Abstract
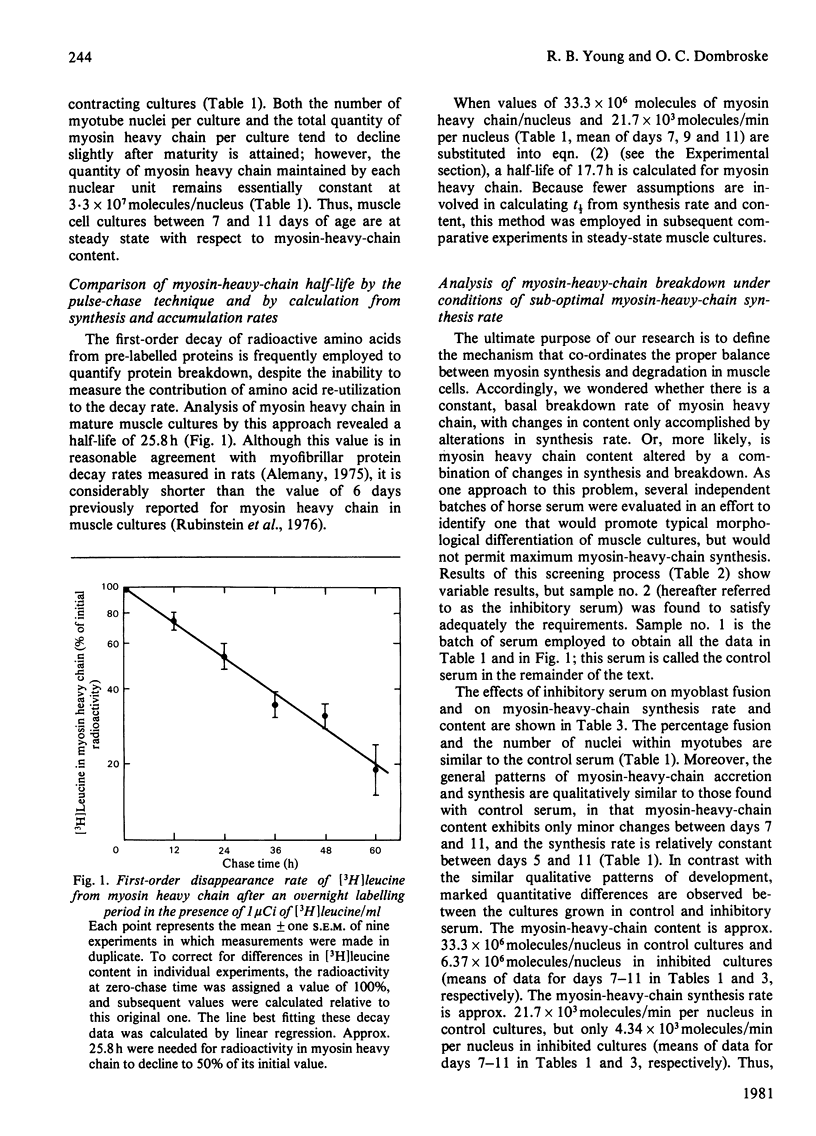
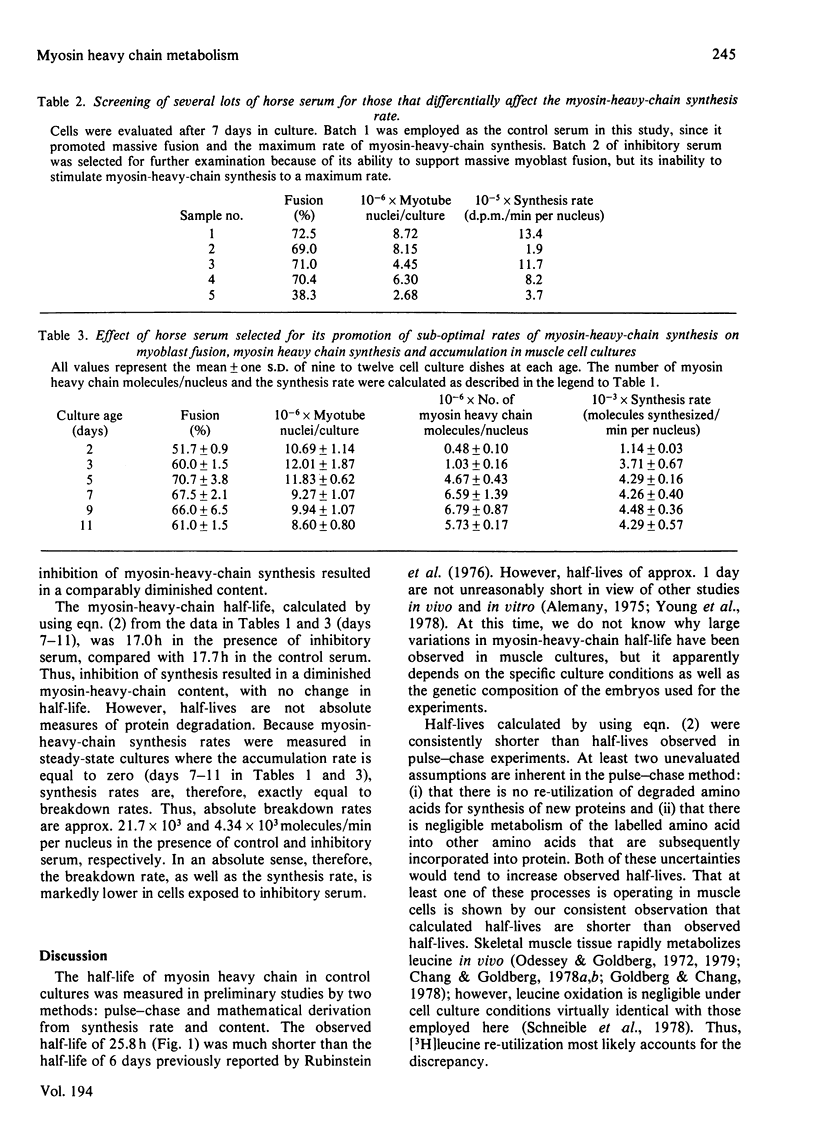
Synthesis, accumulation and breakdown of the 200000-mol.wt. heavy subunit of myosin were analysed over an 11 day period in muscle cell cultures isolated from the leg muscle of 12-day chick embryos. Muscle cells accumulated myosin heavy chain rapidly from days 2 to 5 and maintained a maximum, constant myosin-heavy-chain concentration between days 7 and 11. Myosin-heavy-chain content and breakdown rate were compared in steady-state muscle cultures grown either in the presence of an optimum batch of horse serum (control) or in the presence of horse serum that had been pre-selected for its ability to inhibit several-fold the rate of synthesis of myosin heavy chain (inhibitory). The quantity of myosin heavy chain in the inhibited cultures was decreased in direct proportion to the decrease in the rate of synthesis of myosin heavy chain; however, the half-lives of myosin heavy chain (control, 17.7h; inhibitory, 17.0h) were virtually identical. In contrast, the absolute rate of breakdown of myosin heavy chain, expressed as molecules/min per nucleus, was approx. 5-fold lower in the inhibited cultures (4.3 X 10(3) molecules/min per nucleus) than in the control cultures (21.7 X 10(3) molecules/min per nucleus). Thus, inhibition of myosin-heavy-chain synthesis in this case was accompanied by diminished myosin-heavy-chain concentration and absolute breakdown rate at the altered steady state, but relative myosin-heavy-chain breakdown rates were unchanged.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Airhart J., Kelley J., Brayden J. E., Low R. B., Stirewalt W. S. An ultramicro method of amino acid analysis: application to studies of protein metabolism in cultured cells. Anal Biochem. 1979 Jul 1;96(1):45–55. doi: 10.1016/0003-2697(79)90552-9. [DOI] [PubMed] [Google Scholar]
- Alemany M. Effect of amino acid reutilization in the determination of protein turnover in mice. Horm Metab Res. 1976 Jan;8(1):70–73. doi: 10.1055/s-0028-1093676. [DOI] [PubMed] [Google Scholar]
- Chang T. W., Goldberg A. L. The metabolic fates of amino acids and the formation of glutamine in skeletal muscle. J Biol Chem. 1978 May 25;253(10):3685–3693. [PubMed] [Google Scholar]
- Chang T. W., Goldberg A. L. The origin of alanine produced in skeletal muscle. J Biol Chem. 1978 May 25;253(10):3677–3684. [PubMed] [Google Scholar]
