislav Weiner, The Cleveland Clinic Foundation, Cleveland, OH.
Samir Kapadia, Memorial Healthcare System, Hollywood, FL.
Priyanka Gosain, Piedmont Hospital Atlanta, Atlanta, GA.
Rajeev Singh, Texas Heart Institute, Houston, TX.
Zvonimir Krejcer, Vanderbilt Heart and Vascular Institute, Nashville, TN.
Lynn Punnoose, Wake Med, Raleigh, NC.
Stuart Russell, Weill Cornell Medical College, The New York Presbyterian, New York, NY.
Maria Karas, Atlanta VA Health System; Atlanta, GA.
Gautam Kumar, Baylor College of Medicine (Houston), Houston, TX.
Ajith Nair, Baylor Scott and White, Temple, TX.
Robert Widmer, Chippenham and Johnston Willis Hospital, Richmond, VA.
Ramesh Kundur, Dignity Health - Mercy Gilbert Medical Center, Gilbert, AZ.
Nabil Dib, Northeast Georgia Medical Center; Gainesville, GA.
Ugochukwu Egolum, Northwell Health - Lenox Hill Hospital, NY, NY.
Miguel Alvarez Villela, Penn State Health, Milton S. Hershey Medical Center, Hershey, PA.
John Boehmer, St. Francis Hospital, Roslyn, NY.
George Petrossian, Stanford University Hospital and Clinics, Stanford, CA.
Jeffrey Teuteberg, Summa Health, Akron, OH.
Peter Bittenbender, Hospital Universitario Germans Trias i Pujol, Badalona, Barcelona.
Ignacio Amat Santos, Hospital Clinic of Barcelona, Barcelona.
Ana Garcia, Hospital Universitario Puerta de Hierro, Majadahonda, Madrid.
Maria del Trigo, Hospital de la Santa Creu I Sant Pau, Barcelona.
Sònia Pérez Mirabet, Hospital Clinico San Carlos, Madrid.
Luis Nombela, Franco University Hospital Virgen de la Arrixaca, El Palmar, Murcia.
Domingo Pascual Figal, Shamir Medical Center, Be’er Ya’akov.
Israel Gotsman, The Baruch Padeh Medical Center, Tiberias.
Wadi Kinany, Kaplan Medical Center, Rehovot.
Sorel Goland, Sheba Medical Center at Tel Ha’shomer, Ramat Gan.
Dov Freimark, Shaare Zedek Medical Center, Jerusalem.
Tal Hasin, Rambam Medical Center, Haifa.
Oren Caspi, University Hospital Samson Assuta Ashdod, Ashdod.
Eli Lev, Soroka University Medical Center, Be’er Sheva.
Hilmi Alnsasra, Sana-Klinikum Hospital, Remscheid.
Burkhard Sievers, Vivantes Clinic in Friedrichs Hain, Berlin.
Mohammad Sherif, Heart Center Leipzig, Leipzig.
Karl Fengler, University of Leipzig, Leipzig.
Rolf Wachter, Vivantes Hospital Am Urban, Berlin.
Ince Hüseyin, Ludwig Maximilian University, München.
Jörg Hausleiter, University Medicine Mainz, Mainz.
Ralf Stephan Von Bardeleben, University Hospital of Rostock, Rostock.
Ince Hüseyin, (Primary Regulatory site for Germany EC).
Reda Ibrahim, University Health Network - Toronto General Hospital, Toronto, Ontario.
Eric Horlick, The 4th Military Hospital in Wroclaw, Wroclaw.
Bartosz Krakowiak, Institute of Cardiology, Warsaw.
Adam Witkowski, Upper Silesian Medical Center, Medical University of Katowice, Katowice.
Wojciech Wojakowski, AZ Sint-Jan Brugge, Bruges.
Jan Van Der Heyden, Erasmus Medical Center, Rotterdam.
Nicholas Van Mieghem, St. Antonius Hospital, Nieuwegein.
Martijn Post, Flinders Medical Centre, Bedford Park, SA.
