Abstract
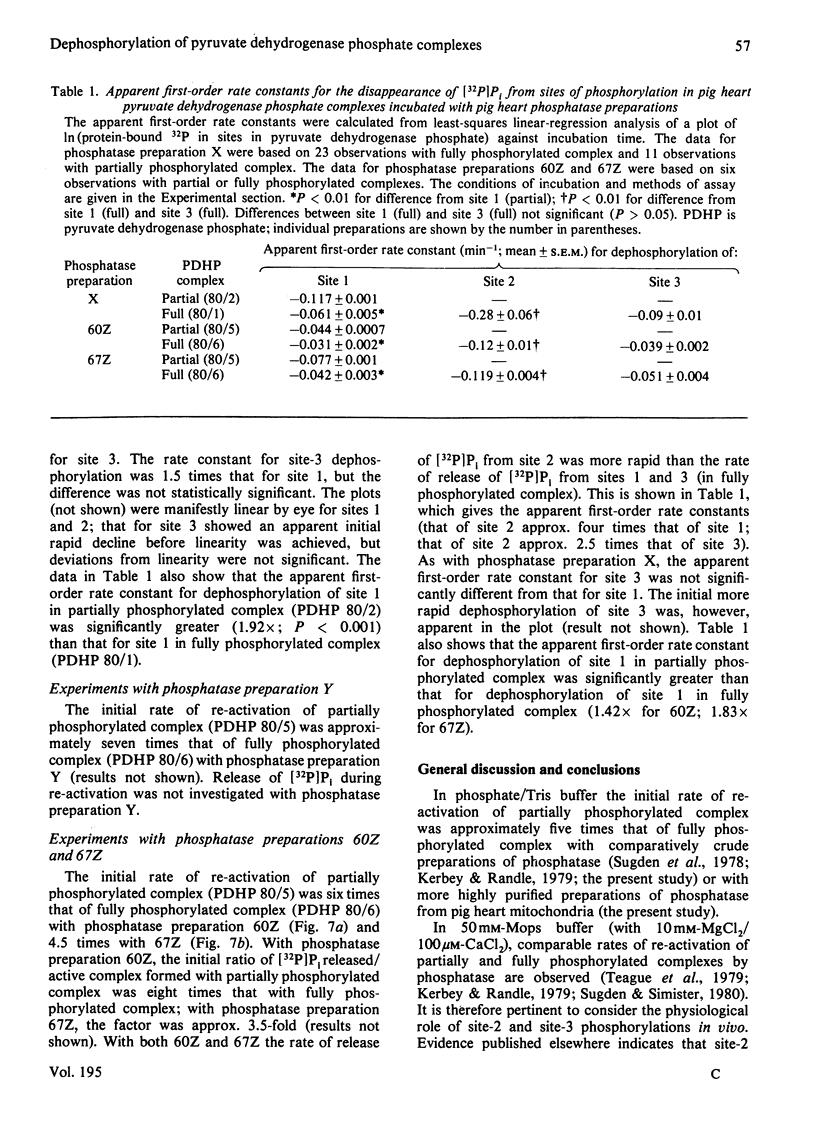
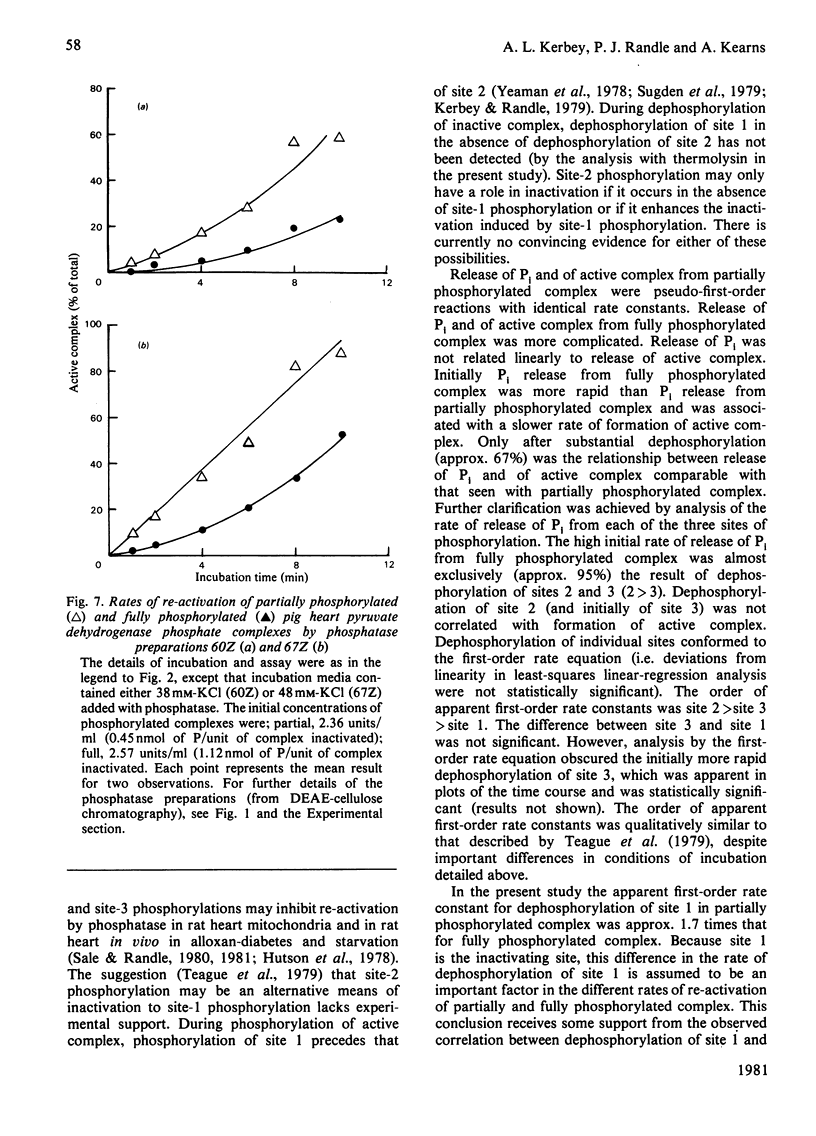
1. Pig heart pyruvate dehydrogenase phosphate complex in which all three sites of phosphorylation were completely phosphorylated was re-activated at a slower rate by phosphatase than complex predominantly phosphorylated in site 1. The ratio of initial rates of re-activation was approx. 1:5 with a comparatively crude preparation of phosphatase and with phosphatase purified by gel filtration and ion-exchange chromatography. 2. The ratio of apparent first-order rate constants during dephosphorylation of fully phosphorylated complex averaged 1/3.8/1.3 for site 1/site 2/site 3. Only site-1 dephosphorylation was linearly correlated with re-activation of the complex throughout dephosphorylation. Dephosphorylation of site 3 was linearly correlated with re-activation after an initial burst of dephosphorylation. 3. Because dephosphorylation of site 1 was always associated with dephosphorylation of site 2, it is concluded that dephosphorylation cannot be purely random. 4. The ratio of apparent first-order rate constants for dephosphorylation of site 1 (partially/fully phosphorylated complexes) averaged 1.72. This ratio is smaller than the ratio of approx. 5 for the initial rates of re-activation. Possible mechanisms involved in the diminished rate of re-activation of fully phosphorylated complex are discussed.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Butler J. R., Pettit R. H., Davis P. F., Reed L. J. Binding of thiamin thiazolone pyrophosphate to mammalian pyruvate dehydrogenase and its effects of kinase and phosphatase activities. Biochem Biophys Res Commun. 1977 Feb 21;74(4):1667–1674. doi: 10.1016/0006-291x(77)90636-2. [DOI] [PubMed] [Google Scholar]
- Hutson N. J., Kerbey A. L., Randle P. J., Sugden P. H. Conversion of inactive (phosphorylated) pyruvate dehydrogenase complex into active complex by the phosphate reaction in heart mitochondria is inhibited by alloxan-diabetes or starvation in the rat. Biochem J. 1978 Aug 1;173(2):669–680. doi: 10.1042/bj1730669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerbey A. L., Radcliffe P. M., Randle P. J., Sugden P. H. Regulation of kinase reactions