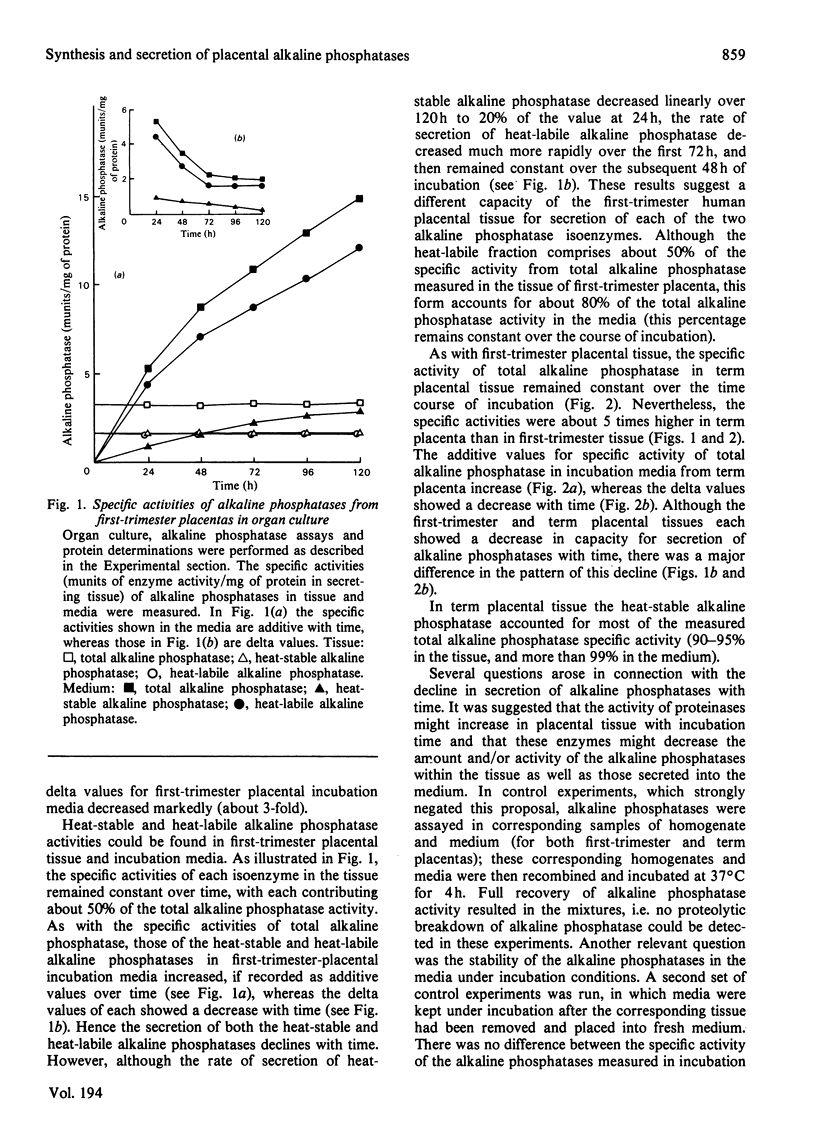
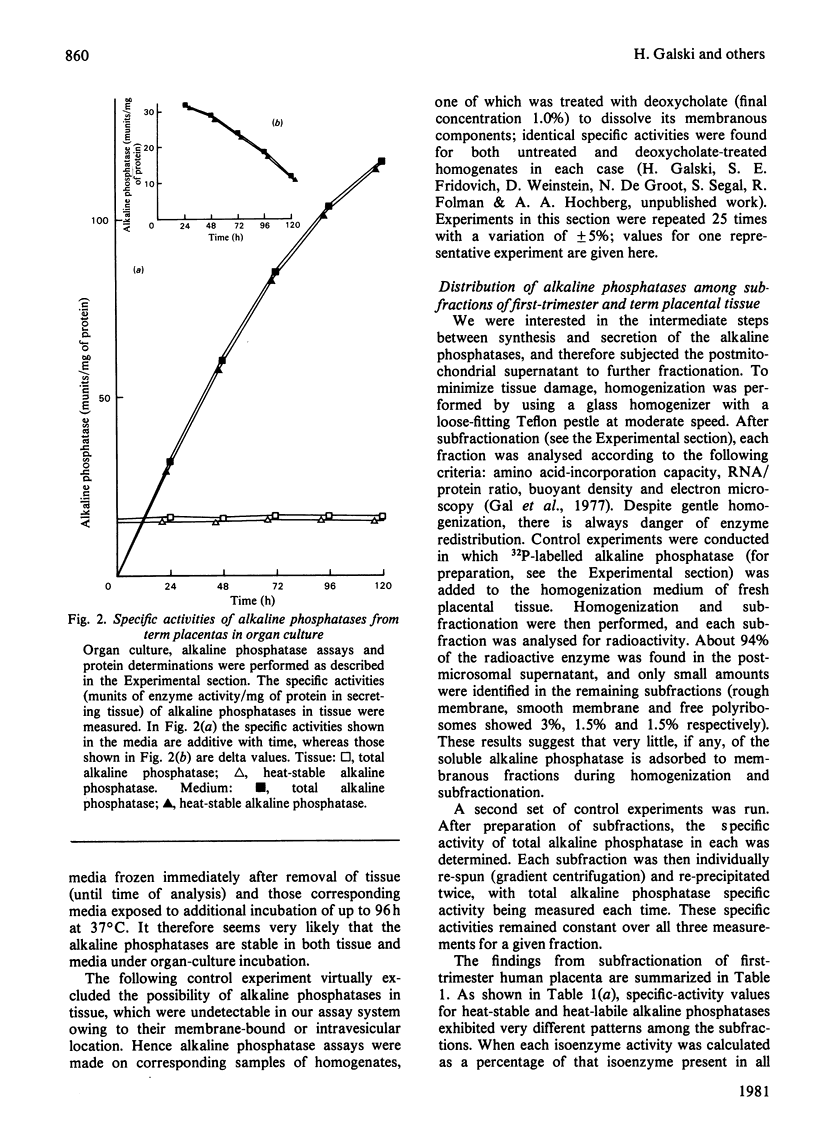
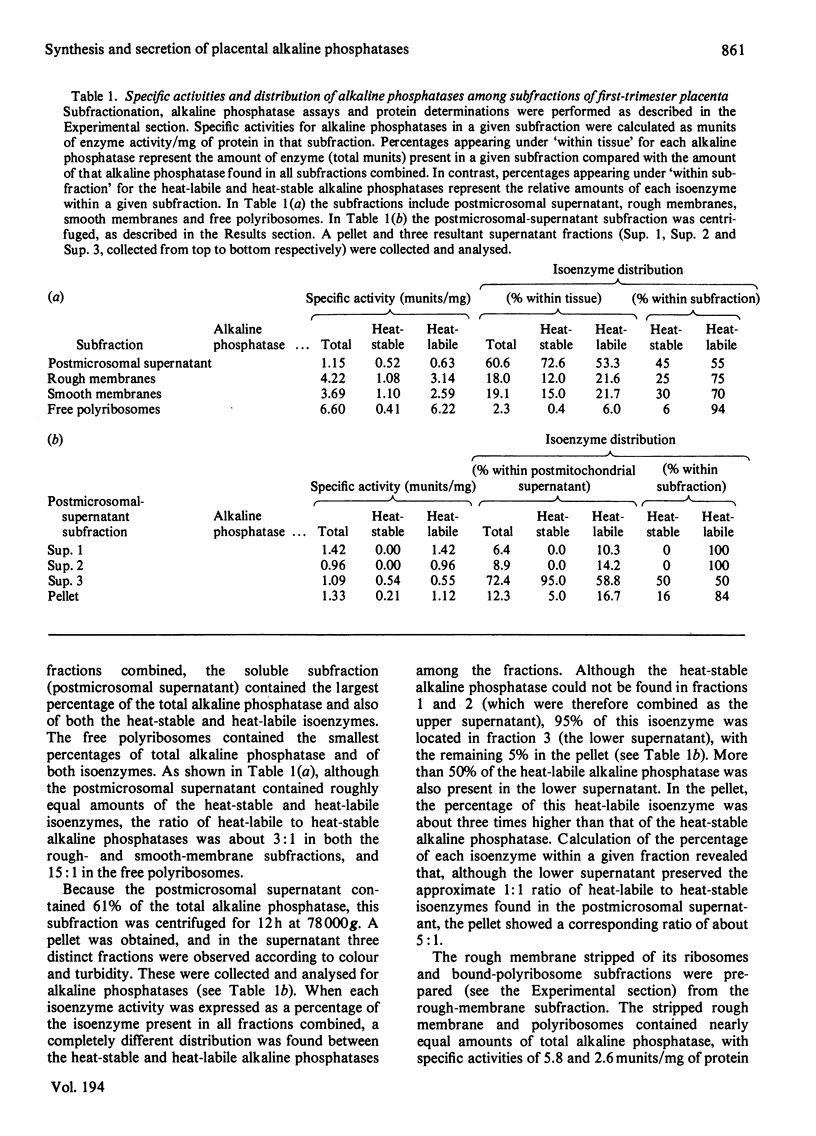
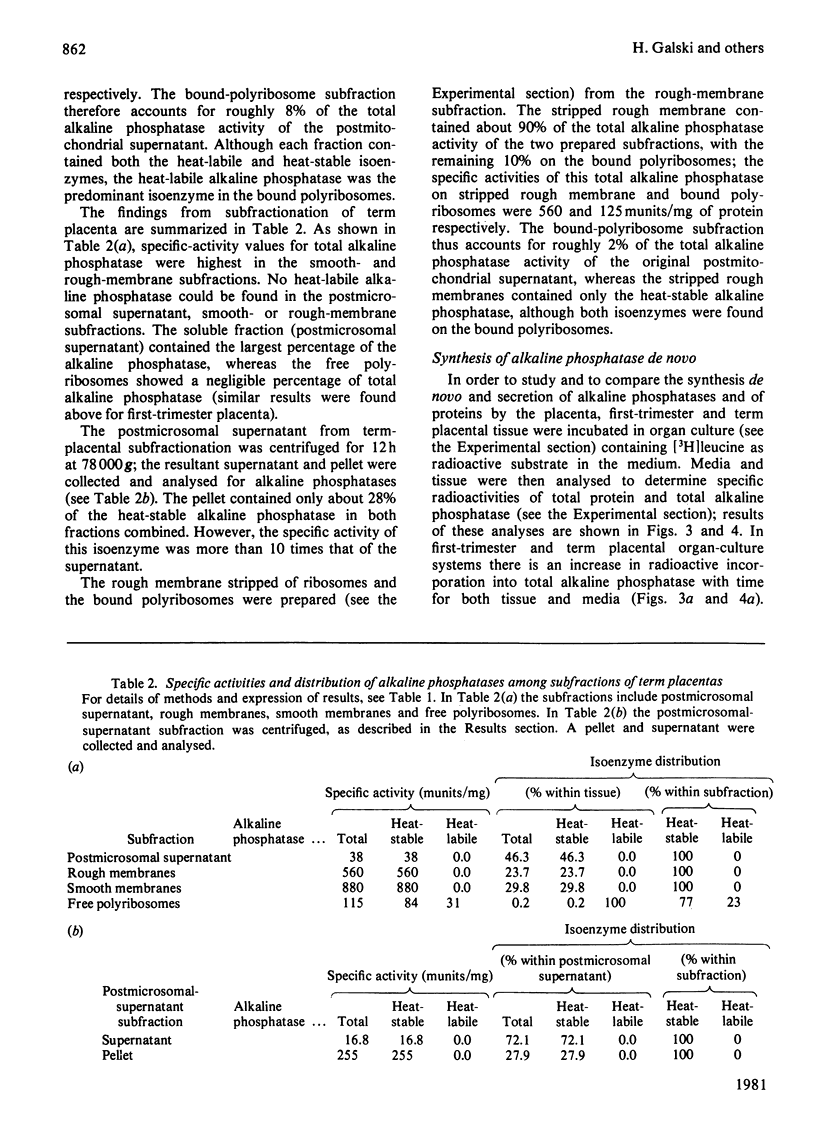
Abstract
The synthesis and secretion of alkaline phosphatases in vitro by human placental tissue incubated in organ culture were studied. First-trimester placenta synthesizes and secretes two different alkaline phosphatase isoenzymes (heat-labile and heat-stable), whereas in term placenta nearly all the alkaline phosphatase synthesized and secreted is heat-stable. The specific activities of alkaline phosphatases in first-trimester and term placental tissue remain constant throughout the time course of incubation. In the media, specific activities increase with time. Hence, alkaline phosphatase synthesis seems to be the driving force for its own secretion. The rates of synthesis de novo and of alkaline phosphatases were measured. The specific radioactivities of the secreted alkaline phosphatases were higher than the corresponding specific radioactivities in the tissue throughout the entire incubation period. The intracellular distribution of the alkaline phosphatase isoenzymes was compared.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Badger K. S., Sussman H. H. Structural evidence that human liver and placental alkaline phosphatase isoenzymes are coded by different genes. