Abstract
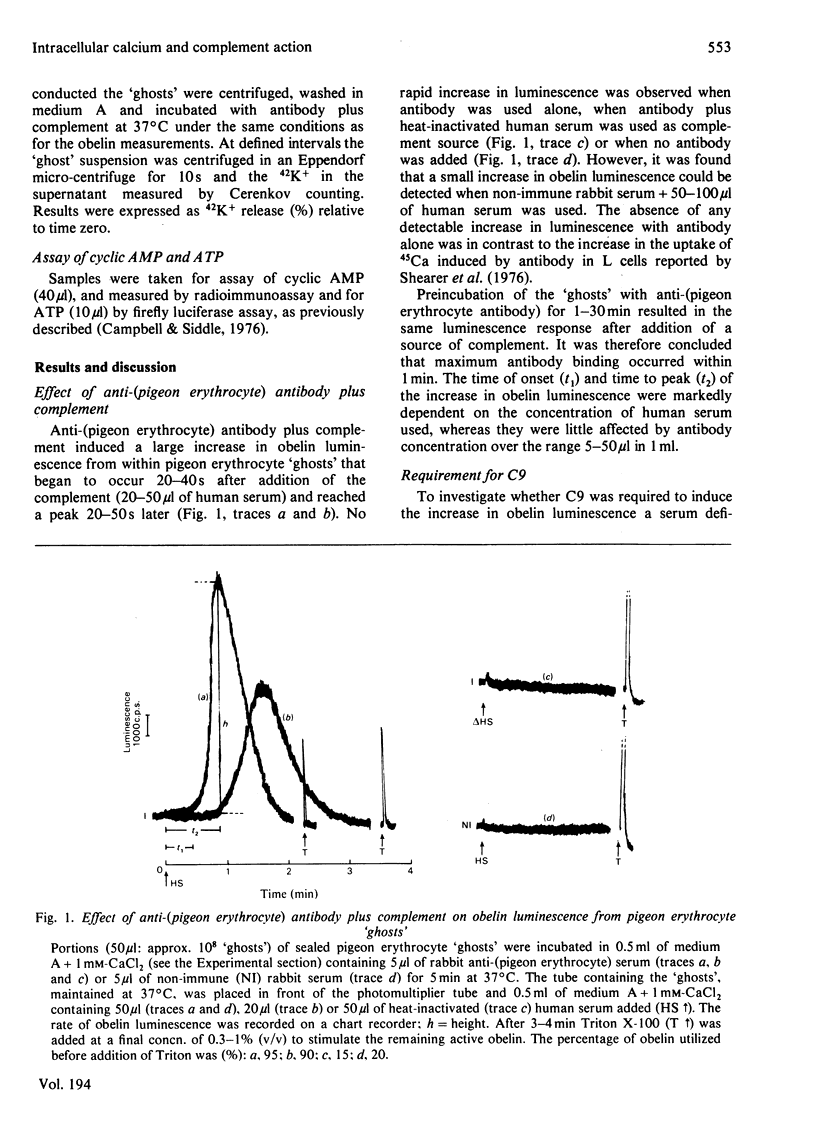
1. The effect of rabbit anti-(pigeon erythrocyte) antibodies plus human complement on the concentration of intracellular free Ca2+ in sealed pigeon erythrocyte 'ghosts' was investigated with the photoprotein obelin. 2. The addition of human serum, as a source of complement, to 'ghosts' coated with antibody caused a rapid increase in intracellular free Ca2+ after a lag of 20-40 s, as detected by an increase in obelin luminescence. 3. The increase in obelin luminescence could not be explained by release of obelin into the medium. It was also Ca2+-dependent in that extracellular EGTA abolished the effect and intracellular EGTA inhibited it and required the complete terminal complex (C56789). No effect was seen with C5678. 4. The concentration of intracellular free Ca2+ before addition of complement was approx. 0.3 microM. This increased to a maximum of 5-30 microM after complement addition and then remained constant for at least 1-2 min. 5. Antibody plus complement induced a rapid increase in 42K+ efflux and an inhibition of cyclic AMP formation. 6. When partially purified complement components (C5b-9) were used in 'reactive lysis' it was possible to inhibit the release of macromolecules from pigeon erythrocyte 'ghosts' by extracellular EGTA. 7. It was concluded that the increase in intracellular free Ca2+ concentration caused by anti-cell antibody plus complement occurred before cell lysis and may be involved in the mechanism of complement-induced cell injury.
