Abstract
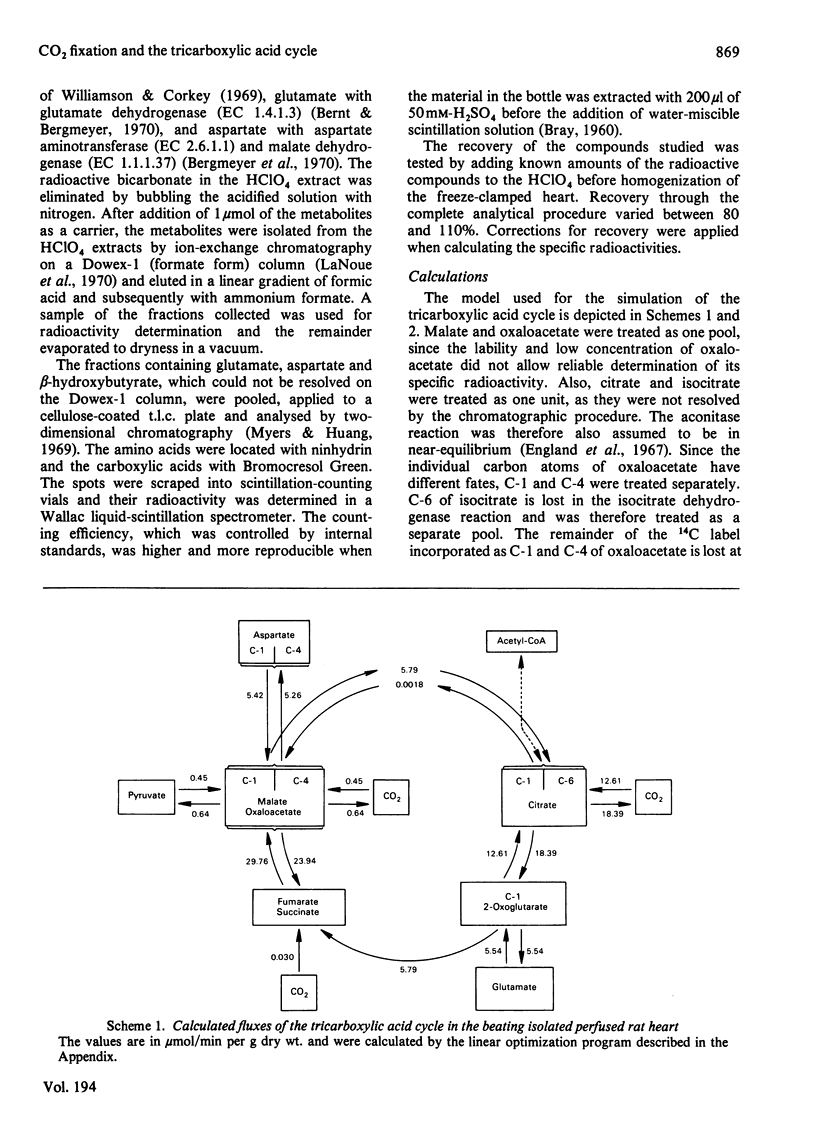
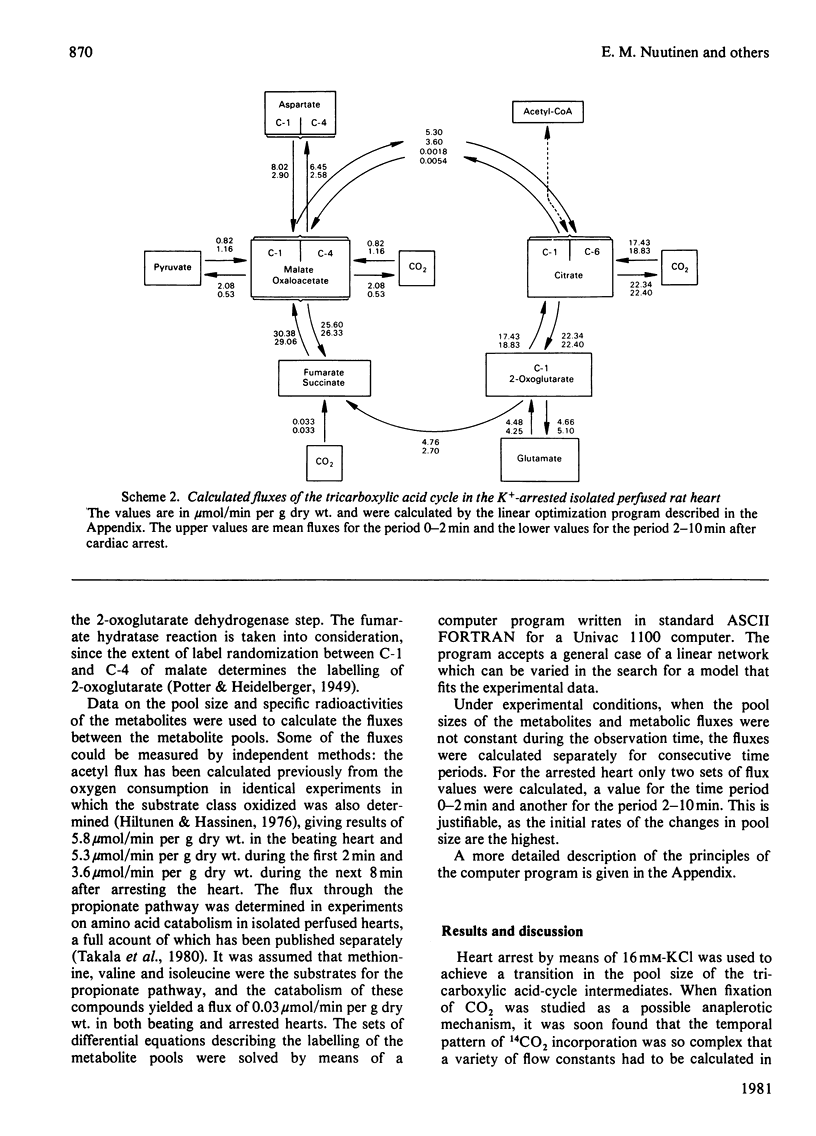
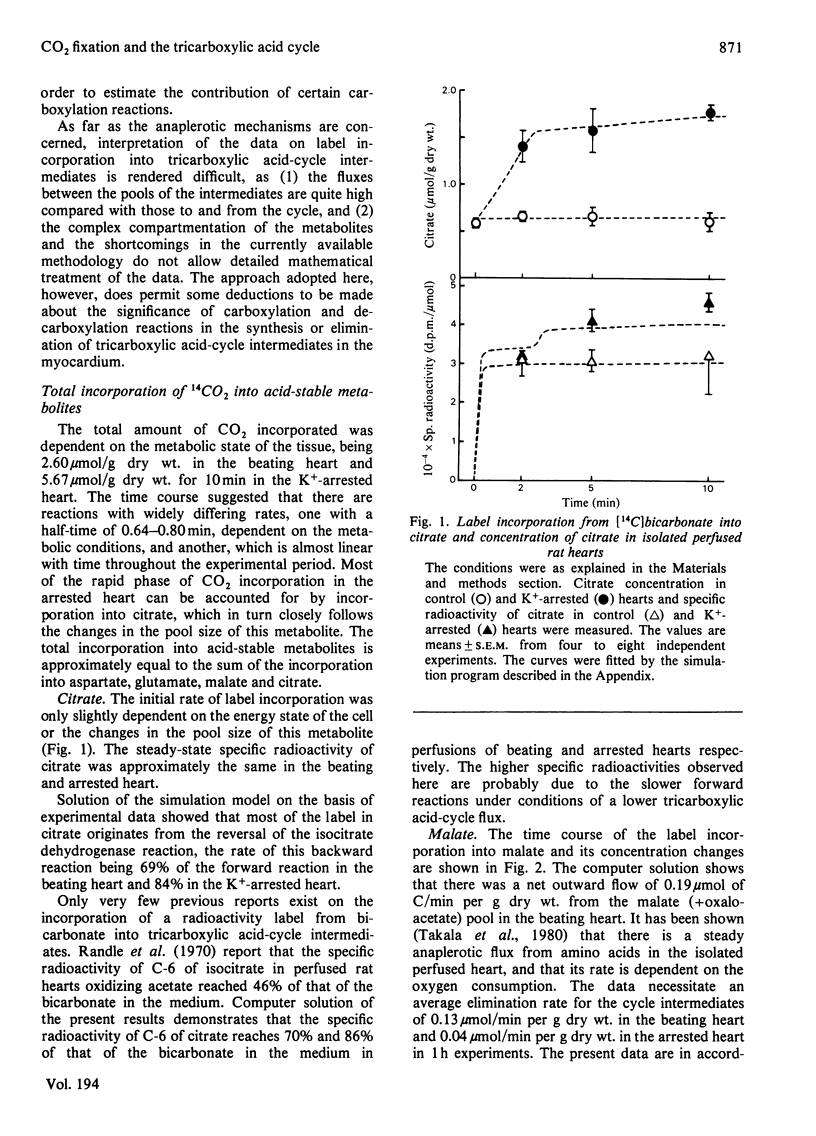
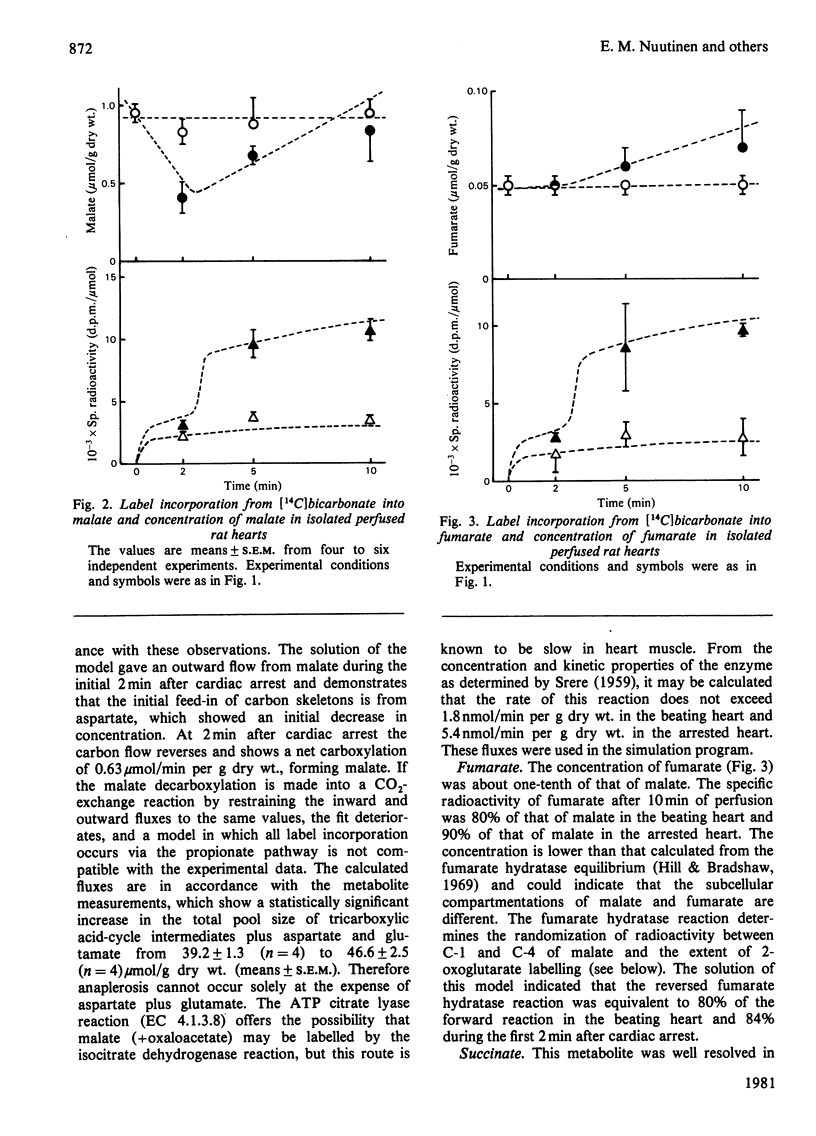
1. The contribution of Co2 fixation to the anaplerotic mechanisms in the myocardium was investigated in isolated perfused rat hearts. 2. K+-induced arrest of the heart was used to elicit a transition in the concentrations of the intermediates of the tricarboxylic acid cycle. 3. Incorporation of 14C from [14]bicarbonate into tricarboxylic acid-cycle intermediates was measured and the rates of the reactions of the cycle were estimated by means of a linear optimization program which solves the differential equations describing a simulation model of the tricarboxylic acid cycle and related reactions. 4. The results showed that the rate of CO2 fixation is dependent on the metabolic state of the myocardium. Upon a sudden diminution of cellular ATP consumption, the pool size of the tricarboxylic acid-cycle