Collaborators: John Gorcsan, Marrick Kukin, Kunjan Bhatt, David Shavelle, Liviu Klein, Alicia Romero, Garrie Haas, Vijay Swarup, Elizabeth Volz, Fareed Collado, Amit Badiye, Luanda Grazette, Michelle Hamilton, Tom McRae, Sumant Lamba, Steven Krueger, Timothy Byrne, Leslie Miller, Youssef Al-Saghir, Robin Chand, David Shavelle, Saeb Khoury, Ira Dauber, Sula Mazimba, Suneet Mittal, Steven Driver, Stanislav Weiner, Samir Kapadia, Priyanka Gosain, Rajeev Singh, Zvonimir Krejcer, Lynn Punnoose, Stuart Russell, Maria Karas, Gautam Kumar, Ajith Nair, Robert Widmer, Ramesh Kundur, Nabil Dib, Ugochukwu Egolum, Miguel Alvarez Villela, John Boehmer, George Petrossian, Jeffrey Teuteberg, Peter Bittenbender, Ignacio Amat Santos, Ana Garcia, Maria del Trigo, Sònia Pérez Mirabet, Luis Nombela, Domingo Pascual Figal, Israel Gotsman, Wadi Kinany, Sorel Goland, Dov Freimark, Tal Hasin, Oren Caspi, Eli Lev, Hilmi Alnsasra, Burkhard Sievers, Mohammad Sherif, Karl Fengler, Rolf Wachter, Ince Hüseyin, Jörg Hausleiter, Ralf Stephan Von Bardeleben, Ince Hüseyin, Reda Ibrahim, Eric Horlick, Bartosz Krakowiak, Adam Witkowski, Wojciech Wojakowski, Jan Van Der Heyden, Nicholas Van Mieghem, Martijn Post, Carmine DePasquale, Frank Ruschitzka, and Richard Troughton
References
- 1.Ritzema J, Troughton R, Melton I, Crozier I, Doughty R, Krum H, Walton A, Adamson P, Kar S, Shah PK, et al. ; Hemodynamically Guided Home Self-Therapy in Severe Heart Failure Patients (HOMEOSTASIS) Study Group. Physician-directed patient self-management of left atrial pressure in advanced chronic heart failure. Circulation. 2010;121:1086–1095. doi: 10.1161/CIRCULATIONAHA.108.800490 [DOI] [PubMed] [Google Scholar]
- 2.Schiff GD, Fung S, Speroff T, McNutt RA. Decompensated heart failure: symptoms, patterns of onset, and contributing factors. Am J Med. 2003;114:625–630. doi: 10.1016/s0002-9343(03)00132-3 [DOI] [PubMed] [Google Scholar]
- 3.Fonarow GC, Adams KF, Jr, Abraham WT, Yancy CW, Boscardin WJ; ADHERE Scientific Advisory Committee, Study Group, and Investigators. Risk Stratification for in-hospital mortality in acutely decompensated heart failure. JAMA. 2005;293:572–580. doi: 10.1001/jama.293.5.572 [DOI] [PubMed] [Google Scholar]
- 4.Roven RB, Crampton RS, Case RB. Effect of compromising right ventricular function in left ventricular failure by means of interatrial and other shunts: right ventricular success versus left ventricular failure. Am J Cardiol. 1969;24:209–219. doi: 10.1016/0002-9149(69)90405-6 [DOI] [PubMed] [Google Scholar]
- 5.Burkhoff D, Mirsky I, Suga H. Assessment of systolic and diastolic ventricular properties via pressure-volume analysis: a guide for clinical, translational, and basic researchers. Am J Physiol Heart Circ Physiol. 2005;289:H501–H512. doi: 10.1152/ajpheart.00138.2005 [DOI] [PubMed] [Google Scholar]
- 6.Lutembacher R. De la sténose mitrale avec communication interauriculaire. Arch Mal Coeur. 1916;9:237–260. [Google Scholar]
- 7.Viaene D, Vermeersch P, Van den Branden F. Pulmonary oedema after percutaneous ASD-closure. Acta Cardiol. 2010;65:257–260. doi: 10.2143/AC.65.2.2047064 [DOI] [PubMed] [Google Scholar]
- 8.Ewert P, Berger F, Nagdyman N, Kretschmar O, Dittrich S, Abdul-Khaliq H, Lange P. Masked left ventricular restriction in elderly patients with atrial septal defects: a contraindication for closure? Catheter Cardiovasc Interv. 2001;52:177–180. doi: 10.1002/1522-726x(200102)52:2<177::aid-ccd1043>3.0.co;2-g [DOI] [PubMed] [Google Scholar]
- 9.Seib PM, Faulkner SC, Erickson CC, Van Devanter SH, Harrell JE, Fasules JW, Frazier EA, Morrow WR. Blade and balloon atrial septostomy for left heart decompression in patients with severe ventricular dysfunction on extracorporeal membrane oxygenation. Catheter Cardiovasc Interv. 1999;46:179–186. doi: 10.1002/(SICI)1522-726X(199902)46:2<179::AID-CCD13>3.0.CO;2-W [DOI] [PubMed] [Google Scholar]