Full text
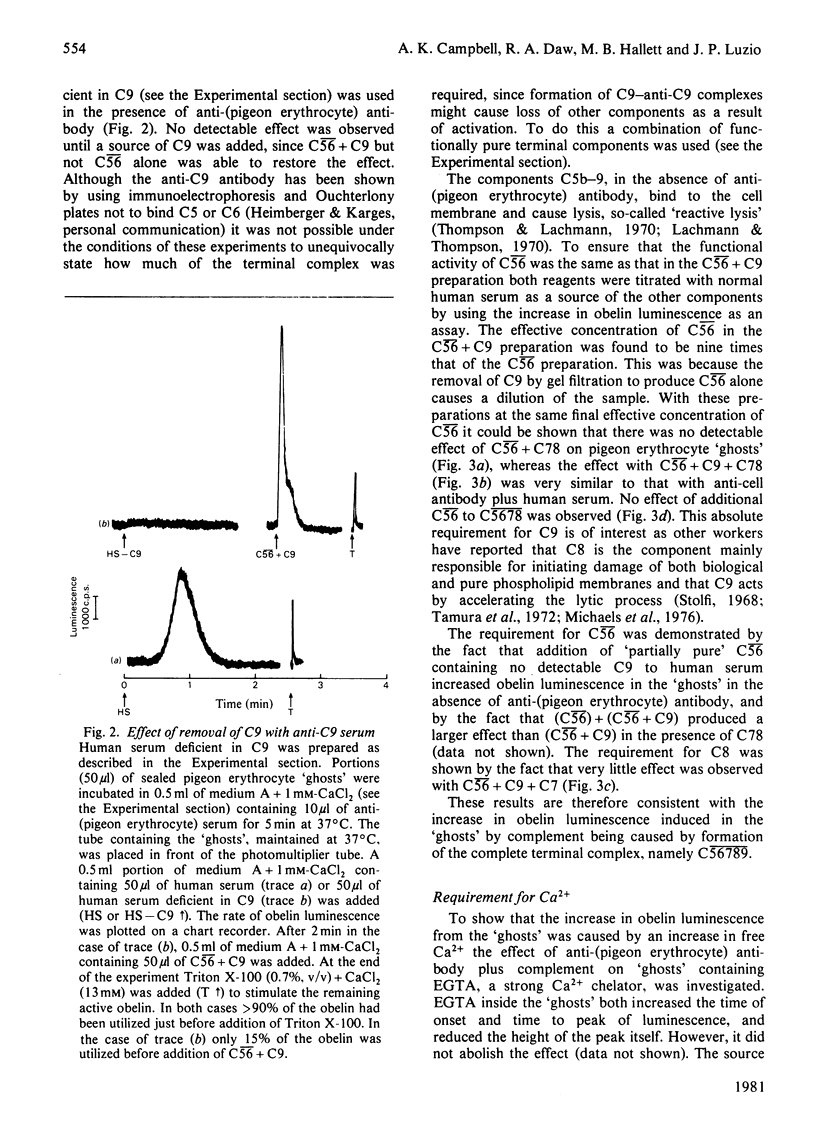
PDF


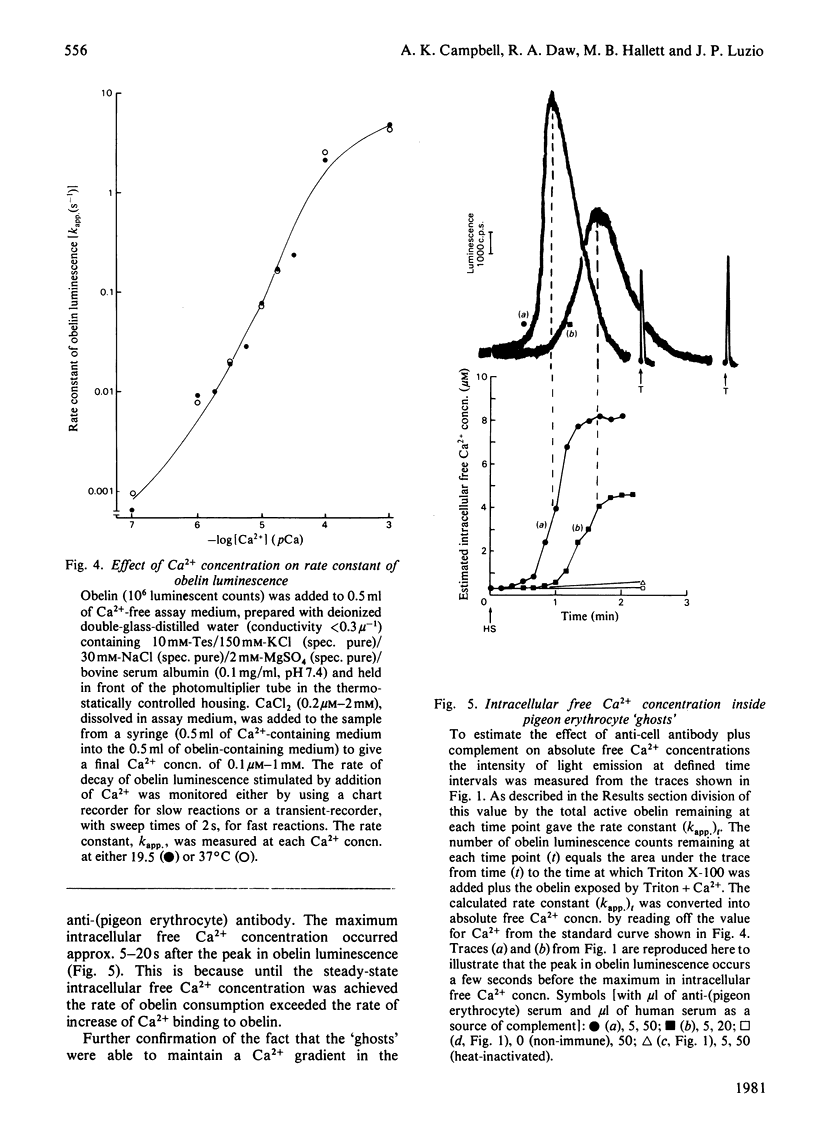
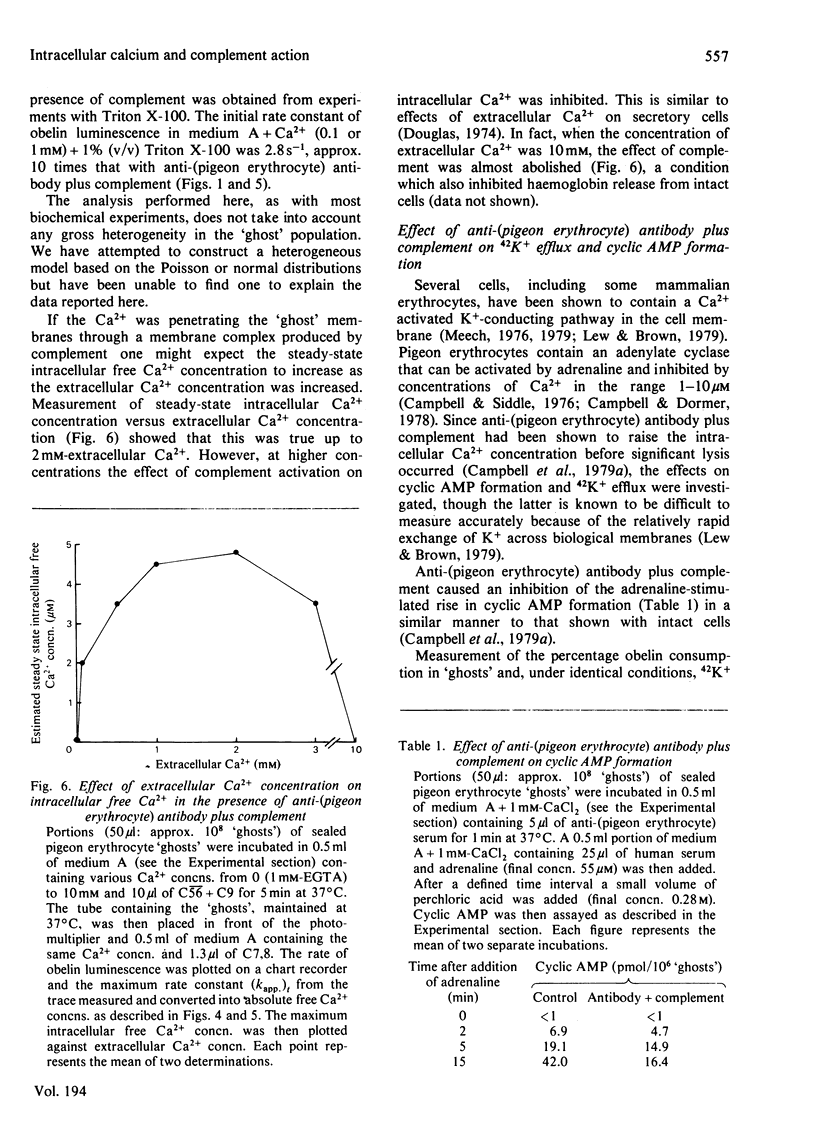


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allan D., Michell R. H. Calcium ion-dependent diacylglycerol accumulation in erythrocytes is associated with microvesiculation but not with efflux of potassium ions. Biochem J. 1977 Sep 15;166(3):495–499. doi: 10.1042/bj1660495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle M. D., Ohanian S. H., Borsos T. Studies on the terminal stages of antibody-complement-mediated killing of a tumor cell. I. Evidence for the existence of an intermediate, T. J Immunol. 1976 May;116(5):1272–1275. [PubMed] [Google Scholar]
- Boyle M. D., Ohanian S. H., Borsos T. Studies on the terminal stages of antibody-complement-mediated killing of a tumor cell. II. Inhibition of transformation of T to dead cells by 3'5' cAMP. J Immunol. 1976 May;116(5):1276–1279. [PubMed] [Google Scholar]
- Campbell A. K., Daw R. A., Luzio J. P. Rapid increase in intracellular free Ca2+ induced by antibody plus complement. FEBS Lett. 1979 Nov 1;107(1):55–60. doi: 10.1016/0014-5793(79)80462-7. [DOI] [PubMed] [Google Scholar]
- Campbell A. K., Dormer R. L. Inhibition by calcium ions of adenosine cyclic monophosphate formation in sealed pigeon erythrocyte 'ghosts'. A study using the photoprotein obelin. Biochem J. 1978 Oct 15;176(1):53–66. doi: 10.1042/bj1760053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell A. K., Dormer R. L. Permeability to calcium of pigeon erythrocyte 'ghosts' studied by using the calcium-activated luminescent protein, obelin. Biochem J. 1975 Nov;152(2):255–265. doi: 10.1042/bj1520255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell A. K., Hallett M. B., Daw R. A., Luzio J. P., Siddle K. The importance of measuring intracellular free Ca2+. Biochem Soc Trans. 1979 Oct;7(5):865–869. doi: 10.1042/bst0070865. [DOI] [PubMed] [Google Scholar]