metabolites increased and the rate of label incorporation from [14C]bicarbonate into the cycle metabolites increased simultaneously. The computer model was necessary to separate the rapid equilibration between bicarbonate and some metabolites from the potentially anaplerotic reactions. The main route of anaplerosis during metabolite accumulation was through malate + oxaloacetate. Under steady-state conditions there was a constant net outward flow from the tricarboxylic acid cycle via the malate + oxaloacetate pool, with a concomitant anaplerotic flow from metabolites forming succinyl-CoA (3-carboxypropionyl-CoA).
Full text
PDF

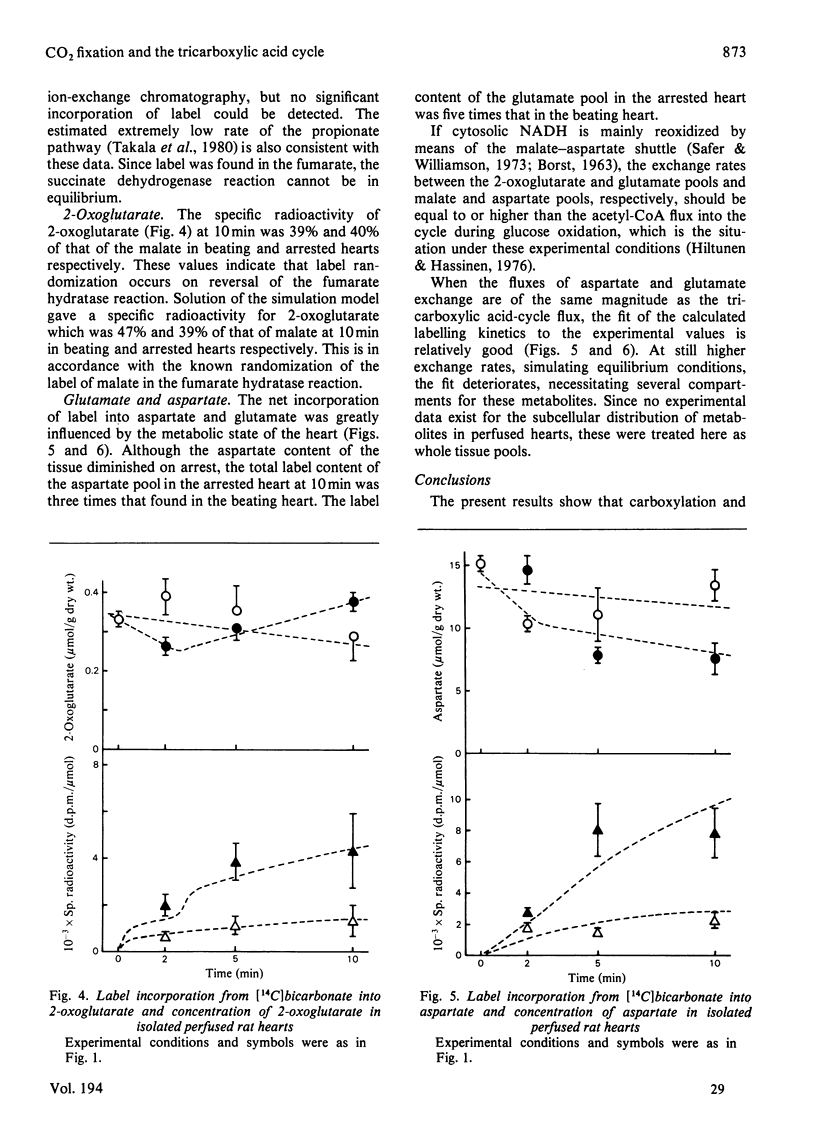
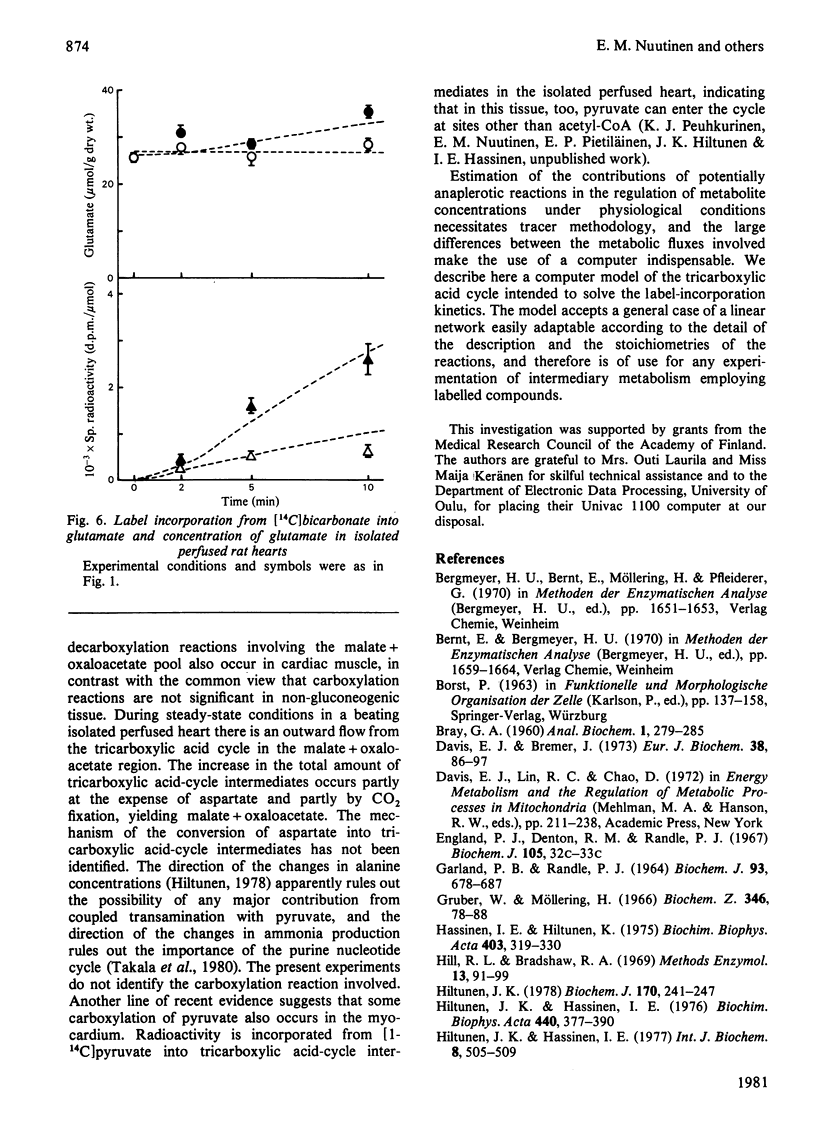

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Davis E. J., Bremer J. Studies with isolated surviving rat hearts. Interdependence of free amino acids and citric-acid-cycle intermediates. Eur J Biochem. 1973 Sep 21;38(1):86–97. doi: 10.1111/j.1432-1033.1973.tb03037.x. [DOI] [PubMed] [Google Scholar]
