Abstract
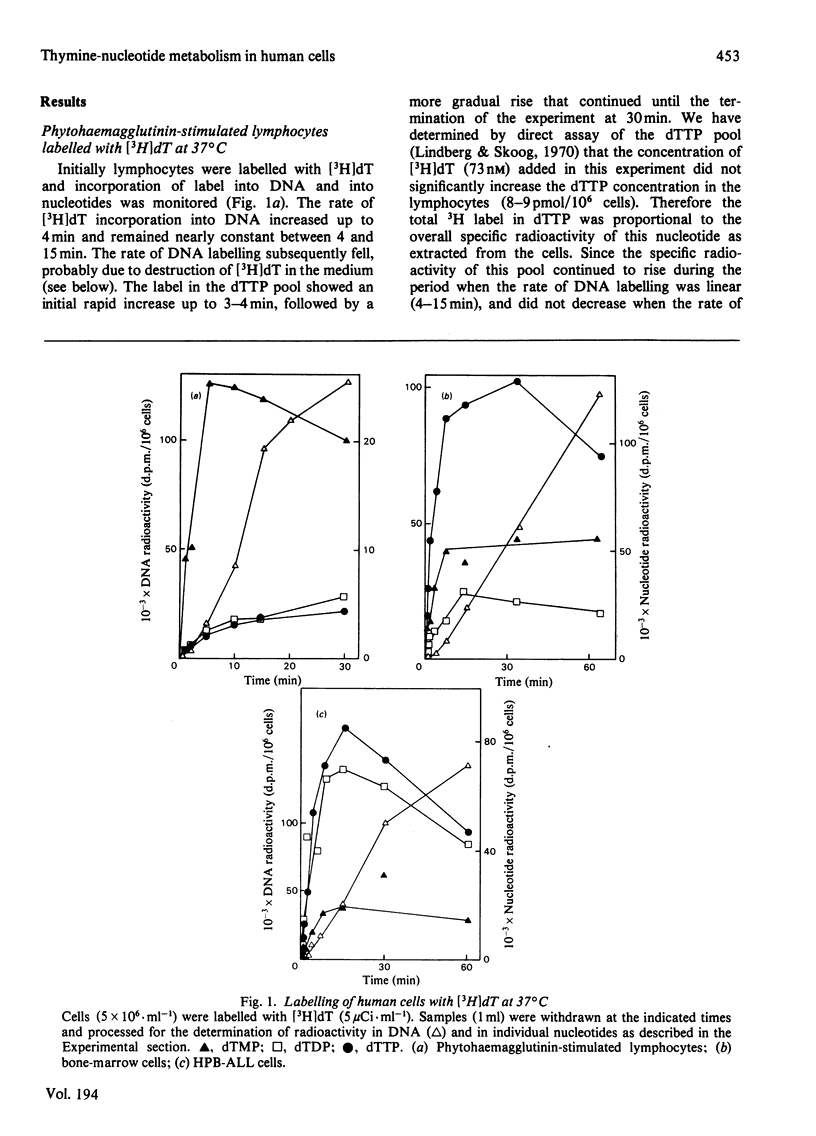
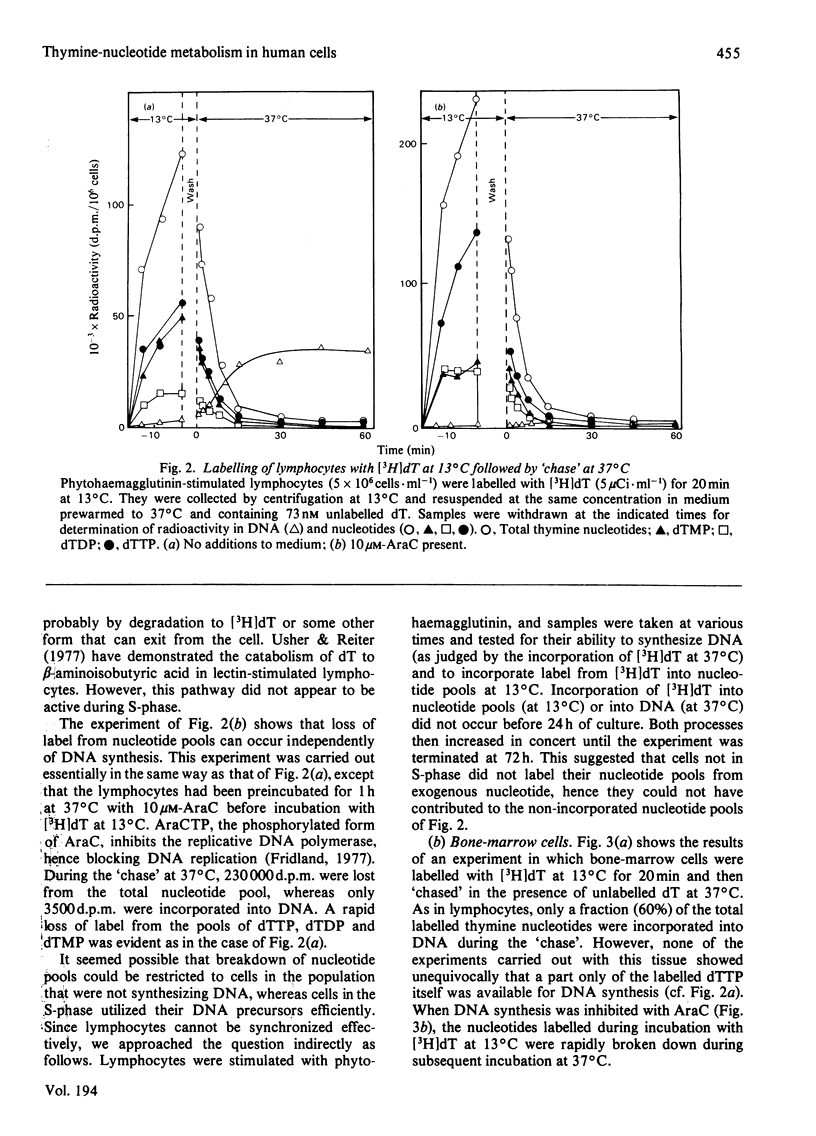
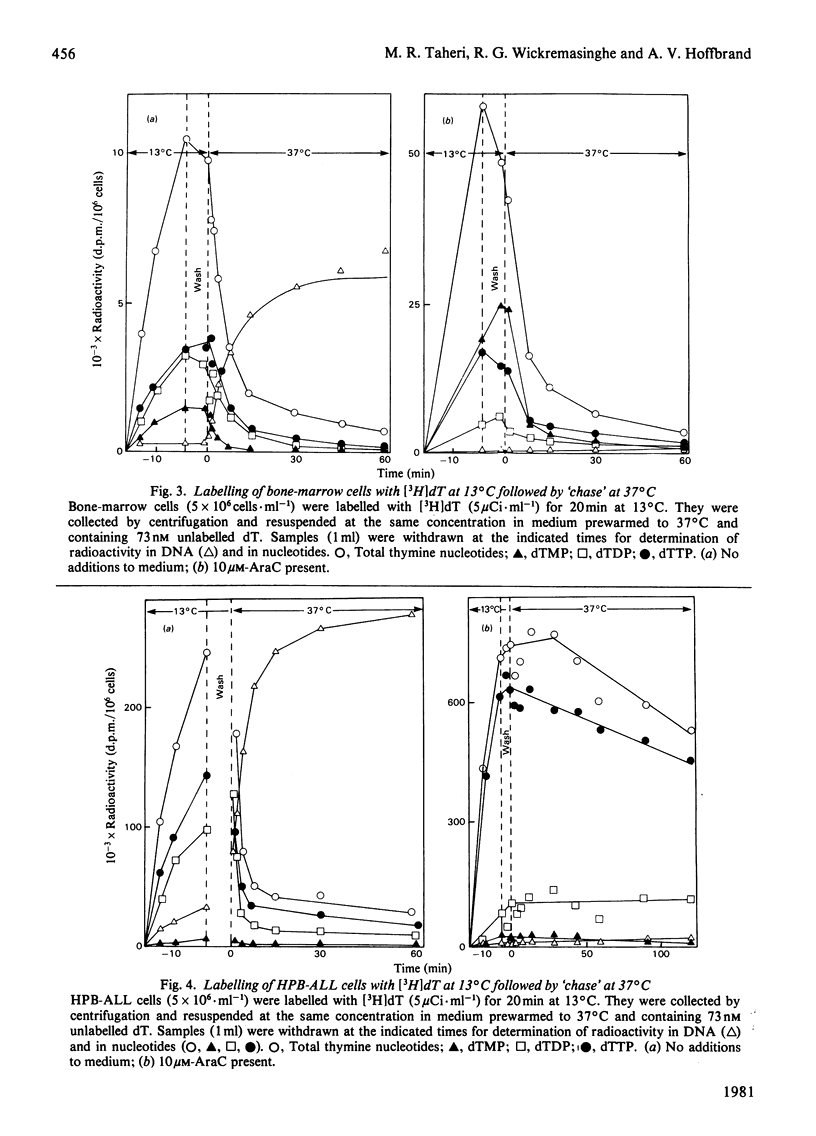
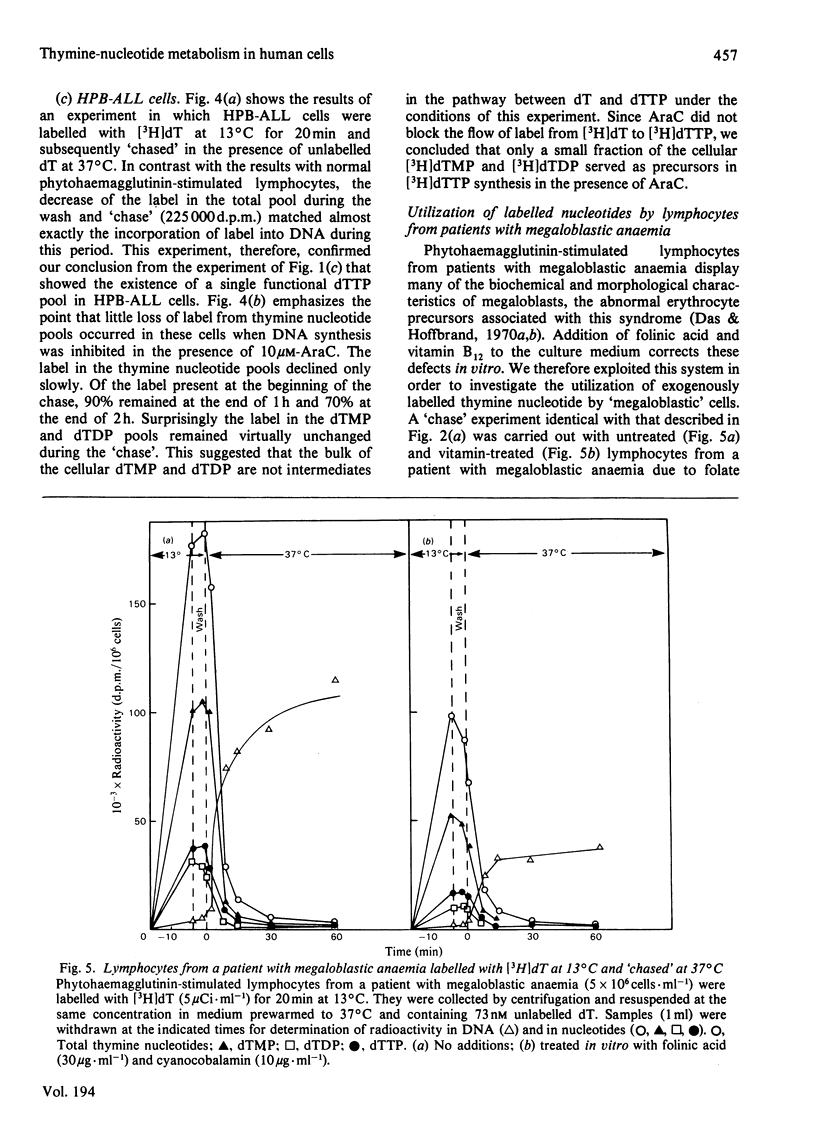
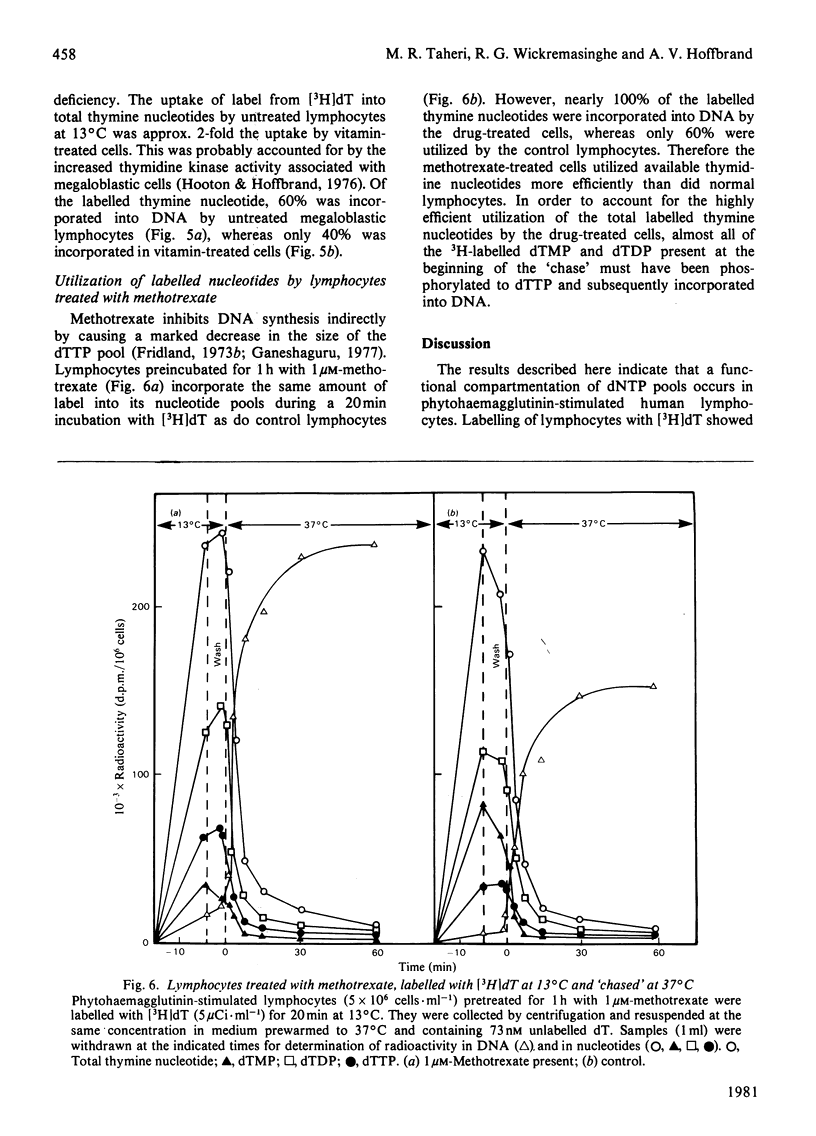
Three types of experiments have been used to study the metabolism of thymine nucleotides by human cells. (1) Cells were labelled continuously with [3H]thymidine and the incorporation of label into DNA compared with the specific radioactivities of pools of individual thymine nucleotides separated by chromatography on polyethylene-imine-cellulose. (2) Cellular thymine nucleotides were labelled with [3H]thymidine at 13 degrees C, followed by incubation at 37 degrees C in unlabelled medium. Incorporation of label into DNA and loss of label from the nucleotide pools were monitored during the 'chase' period at 37 degrees C. (3) The experiments described in (2) above were repeated in the presence of the DNA-synthesis inhibitor cytosine arabinoside, in order to demonstrate more clearly and to quantify degradative pathways for thymine nucleotides. In phytohaemagglutinin-stimulated lymphocytes and in bone-marrow cells, only a proportion (25-60%) of labelled thymine nucleotide was incorporated into DNA, the rest being rapidly degraded and lost from the cell. In contrast, an established cell line (HPB-ALL) from a patient with acute lymphoblastic leukaemia of thymic origin incorporated 