- Fouts D. L., Wolstenholme D. R., Boyer H. W. Heterogeneity in sensitivity to cleavage by the restriction endonucleases ECORI and HindIII of circular kinetoplast DNA molecules of Crithidia acanthocephali. J Cell Biol. 1978 Nov;79(2 Pt 1):329–341. doi: 10.1083/jcb.79.2.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulks R. M., Li J. B., Goldberg A. L. Effects of insulin, glucose, and amino acids on protein turnover in rat diaphragm. J Biol Chem. 1975 Jan 10;250(1):290–298. [PubMed] [Google Scholar]
- Goldberg A. L., Chang T. W. Regulation and significance of amino acid metabolism in skeletal muscle. Fed Proc. 1978 Jul;37(9):2301–2307. [PubMed] [Google Scholar]
- Goldberg A. L. Protein turnover in skeletal muscle. I. Protein catabolism during work-induced hypertrophy and growth induced with growth hormone. J Biol Chem. 1969 Jun 25;244(12):3217–3222. [PubMed] [Google Scholar]
- Goldberg A. L. Protein turnover in skeletal muscle. II. Effects of denervation and cortisone on protein catabolism in skeletal muscle. J Biol Chem. 1969 Jun 25;244(12):3223–3229. [PubMed] [Google Scholar]
- Harris S. E., Rosen J. M., Means A. R., O'Malley B. W. Use of a specific probe for ovalbumin messenger RNA to quantitate estrogen-induced gene transcripts. Biochemistry. 1975 May 20;14(10):2072–2081. doi: 10.1021/bi00681a006. [DOI] [PubMed] [Google Scholar]
- Kameyama T., Etlinger J. D. Calcium-dependent regulation of protein synthesis and degradation in muscle. Nature. 1979 May 24;279(5711):344–346. doi: 10.1038/279344a0. [DOI] [PubMed] [Google Scholar]
- Laurent G. J., Sparrow M. P., Bates P. C., Millward D. J. Turnover of muscle protein in the fowl (Gallus domesticus). Rates of protein synthesis in fast and slow skeletal, cardiac and smooth muscle of the adult fowl. Biochem J. 1978 Nov 15;176(2):393–401. doi: 10.1042/bj1760393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurent G. J., Sparrow M. P., Bates P. C., Millward D. J. Turnover of muscle protein in the fowl. Collagen content and turnover in cardiac and skeletal muscles of the adult fowl and the changes during stretch-induced growth. Biochem J. 1978 Nov 15;176(2):419–427. doi: 10.1042/bj1760419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurent G. J., Sparrow M. P., Millward D. J. Turnover of muscle protein in the fowl. Changes in rates of protein synthesis and breakdown during hypertrophy of the anterior and posterior latissimus dorsi muscles. Biochem J. 1978 Nov 15;176(2):407–417. doi: 10.1042/bj1760407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruyama K., Sunde M. L., Swick R. W. Growth and muscle protein turnover in the chick. Biochem J. 1978 Nov 15;176(2):573–582. doi: 10.1042/bj1760573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee E. E., Cheung J. Y., Rannels D. E., Morgan H. E. Measurement of the rate of protein synthesis and compartmentation of heart phenylalanine. J Biol Chem. 1978 Feb 25;253(4):1030–1040. [PubMed] [Google Scholar]