- Campbell A. K., Siddle K. The effect of intracellular calcium ions on adrenaline-stimulated adenosine 3':5'-cyclic monophosphate concentrations in pigeon erythrocytes, studied by using the ionophore A23187. Biochem J. 1976 Aug 15;158(2):211–221. doi: 10.1042/bj1580211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dormer R. L., Newman G. R., Campbell A. K. Preparation and characterization of liposomes containing the Ca2+-activated photoprotein, obelin. Biochim Biophys Acta. 1978 Jan 3;538(1):87–105. doi: 10.1016/0304-4165(78)90254-4. [DOI] [PubMed] [Google Scholar]
- Douglas W. W. Involvement of calcium in exocytosis and the exocytosis--vesiculation sequence. Biochem Soc Symp. 1974;(39):1–28. [PubMed] [Google Scholar]
- Dourmashkin R. R. The structural events associated with the attachment of complement components to cell membranes in reactive lysis. Immunology. 1978 Aug;35(2):205–212. [PMC free article] [PubMed] [Google Scholar]
- FRANK M. M., RAPP H. J., BORSOS T. STUDIES ON THE TERMINAL STEPS OF IMMUNE HEMOLYSIS. I. INHIBITION BY TRISODIUM ETHYLENEDIAMINETETRAACETATE (EDTA). J Immunol. 1964 Sep;93:409–413. [PubMed] [Google Scholar]
- FRANK M. M., RAPP H. J., BORSOS T. STUDIES ON THE TERMINAL STEPS OF IMMUNE HEMOLYSIS. II. RESOLUTION OF THE E TRANSFORMATION REACTION INTO MULTIPLE STEPS. J Immunol. 1965 Feb;94:295–300. [PubMed] [Google Scholar]
- Fell H. B., Coombs R. R., Dingle J. T. The breakdown of embryonic (chick) cartilage and bone cultivated in the presence of complement-sufficient antiserum. I. Morphological changes, their reversibility and inhibition. Int Arch Allergy Appl Immunol. 1966;30(2):146–176. doi: 10.1159/000229803. [DOI] [PubMed] [Google Scholar]
- Fine D. P., Marney S. R., Jr, Colley D. G., Sergent J. S., Des Prez R. M. C3 shunt activation in human serum chelated with EGTA. J Immunol. 1972 Oct;109(4):807–809. [PubMed] [Google Scholar]