100% of its exogenously labelled thymine nucleotides into DNA. These results indicated that alternative metabolic routes are open to thymine nucleotides in human cells. In lymphocytes from patients with megaloblastic anaemia and in normal lymphocytes treated with methotrexate, the utilization of labelled thymine nucleotides for DNA synthesis was more efficient than in controls. These results offer an explanation for the observation of a normal pool of thymidine triphosphate in the cells of patients with untreated megaloblastic anaemia even though the amount of this compound available for DNA synthesis appears to be decreased.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams R. L. Phosphorylation of tritiated thymidine by L929 mouse fibroblasts. Exp Cell Res. 1969 Jul;56(1):49–54. doi: 10.1016/0014-4827(69)90392-9. [DOI] [PubMed] [Google Scholar]
- Adams R. L. The effect of endogenous pools of thymidylate on the apparent rate of DNA synthesis. Exp Cell Res. 1969 Jul;56(1):55–58. doi: 10.1016/0014-4827(69)90393-0. [DOI] [PubMed] [Google Scholar]
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumunk C. N., Friedman D. L. Absence of an effect of amethopterin and 5-fluorodeoxyuridine upon levels of thymidine triphosphate in HeLa cells. Cancer Res. 1971 Dec;31(12):1930–1935. [PubMed] [Google Scholar]