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2201–2205. doi: 10.1073/pnas.73.7.2201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer S. H. Alkaline Phosphatase in Human Sera and Placentae: Starch gel electrophoresis reveals many phosphatase components including a polymorphism in placentae. Science. 1961 Oct 6;134(3484):1002–1004. doi: 10.1126/science.134.3484.1002. [DOI] [PubMed] [Google Scholar]
- Curzen P., Morris I. Heat-stable alkaline phosphatase in maternal serum. J Obstet Gynaecol Br Commonw. 1968 Feb;75(2):151–157. doi: 10.1111/j.1471-0528.1968.tb02025.x. [DOI] [PubMed] [Google Scholar]
- Doellgast G. J., Spiegel J., Guenther R. A., Fishman W. H. Studies on human placental alkaline phosphatase. Purification by immunoabsorption and comparison of the "A" band "B" forms of the enzyme. Biochim Biophys Acta. 1977 Sep 15;484(1):59–78. doi: 10.1016/0005-2744(77)90113-9. [DOI] [PubMed] [Google Scholar]
- ENGSTROM L. Studies on calf-intestinal alkaline phosphatase. II. Incorporation of inorganic phosphate into a highly purified enzyme preparation. Biochim Biophys Acta. 1961 Sep 2;52:49–59. doi: 10.1016/0006-3002(61)90902-7. [DOI] [PubMed] [Google Scholar]
- Fishman L., Miyayama H., Driscoll S. G., Fishman W. H. Developmental phase-specific alkaline phosphatase isoenzymes of human placenta and their occurrence in human cancer. Cancer Res. 1976 Jul;36(7 Pt 1):2268–2273. [PubMed] [Google Scholar]
- Fishman W. H. Immunologic and biochemical approaches to alkaline phosphatase isoenzyme analysis: the Regan isoenzyme. Ann N Y Acad Sci. 1969 Oct 14;166(2):745–759. doi: 10.1111/j.1749-6632.1969.tb46432.x. [DOI] [PubMed] [Google Scholar]