- Gigli I., Porter R. R., Sim R. B. The unactivated form of the first component of human complement, C1. Biochem J. 1976 Sep 1;157(3):541–548. doi: 10.1042/bj1570541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadding U., Müller-Eberhard H. J. Complement: substitution of the terminal component in immune hemolysis by 1,10-phenanthroline. Science. 1967 Jul 28;157(3787):442–443. doi: 10.1126/science.157.3787.442. [DOI] [PubMed] [Google Scholar]
- Hallett M. B., Campbell A. K. Uptake of liposomes containing the photoprotein obelin by rat isolated adipocytes. Adhesion, endocytosis or fusion? Biochem J. 1980 Nov 15;192(2):587–596. doi: 10.1042/bj1920587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser N., Edelman I. S. Calcium dependence of glucocorticoid-induced lymphocytolysis. Proc Natl Acad Sci U S A. 1977 Feb;74(2):638–642. doi: 10.1073/pnas.74.2.638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachmann P. J., Bowyer D. E., Nicol P., Dawson R. M., Munn E. A. Studies on the terminal stages of complement lysis. Immunology. 1973 Jan;24(1):135–145. [PMC free article] [PubMed] [Google Scholar]
- Lachmann P. J., Thompson R. A. Reactive lysis: the complement-mediated lysis of unsensitized cells. II. The characterization of activated reactor as C56 and the participation of C8 and C9. J Exp Med. 1970 Apr 1;131(4):643–657. doi: 10.1084/jem.131.4.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luzio J. P., Daw R. A., Hallett M. B., Richardson P. J., Campbell A. K. The rapid increase in intracellular free calcium ion concentration induced by complement and its role in cell damage [proceedings]. Biochem Soc Trans. 1979 Oct;7(5):1066–1068. doi: 10.1042/bst0071066. [DOI] [PubMed] [Google Scholar]
- Luzio J. P., Newby A. C., Hales C. N. A rapid immunological procedure for the isolation of hormonally sensitive rat fat-cell plasma membrane. Biochem J. 1976 Jan 15;154(1):11–21. doi: 10.1042/bj1540011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchesi V. T., Furthmayr H., Tomita M. The red cell membrane. Annu Rev Biochem. 1976;45:667–698. doi: 10.1146/annurev.bi.45.070176.003315. [DOI] [PubMed] [Google Scholar]