- Garland P. B., Randle P. J. Regulation of glucose uptake by muscles. 10. Effects of alloxan-diabetes, starvation, hypophysectomy and adrenalectomy, and of fatty acids, ketone bodies and pyruvate, on the glycerol output and concentrations of free fatty acids, long-chain fatty acyl-coenzyme A, glycerol phosphate and citrate-cycle intermediates in rat heart and diaphragm muscles. Biochem J. 1964 Dec;93(3):678–687. doi: 10.1042/bj0930678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassinen I. E., Hiltunen K. Respiratory control in isolated perfused rat heart. Role of the equilibrium relations between the mitochondrial electron carriers and the adenylate system. Biochim Biophys Acta. 1975 Dec 11;408(3):319–330. doi: 10.1016/0005-2728(75)90133-4. [DOI] [PubMed] [Google Scholar]
- Hiltunen J. K., Hassinen I. E. Energy-linked regulation of glucose and pyruvate oxidation in isolated perfused rat heart. Role of pyruvate dehydrogenase. Biochim Biophys Acta. 1976 Aug 13;440(2):377–390. doi: 10.1016/0005-2728(76)90072-4. [DOI] [PubMed] [Google Scholar]
- Hiltunen J. K., Jauhonen V. P., Savolainen M. J., Hassinen I. E. Effects of pent-4-enoate on cellular redox state, glycolysis and fatty acid oxidation in isolated perfused rat heart. Biochem J. 1978 Feb 15;170(2):235–240. doi: 10.1042/bj1700235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiltunen J. K., Kauppinen R. A., Nuutinen E. M., Peuhkurinen K. J., Hassinen I. E. Isolated rat heart mitochondria are able to metabolize pent-4-enoate to tricarboxylic acid-cycle intermediates. Biochem J. 1980 Jun 15;188(3):725–729. doi: 10.1042/bj1880725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiltunen J. K. Metabolic effects of pent-4-enoate in isolated perfused rat heart. Biochem J. 1978 Feb 15;170(2):241–247. doi: 10.1042/bj1700241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirklin J. W., Conti V. R., Blackstone E. H. Prevention of myocardial damage during cardiac operations. N Engl J Med. 1979 Jul 19;301(3):135–141. doi: 10.1056/NEJM197907193010305. [DOI] [PubMed] [Google Scholar]