- Fishman W. H. Perspectives on alkaline phosphatase isoenzymes. Am J Med. 1974 May;56(5):617–650. doi: 10.1016/0002-9343(74)90631-7. [DOI] [PubMed] [Google Scholar]
- Gal A. L., Folman R., Czosnek H. H., Shiklosh Y., de Groot N., Hochberg A. A. The in vitro reconstitution of rough endoplasmic reticulum membrane derived from human placenta. Life Sci. 1977 Sep 15;21(6):779–788. doi: 10.1016/0024-3205(77)90405-2. [DOI] [PubMed] [Google Scholar]
- Greene P. J., Sussman H. H. Structual comparison of ectopic and normal placental alkaline phosphatase. Proc Natl Acad Sci U S A. 1973 Oct;70(10):2936–2940. doi: 10.1073/pnas.70.10.2936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton T. A., Tin A. W., Sussman H. H. Regulation of alkaline phosphatase expression in human choriocarcinoma cell lines. Proc Natl Acad Sci U S A. 1979 Jan;76(1):323–327. doi: 10.1073/pnas.76.1.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harkness E. R. Studies on human placental alkaline phosphatase. I. Purification and crystallization. Arch Biochem Biophys. 1968 Aug;126(2):503–512. doi: 10.1016/0003-9861(68)90435-9. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Ludueña M. A., Sussman H. H. Characterization of KB cell alkaline phosphatase. Evidence of similarity to placental alkaline phosphatase. J Biol Chem. 1976 May 10;251(9):2620–2628. [PubMed] [Google Scholar]