- May J. E., Frank M. M. A new complement-mediated cytolytic mechanism--the C1-bypass activation pathway. Proc Natl Acad Sci U S A. 1973 Mar;70(3):649–652. doi: 10.1073/pnas.70.3.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meech R. W. Calcium-dependent potassium activation in nervous tissues. Annu Rev Biophys Bioeng. 1978;7:1–18. doi: 10.1146/annurev.bb.07.060178.000245. [DOI] [PubMed] [Google Scholar]
- Michaels D. W., Abramovitz A. S., Hammer C. H., Mayer M. M. Increased ion permeability of planar lipid bilayer membranes after treatment with the C5b-9 cytolytic attack mechanism of complement. Proc Natl Acad Sci U S A. 1976 Aug;73(8):2852–2856. doi: 10.1073/pnas.73.8.2852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto M., Mayer M. M. Studies on the mechanism of action of guinea pig lymphotoxin. II. Increase of calcium uptake rate in LT-damaged target cells. J Immunol. 1978 Jan;120(1):279–285. [PubMed] [Google Scholar]
- Podack E. R., Biesecker G., Müller-Eberhard H. J. Membrane attack complex of complement: generation of high-affinity phospholipid binding sites by fusion of five hydrophilic plasma proteins. Proc Natl Acad Sci U S A. 1979 Feb;76(2):897–901. doi: 10.1073/pnas.76.2.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter R. R., Reid K. B. The biochemistry of complement. Nature. 1978 Oct 26;275(5682):699–704. doi: 10.1038/275699a0. [DOI] [PubMed] [Google Scholar]
- Raisz L. G., Sandberg A. L., Goodson J. M., Simmons H. A., Mergenhagen S. E. Complement-dependent stimulation of prostaglandin synthesis and bone resorption. Science. 1974 Aug 30;185(4153):789–791. doi: 10.1126/science.185.4153.789. [DOI] [PubMed] [Google Scholar]
- Rasmussen H., Goodman D. B. Relationships between calcium and cyclic nucleotides in cell activation. Physiol Rev. 1977 Jul;57(3):421–509. doi: 10.1152/physrev.1977.57.3.421. [DOI] [PubMed] [Google Scholar]