- Carson D. A., Kaye J., Matsumoto S., Seegmiller J. E., Thompson L. Biochemical basis for the enhanced toxicity of deoxyribonucleosides toward malignant human T cell lines. Proc Natl Acad Sci U S A. 1979 May;76(5):2430–2433. doi: 10.1073/pnas.76.5.2430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das K. C., Hoffbrand A. V. Lymphocyte transformation in megaloblastic anaemia: morphology and DNA synthesis. Br J Haematol. 1970 Oct;19(4):459–468. doi: 10.1111/j.1365-2141.1970.tb06973.x. [DOI] [PubMed] [Google Scholar]
- Das K. C., Hoffbrand A. V. Studies of folate uptake by phytohaemagglutinin-stimulated lymphocytes. Br J Haematol. 1970 Aug;19(2):203–221. doi: 10.1111/j.1365-2141.1970.tb01617.x. [DOI] [PubMed] [Google Scholar]
- Davis R. H. Metabolite distribution in cells. Science. 1972 Nov 24;178(4063):835–840. doi: 10.1126/science.178.4063.835. [DOI] [PubMed] [Google Scholar]
- Fox R. M., Piddington S. K., Tripp E. H., Dudman N. P., Tattersall M. H. Thymidine sensitivity of cultured leukaemic lymphocytes. Lancet. 1979 Aug 25;2(8139):391–393. doi: 10.1016/s0140-6736(79)90405-7. [DOI] [PubMed] [Google Scholar]
- Fridland A. DNA precursors in eukaryotic cells. Nat New Biol. 1973 May 23;243(125):105–107. [PubMed] [Google Scholar]
- Fridland A. Effect of methotrexate on deoxyribonucleotide pools and DNA synthesis in human lymphocytic cells. Cancer Res. 1974 Aug;34(8):1883–1888. [PubMed] [Google Scholar]