- McKenna M. J., Hamilton T. A., Sussman H. H. Comparison of human alkaline phosphatase isoenzymes. Structural evidence for three protein classes. Biochem J. 1979 Jul 1;181(1):67–73. doi: 10.1042/bj1810067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milstein C. The amino acid sequence around the reactive serine residue in alkaline phosphatase from Escherichia coli. Biochem J. 1964 Aug;92(2):410–421. doi: 10.1042/bj0920410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulivor R. A., Plotkin L. I., Harris H. Differential inhibition of the products of the human alkaline phosphatase loci. Ann Hum Genet. 1978 Jul;42(1):1–13. doi: 10.1111/j.1469-1809.1978.tb00927.x. [DOI] [PubMed] [Google Scholar]
- Placental proteins and their subunits as tumor markers. Ann Intern Med. 1975 Jan;82(1):71–83. doi: 10.7326/0003-4819-82-1-71. [DOI] [PubMed] [Google Scholar]
- Redman C. M., Sabatini D. D. Vectorial discharge of peptides released by puromycin from attached ribosomes. Proc Natl Acad Sci U S A. 1966 Aug;56(2):608–615. doi: 10.1073/pnas.56.2.608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakiyama T., Robinson J. C., Chou J. Y. Characterization of alkaline phosphatases from human first trimester placentas. J Biol Chem. 1979 Feb 10;254(3):935–938. [PubMed] [Google Scholar]
- Sakiyama T., Robinson J. C., Chou J. Y. Multiple forms of alkaline phosphatase in untreated and 5-bromo-2'-deoxyuridine-treated choriocarcinoma cells. Arch Biochem Biophys. 1978 Dec;191(2):782–791. doi: 10.1016/0003-9861(78)90420-4. [DOI] [PubMed] [Google Scholar]