- Richardson P. J., Luzio J. P. Complement-mediated production of plasma-membrane vesicles from rat fat-cells. Biochem J. 1980 Mar 15;186(3):897–906. doi: 10.1042/bj1860897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schanne F. A., Kane A. B., Young E. E., Farber J. L. Calcium dependence of toxic cell death: a final common pathway. Science. 1979 Nov 9;206(4419):700–702. doi: 10.1126/science.386513. [DOI] [PubMed] [Google Scholar]
- Shearer W. T., Atkinson J. P., Parker C. W. Humoral immunostimulation. VI. Increased calcium uptake by cells treated with antibody and complement. J Immunol. 1976 Sep;117(3):973–980. [PubMed] [Google Scholar]
- Sims P. J., Lauf P. K. Steady-state analysis of tracer exchange across the C5b-9 complement lesion in a biological membrane. Proc Natl Acad Sci U S A. 1978 Nov;75(11):5669–5673. doi: 10.1073/pnas.75.11.5669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolfi R. L. Immune lytic transformation: a state of irreversible damage generated as a result of the reaction of the eighth component in the guinea pig complement system. J Immunol. 1968 Jan;100(1):46–54. [PubMed] [Google Scholar]
- Tamura N., Shimada A., Chang S. Further evidence for immune cytolysis by antibody and the first eight components of complement in the absence of C9. Immunology. 1972 Jan;22(1):131–140. [PMC free article] [PubMed] [Google Scholar]
- Thompson R. A., Lachmann P. J. Reactive lysis: the complement-mediated lysis of unsensitized cells. I. The characterization of the indicator factor and its identification as C7. J Exp Med. 1970 Apr 1;131(4):629–641. doi: 10.1084/jem.131.4.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White J. G. Effects of an ionophore, A23187, on the surface morphology of normal erythrocytes. Am J Pathol. 1974 Dec;77(3):507–518. [PMC free article] [PubMed] [Google Scholar]