- Fridland A. Inhibition of deoxyribonucleic acid chain initiation: a new mode of action for 1-beta-D-arabinofuranosylcytosine in human lymphoblasts. Biochemistry. 1977 Nov 29;16(24):5308–5312. doi: 10.1021/bi00643a023. [DOI] [PubMed] [Google Scholar]
- Ganeshaguru K., Hoffbrand A. V. The effect of deoxyuridine, vitamin B12, folate and alcohol on the uptake of thymidine and on the deoxynucleoside triphosphate concentrations in normal and megaloblastic cells. Br J Haematol. 1978 Sep;40(1):29–41. doi: 10.1111/j.1365-2141.1978.tb03636.x. [DOI] [PubMed] [Google Scholar]
- Giblett E. R., Anderson J. E., Cohen F., Pollara B., Meuwissen H. J. Adenosine-deaminase deficiency in two patients with severely impaired cellular immunity. Lancet. 1972 Nov 18;2(7786):1067–1069. doi: 10.1016/s0140-6736(72)92345-8. [DOI] [PubMed] [Google Scholar]
- Hoffbrand A. V., Ganeshaguru K., Lavoie A., Tattersall M. H., Tripp E. Thymidylate concentration in megaloblastic anaemia. Nature. 1974 Apr 12;248(449):602–604. doi: 10.1038/248602a0. [DOI] [PubMed] [Google Scholar]
- Hooton J. W., Hoffbrand A. V. Thymidine kinase in megaloblastic anaemia. Br J Haematol. 1976 Aug;33(4):527–537. doi: 10.1111/j.1365-2141.1976.tb03571.x. [DOI] [PubMed] [Google Scholar]
- Kuebbing D., Werner R. A model for compartmentation of de novo and salvage thymidine nucleotide pools in mammalian cells. