Abstract
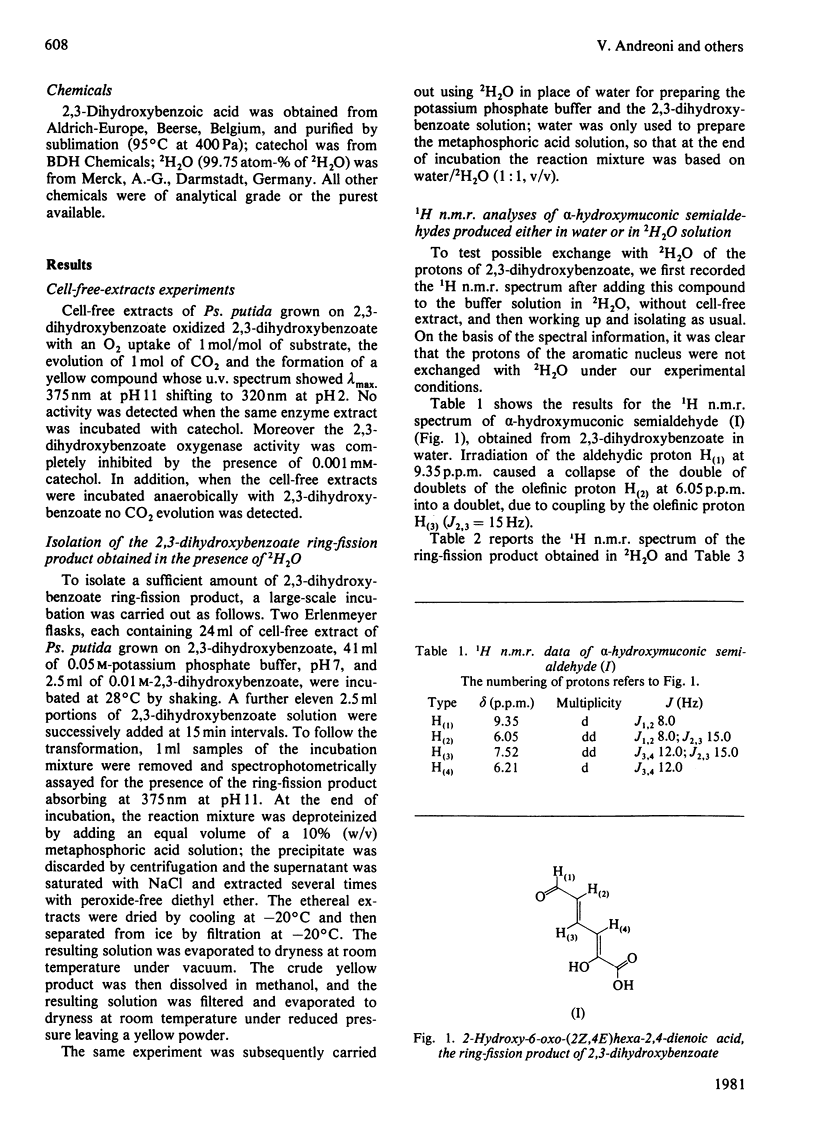
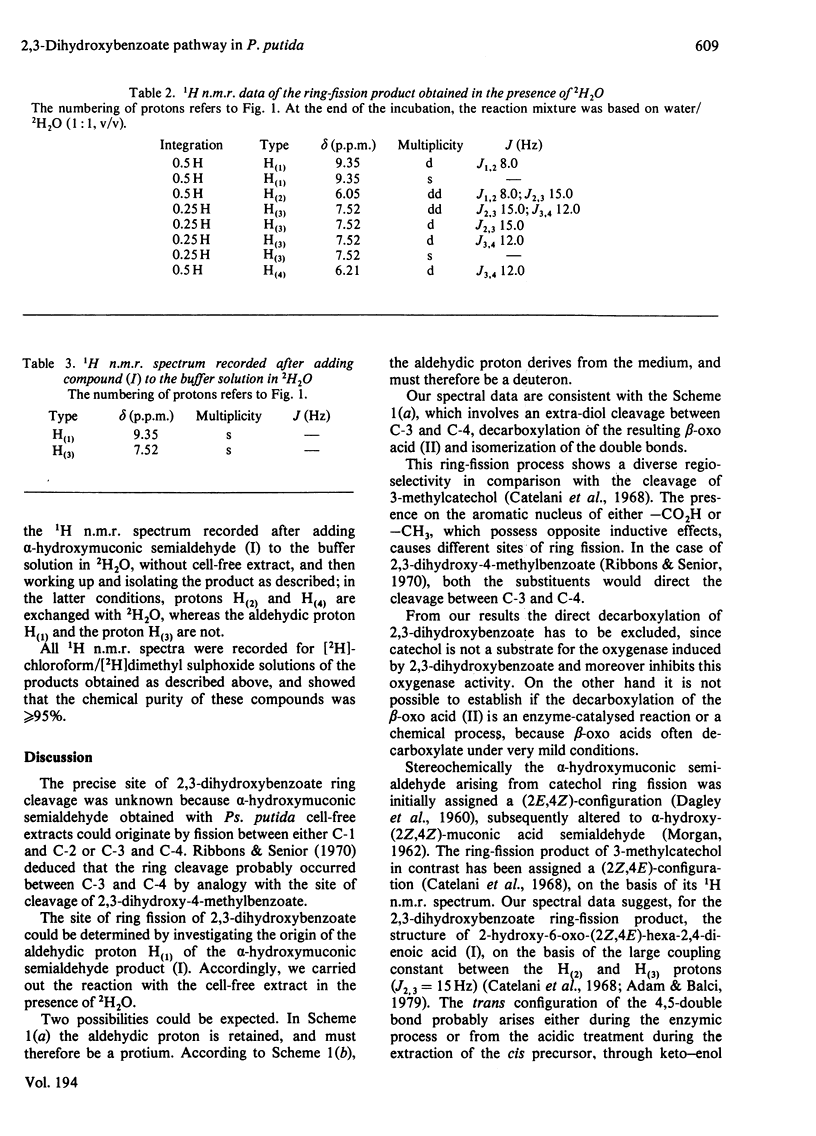
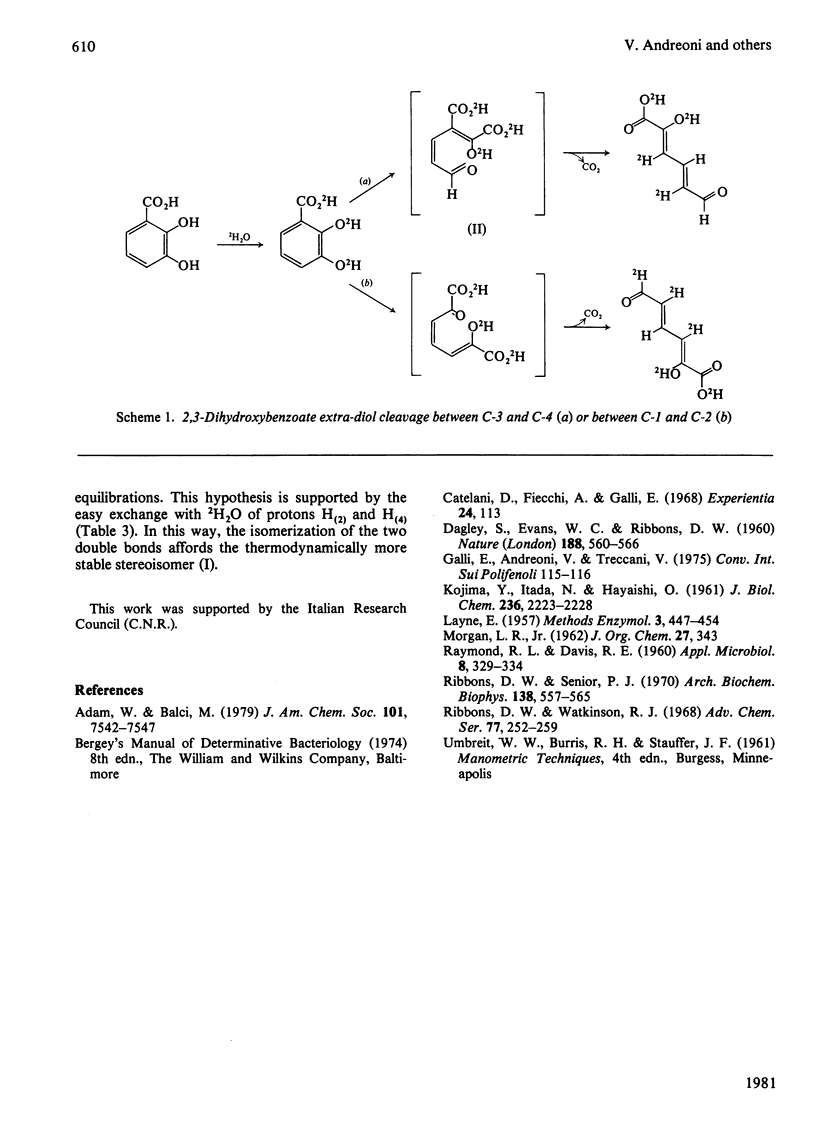
1. Ring cleavage of 2,3-dihydroxybenzoate by cell-free extracts of Pseudomonas putida leads to 2-hydroxy-6-oxo-(2Z,4E)-hexa-2,4-dienoic acid and CO2. 2. The 1H n.m.r. spectrum of the ring-fission product obtained in a 2H2O solution suggests that the extra-diol cleavage occurs between C-3 and C-4.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Catelani D., Fiecchi A., Galli E. Formation of 2-hydroxy-6-oxo-2, trans-4, trans-heptad-ienoic acid from 3-methylcatechol by a Pseudomonas. Experientia. 1968 Feb 15;24(2):113–113. doi: 10.1007/BF02146927. [DOI] [PubMed] [Google Scholar]
- DAGLEY S., EVANS W. C., RIBBONS D. W. New pathways in the oxidative metabolism of aromatic compounds by microorganisms. Nature. 1960 Nov 12;188:560–566. doi: 10.1038/188560a0. [DOI] [PubMed] [Google Scholar]
- KOJIMA Y., ITADA N., HAYAISHI O. Metapyrocatachase: a new catechol-cleaving enzyme. J Biol Chem. 1961 Aug;236:2223–2228. [PubMed] [Google Scholar]
- RAYMOND R. L., DAVIS J. B. n-Alkane utilization and lipid formation by a Nocardia. Appl Microbiol. 1960 Nov;8:329–334. doi: 10.1128/am.8.6.329-334.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribbons D. W., Senior P. J. 2,3-dihydroxybenzoate 3,4-oxygenase from Pseudomonas fluorescens--oxidation of a substrate analog. Arch Biochem Biophys. 1970 Jun;138(2):557–565. doi: 10.1016/0003-9861(70)90381-4. [DOI] [PubMed] [Google Scholar]