- 10.Eigler NL, del Rio CL, Verheye S, McConnell PI, Lilly SM, George R, Hamlin RL, Ueyama Y, Youngblood BL, Shkurovich S, et al. Cardiac unloading with an implantable interatrial shunt in heart failure: serial observations in an ovine model of ischemic cardiomyopathy. Structural Heart. 2017;1:40–48. doi: 10.1080/24748706.2017.1326647 [Google Scholar]
- 11.Rodés-Cabau J, Bernier M, Amat-Santos IJ, Ben Gal T, Nombela-Franco L, García Del Blanco B, Kerner A, Bergeron S, Del Trigo M, Pibarot P, et al. Interatrial shunting for heart failure: early and late results from the first-in-human experience with the V-wave system. JACC Cardiovasc Interv. 2018;11:2300–2310. doi: 10.1016/j.jcin.2018.07.001 [DOI] [PubMed] [Google Scholar]
- 12.Guimarães L, Bergeron S, Bernier M, Ben Gal T, Nombela-Franco L, García Del Blanco B, Kerner A, Bergeron S, Del Trigo M, Pibarot P, et al. Interatrial shunt with the second-generation V-Wave system for patients with advanced chronic heart failure. EuroIntervention. 2020;15:1426–1428. doi: 10.1016/j.jcin.2018.07.001 [DOI] [PubMed] [Google Scholar]
- 13.Heidenreich PA, Bozkurt B, Aguilar D, Allen LA, Byun JJ, Colvin MM, Deswal A, Drazner MH, Dunlay SM, Evers LR, et al. 2022 AHA/ACC/HFSA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association joint committee on clinical practice guidelines. J Am Coll Cardiol. 2022;79:e263–e421. doi: 10.1016/j.jacc.2021.12.012 [DOI] [PubMed] [Google Scholar]
- 14.McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, Burri H, Butler J, Čelutkienė J, Chioncel O, et al. ; Authors/Task Force Members. 2023 Focused update of the 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: developed by the task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. 2024;26:5–17. doi: 10.1002/ejhf.3024 [DOI] [PubMed] [Google Scholar]
- 15.Finkelstein DM, Schoenfeld DA. Combining mortality and longitudinal measures in clinical trials. Stat Med. 1999;18:1341–1354. doi: 10.1002/(sici)1097-0258(19990615)18:11<1341::aid-sim129>3.0.co;2-7 [DOI] [PubMed] [Google Scholar]
- 16.Pocock SJ, Ariti CA, Collier TJ, Wang D. The win ratio: a new approach to the analysis of composite end points in clinical trials based on clinical priorities. Eur Heart J. 2012;33:176–182. doi: 10.1093/eurheartj/ehr352 [DOI] [PubMed] [Google Scholar]
- 17.Cui L, Hung HMJ, Wang S-J. Modification of sample size in group sequential clinical trials. Biometrics. 1999;55:853–857. doi: 10.1111/j.0006-341x.1999.00853.x [DOI] [PubMed] [Google Scholar]
- 18.Shah SJ, Borlaug BA, Chung ES, Cutlip DE, Debonnaire P, Fail PS, Gao Q, Hasenfuß G, Kahwash R, Kaye DM, et al. ; REDUCE LAP-HF II investigators. Atrial shunt device for heart failure with preserved and mildly reduced ejection fraction (REDUCE LAP-HF II): a randomized, multicentre, blinded, sham-controlled trial. Lancet. 2022;399:1130–1140. doi: 10.1016/S0140-6736(22)00016-2 [DOI] [PubMed] [Google Scholar]
- 19.Jagadeesan V, Gray WA, Shah SJ. Shunt therapy for heart failure: an update. J Soc Cardiovasc Angio Intervent. 2023;2:101203. doi: https://doi.org/10.1016/j.jscai.2023.101203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Anker SD, Butler J, Filippatos G, Ferreira JP, Bocchi E, Böhm M, Brunner-La Rocca HP, Choi DJ, Chopra V, Chuquiure-Valenzuela E, et al. ; EMPEROR-Preserved Trial Investigators. Empagliflozin in heart failure with a preserved ejection fraction. N Engl J Med. 2021;385:1451–1461. doi: 10.1056/NEJMoa2107038 [DOI] [PubMed] [Google Scholar]
- 21.Borlaug BA, Blair J, Bergmann MW, Bugger H, Burkhoff D, Bruch L, Celermajer DS, Claggett B, Cleland JGF, Cutlip DE, et al. ; REDUCE LAP-HF-II Investigators. Latent pulmonary vascular disease may alter the response to therapeutic atrial shunt device in heart failure. Circulation. 2022;145:1592–1604. doi: 10.1161/CIRCULATIONAHA.122.059486 [DOI] [PMC free article] [PubMed] [Google Scholar]