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3333–3336. doi: 10.1073/pnas.72.9.3333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S. S., Giovanella B. C., Stehlin J. S., Jr Selective lethal effect of thymidine on human and mouse tumor cells. J Cell Physiol. 1977 Sep;92(3):401–405. doi: 10.1002/jcp.1040920308. [DOI] [PubMed] [Google Scholar]
- Lindberg U., Skoog L. A method for the determination of dATP and dTTP in picomole amounts. Anal Biochem. 1970 Mar;34:152–160. doi: 10.1016/0003-2697(70)90096-5. [DOI] [PubMed] [Google Scholar]
- MILLER O. L., Jr, STONE G. E., PRESCOTT D. M. AUTORADIOGRAPHY OF SOLUBLE MATERIALS. J Cell Biol. 1964 Dec;23:654–658. doi: 10.1083/jcb.23.3.654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metz J., Kelly A., Swett V. C., Waxman S., Herbert V. Deranged DNA synthesis by bone marrow from vitamin B-12-deficient humans. Br J Haematol. 1968 Jun;14(6):575–592. doi: 10.1111/j.1365-2141.1968.tb00364.x. [DOI] [PubMed] [Google Scholar]
- Ochs U. H., Chen S. H., Ochs H. D., Osborne W. R., Scott C. R. Purine nucleoside phosphorylase deficiency: a molecular model for selective loss of T cell function. J Immunol. 1979 Jun;122(6):2424–2429. [PubMed] [Google Scholar]
- Prem veer Reddy G., Pardee A. B. Multienzyme complex for metabolic channeling in mammalian DNA replication. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3312–3316. doi: 10.1073/pnas.77.6.3312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy G. P., Mathews C. K. Functional compartmentation of DNA precursors in T4 phage-infected bacteria. J Biol Chem. 1978 May 25;253(10):3461–3467. [PubMed] [Google Scholar]