- Millward D. J., Garlick P. J., Stewart R. J., Nnanyelugo D. O., Waterlow J. C. Skeletal-muscle growth and protein turnover. Biochem J. 1975 Aug;150(2):235–243. doi: 10.1042/bj1500235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odessey R., Goldberg A. L. Leucine degradation in cell-free extracts of skeletal muscle. Biochem J. 1979 Feb 15;178(2):475–489. doi: 10.1042/bj1780475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odessey R., Goldberg A. L. Oxidation of leucine by rat skeletal muscle. Am J Physiol. 1972 Dec;223(6):1376–1383. doi: 10.1152/ajplegacy.1972.223.6.1376. [DOI] [PubMed] [Google Scholar]
- Riebow J. F., Young R. B. Effect of leupeptin on protein turnover in normal and dystrophic chicken skeletal muscle cells in culture. Biochem Med. 1980 Jun;23(3):316–323. doi: 10.1016/0006-2944(80)90042-3. [DOI] [PubMed] [Google Scholar]
- Rourke A. W. Myosin in developing normal and dystrophic chicken pectoralis. I. Synthesis and degradation. J Cell Physiol. 1975 Oct;86(2 Pt 2 Suppl 1):343–351. doi: 10.1002/jcp.1040860406. [DOI] [PubMed] [Google Scholar]
- Rourke A. W. Myosin in developing normal and dystrophic chicken pectoralis. II. The relationship between intracellular and extracellular aspartate pools and myosin synthesis. J Cell Physiol. 1975 Oct;86(2 Pt 2 Suppl 1):353–358. doi: 10.1002/jcp.1040860407. [DOI] [PubMed] [Google Scholar]
- Rubinstein N., Chi J., Holtzer H. Coordinated synthesis and degradation of actin and myosin in a variety of myogenic and non-myogenic cells. Exp Cell Res. 1976 Feb;97(2):387–393. doi: 10.1016/0014-4827(76)90630-3. [DOI] [PubMed] [Google Scholar]
- Segal H. I., Winkler J. R., Miyagi M. P. Relationship between digradation rates of proteins in vivo and their susceptibility to lysosomal proteases. J Biol Chem. 1974 Oct 10;249(19):6364–6365. [PubMed] [Google Scholar]
- Young R. B., Allen R. E. Transitions in gene activity during development of muscle fibers. J Anim Sci. 1979 Apr;48(4):837–852. doi: 10.2527/jas1979.484837x. [DOI] [PubMed] [Google Scholar]
- Young R. B., Bergen W. G., Blauwiekel P. B. Myosin synthesis in embryonic chicken fibroblasts. J Cell Biol. 1979 Apr;81(1):115–122. doi: 10.1083/jcb.81.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young R. B., Goll D. E., Stromer M. H. Isolation of myosin-synthesizing polysomes from cultures of embryonic chicken myoblasts before fusion. Dev Biol. 1975 Nov;47(1):123–135. doi: 10.1016/0012-1606(75)90268-7. [DOI] [PubMed] [Google Scholar]