in pig heart pyruvate dehydrogenase complex. Biochem J. 1979 Aug 1;181(2):427–433. doi: 10.1042/bj1810427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerbey A. L., Randle P. J., Cooper R. H., Whitehouse S., Pask H. T., Denton R. M. Regulation of pyruvate dehydrogenase in rat heart. Mechanism of regulation of proportions of dephosphorylated and phosphorylated enzyme by oxidation of fatty acids and ketone bodies and of effects of diabetes: role of coenzyme A, acetyl-coenzyme A and reduced and oxidized nicotinamide-adenine dinucleotide. Biochem J. 1976 Feb 15;154(2):327–348. doi: 10.1042/bj1540327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerbey A. L., Randle P. J. Role of multi-site phosphorylation in regulation of pig heart pyruvate dehydrogenase phosphatase. FEBS Lett. 1979 Dec 15;108(2):485–488. doi: 10.1016/0014-5793(79)80594-3. [DOI] [PubMed] [Google Scholar]
- Linn T. C., Pettit F. H., Reed L. J. Alpha-keto acid dehydrogenase complexes. X. Regulation of the activity of the pyruvate dehydrogenase complex from beef kidney mitochondria by phosphorylation and dephosphorylation. Proc Natl Acad Sci U S A. 1969 Jan;62(1):234–241. doi: 10.1073/pnas.62.1.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radcliffe P. M., Kerbey A. L., Randle P. J. Inactivation of pig heart pyruvate dehydrogenase complex by adenosine-5'-O(3-thiotriphosphate). FEBS Lett. 1980 Feb 25;111(1):47–50. doi: 10.1016/0014-5793(80)80758-7. [DOI] [PubMed] [Google Scholar]
- Sale G. J., Randle P. J. Incorporation of [32P]phosphate into the pyruvate dehydrogenase complex in rat heart mitochondria. Biochem J. 1980 May 15;188(2):409–421. doi: 10.1042/bj1880409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sale G. J., Randle P. J. Occupancy of sites of phosphorylation in inactive rat heart pyruvate dehydrogenase phosphate in vivo. Biochem J. 1981 Mar 1;193(3):935–946. doi: 10.1042/bj1930935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Severson D. L., Denton R. M., Pask H. T., Randle P. J. Calcium and magnesium ions as effectors of adipose-tissue pyruvate dehydrogenase phosphate phosphatase. Biochem J. 1974 May;140(2):225–237. doi: 10.1042/bj1400225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siess E. A., Wieland O. H. Purification and characterization of pyruvate-dehydrogenase phosphatase from pig-heart muscle. Eur J Biochem. 1972 Mar 15;26(1):96–105. doi: 10.1111/j.1432-1033.1972.tb01744.x. [DOI] [PubMed] [Google Scholar]