- LaNoue K., Nicklas W. J., Williamson J. R. Control of citric acid cycle activity in rat heart mitochondria. J Biol Chem. 1970 Jan 10;245(1):102–111. [PubMed] [Google Scholar]
- PETTE D., KLINGENBERG M., BUECHER T. Comparable and specific proportions in the mitochondrial enzyme activity pattern. Biochem Biophys Res Commun. 1962 Jun 4;7:425–429. doi: 10.1016/0006-291x(62)90328-5. [DOI] [PubMed] [Google Scholar]
- Randle P. J., England P. J., Denton R. M. Control of the tricarboxylate cycle and its interactions with glycolysis during acetate utilization in rat heart. Biochem J. 1970 May;117(4):677–695. doi: 10.1042/bj1170677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SRERE P. A. The citrate cleavage enzyme. I. Distribution and purification. J Biol Chem. 1959 Oct;234:2544–2547. [PubMed] [Google Scholar]
- Safer B., Williamson J. R. Mitochondrial-cytosolic interactions in perfused rat heart. Role of coupled transamination in repletion of citric acid cycle intermediates. J Biol Chem. 1973 Apr 10;248(7):2570–2579. [PubMed] [Google Scholar]
- Spydevold S., Davis E. J., Bremer J. Replenishment and depletion of citric acid cycle intermediates in skeletal muscle. Indication of pyruvate carboxylation. Eur J Biochem. 1976 Dec;71(1):155–165. doi: 10.1111/j.1432-1033.1976.tb11101.x. [DOI] [PubMed] [Google Scholar]
- Takala T., Hiltunen J. K., Hassinen I. E. The mechanism of ammonia production and the effect of mechanical work load on proteolysis and amino acid catabolism in isolated perfused rat heart. Biochem J. 1980 Oct 15;192(1):285–295. doi: 10.1042/bj1920285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WOLLENBERGER A., RISTAU O., SCHOFFA G. [A simple technic for extremely rapid freezing of large pieces of tissue]. Pflugers Arch Gesamte Physiol Menschen Tiere. 1960;270:399–412. [PubMed] [Google Scholar]