- Tobey R. A., Ley K. D. Isoleucine-mediated regulation of genome repliction in various mammalian cell lines. Cancer Res. 1971 Jan;31(1):46–51. [PubMed] [Google Scholar]
- Usher D. C., Reiter H. Catabolism of thymidine during the lymphocyte cell cycle. Cell. 1977 Oct;12(2):365–370. doi: 10.1016/0092-8674(77)90112-x. [DOI] [PubMed] [Google Scholar]
- Werner R. Nature of DNA precursors. Nat New Biol. 1971 Sep 22;233(38):99–103. doi: 10.1038/newbio233099a0. [DOI] [PubMed] [Google Scholar]
- Wickremasinghe R. G., Hoffbrand A. V. Conversion of partially single-stranded replicating DNA to double-stranded DNA is delayed in megaloblastic anaemia. Biochim Biophys Acta. 1980 May 30;607(3):411–419. doi: 10.1016/0005-2787(80)90151-3. [DOI] [PubMed] [Google Scholar]
- Wickremasinghe R. G., Hoffbrand A. V. Defective DNA synthesis in megaloblastic anaemia. Studies employing velocity sedimentation in alkaline sucrose gradients. Biochim Biophys Acta. 1979 Jun 20;563(1):46–58. doi: 10.1016/0005-2787(79)90006-6. [DOI] [PubMed] [Google Scholar]
- Wickremasinghe R. G., Hoffbrand A. V. Reduced rate of DNA replication fork movement in megaloblastic anemia. J Clin Invest. 1980 Jan;65(1):26–36. doi: 10.1172/JCI109656. [DOI] [PMC free article] [PubMed] [Google Scholar]