- Sasaki M., Fishman W. H. Ultrastructural studies on Regan and non-Regan isoenzymes of alkaline phosphatase in human ovarian cancer cells. Cancer Res. 1973 Nov;33(11):3008–3018. [PubMed] [Google Scholar]
- Singer R. M., Fishman W. H. Characterization of two HeLa sublines: TCRC-1 produces Regan isoenzyme and TCRC-2, non-Regan isoenzyme. J Cell Biol. 1974 Mar;60(3):777–780. doi: 10.1083/jcb.60.3.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speeg K. V., Jr, Azizkhan J. C., Stromberg K. Characteristics of alkaline phosphatase from two continuous lines of human choriocarcinoma cells. Exp Cell Res. 1977 Mar 1;105(1):199–205. doi: 10.1016/0014-4827(77)90166-5. [DOI] [PubMed] [Google Scholar]
- Stolbach L. L., Krant M. J., Fishman W. H. Ectopic production of an alkaline phosphatase isoenzyme in patients with cancer. N Engl J Med. 1969 Oct 2;281(14):757–762. doi: 10.1056/NEJM196910022811403. [DOI] [PubMed] [Google Scholar]
- Sussman H. H., Bowman M., Lewis J. L., Jr Placental alkaline phosphatase in maternal serum during normal and abnormal pregnancy. Nature. 1968 Apr 27;218(5139):359–360. doi: 10.1038/218359a0. [DOI] [PubMed] [Google Scholar]
- Sussman H. H., Gottlieb A. J. Human placental alkaline phosphatase. II. Molecular and subunit properties of the enzyme. Biochim Biophys Acta. 1969 Nov 11;194(1):170–179. doi: 10.1016/0005-2795(69)90192-5. [DOI] [PubMed] [Google Scholar]
- Sussman H. H., Small P. A., Jr, Cotlove E. Human alkaline phosphatase. Immunochemical identification of organ-specific isoenzymes. J Biol Chem. 1968 Jan 10;243(1):160–166. [PubMed] [Google Scholar]