- Stansbie D., Denton R. M., Bridges B. J., Pask H. T., Randle P. J. Regulation of pyruvate dehydrogenase and pyruvate dehydrogenase phosphate phosphatase activity in rat epididymal fat-pads. Effects of starvation, alloxan-diabetes and high-fat diet. Biochem J. 1976 Jan 15;154(1):225–236. doi: 10.1042/bj1540225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugden P. H., Hutson N. J., Kerbey A. L., Randle P. J. Phosphorylation of additional sites on pyruvate dehydrogenase inhibits its re-activation by pyruvate dehydrogenase phosphate phosphatase. Biochem J. 1978 Feb 1;169(2):433–435. doi: 10.1042/bj1690433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugden P. H., Kerbey A. L., Randle P. J., Waller C. A., Reid K. B. Amino acid sequences around the sites of phosphorylation in the pig heart pyruvate dehydrogenase complex. Biochem J. 1979 Aug 1;181(2):419–426. doi: 10.1042/bj1810419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugden P. H., Randle P. J. Regulation of pig heart pyruvate dehydrogenase by phosphorylation. Studies on the subunit and phosphorylation stoicheiometries. Biochem J. 1978 Aug 1;173(2):659–668. doi: 10.1042/bj1730659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugden P. H., Simister N. E. Role of multisite phosphorylation in the regulation of ox kidney pyruvate dehydrogenase complex. FEBS Lett. 1980 Mar 10;111(2):299–302. doi: 10.1016/0014-5793(80)80814-3. [DOI] [PubMed] [Google Scholar]
- Teague W. M., Pettit F. H., Yeaman S. J., Reed L. J. Function of phosphorylation sites on pyruvate dehydrogenase. Biochem Biophys Res Commun. 1979 Mar 15;87(1):244–252. doi: 10.1016/0006-291x(79)91672-3. [DOI] [PubMed] [Google Scholar]
- Yeaman S. J., Hutcheson E. T., Roche T. E., Pettit F. H., Brown J. R., Reed L. J., Watson D. C., Dixon G. H. Sites of phosphorylation on pyruvate dehydrogenase from bovine kidney and heart. Biochemistry. 1978 Jun 13;17(12):2364–2370. doi: 10.1021/bi00605a017. [DOI] [PubMed] [Google Scholar]


