Abstract
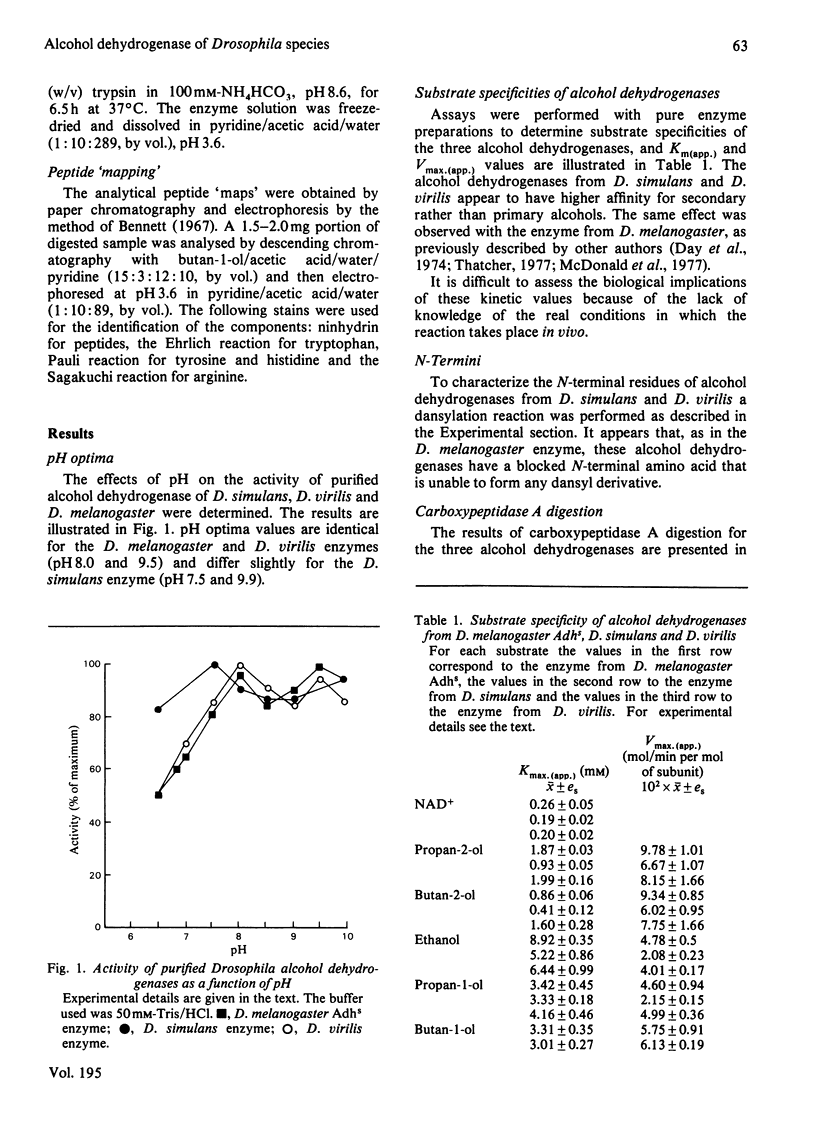
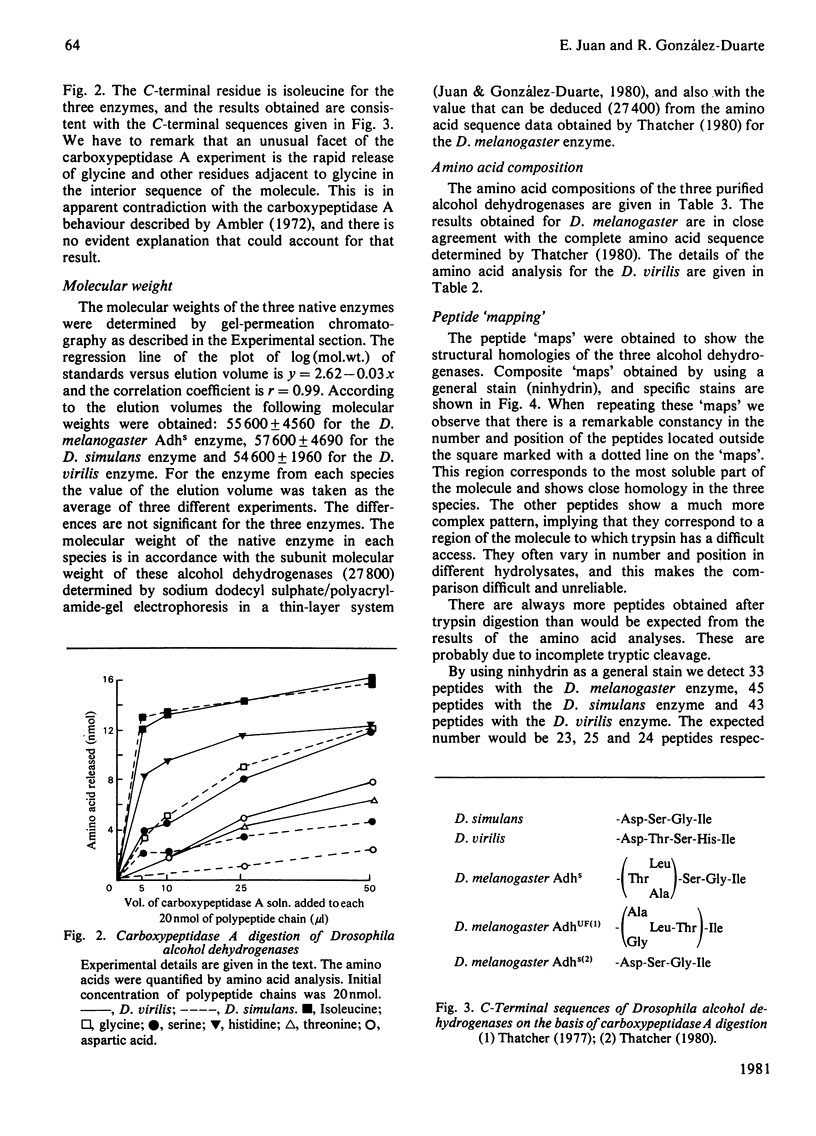
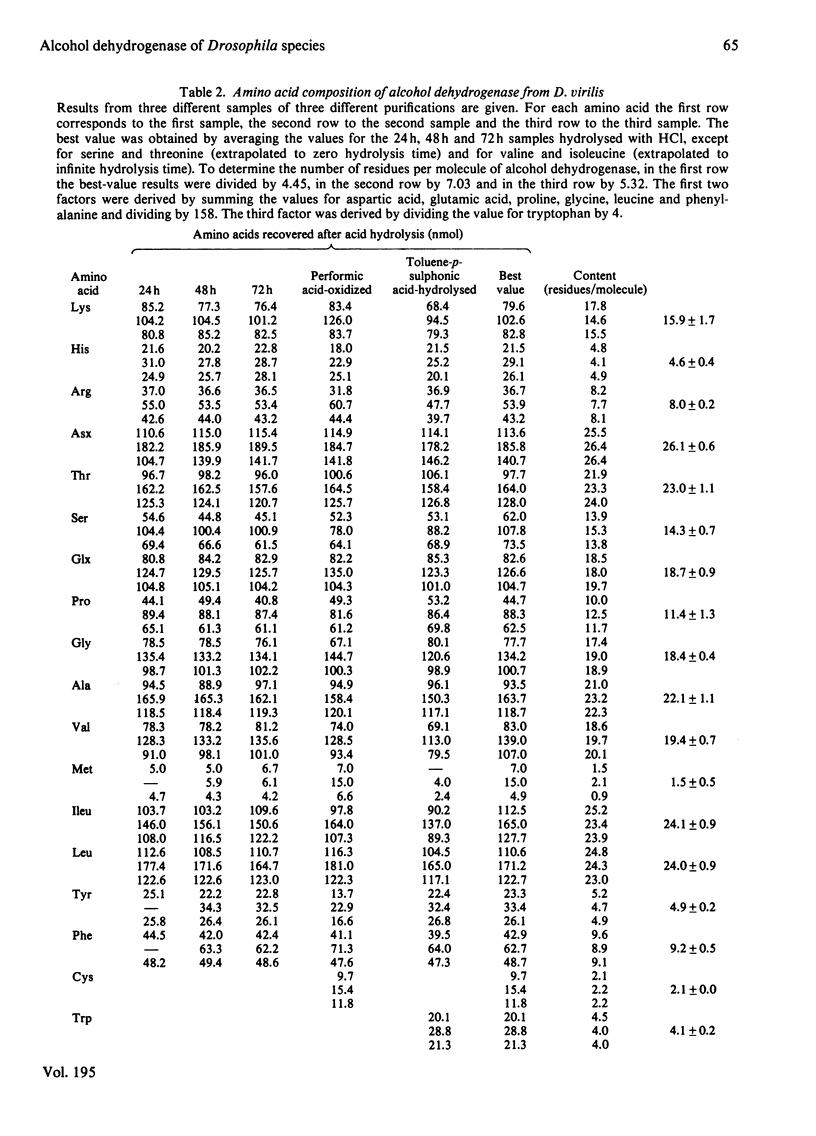
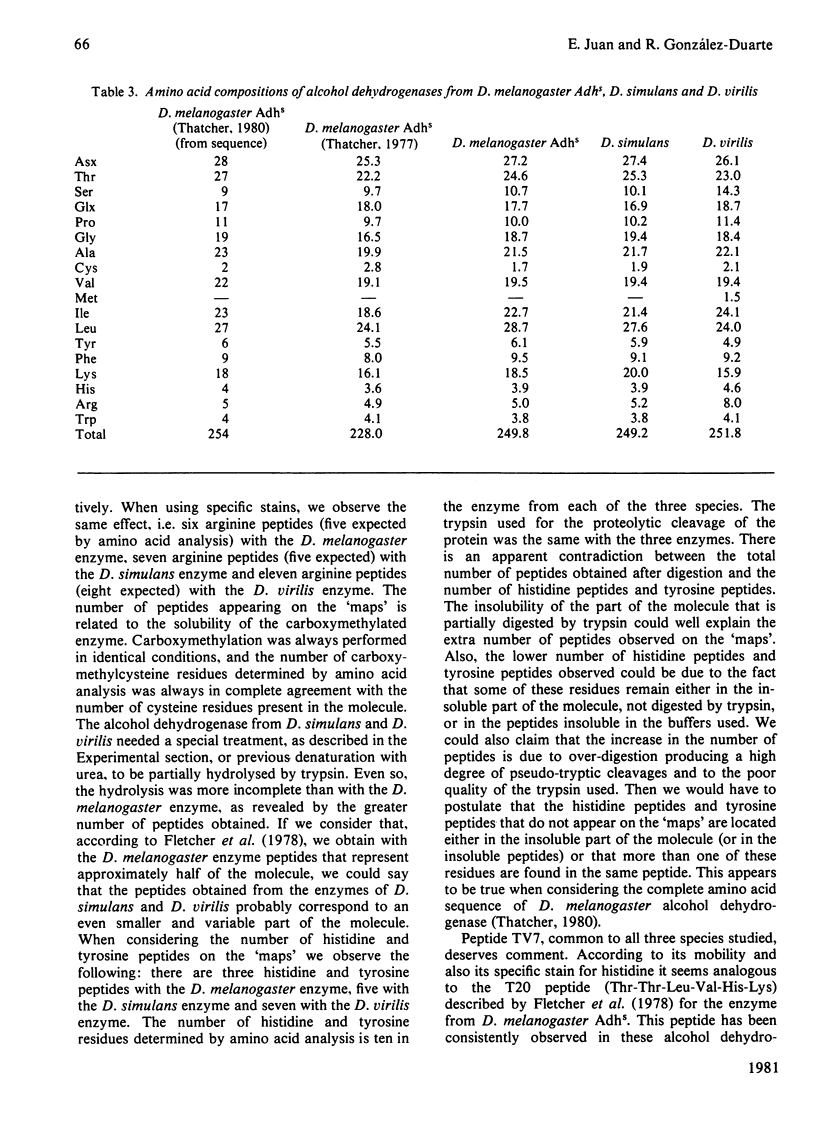
The biochemical properties of the enzyme alcohol dehydrogenase of two different Drosophila species, Drosophila simulans and Drosophila virilis, were studied and compared with those of Drosophila melanogaster Adhs enzyme. All of them consist of two identical subunits of molecular weight 27800 and share significant similarities in function. The substrate specificities of these enzymes were characterized and Km(app.) and Vmax.(app.) values were calculated. All these alcohol dehydrogenases show greater affinity for secondary rather than for primary alcohols. The amino acid compositions of the three enzymes were determined, and there is a close similarity between the D. simulans and the D. melanogaster enzymes, but there are significant differences from the alcohol dehydrogenase of D. virilis. The N-terminal amino acid is blocked and the C-terminal amino acid is the same for all three alcohol dehydrogenases. The enzymes from the three species were carboxymethylated and digested with trypsin. The peptide 'maps' reveal, as expected, more homologies between the enzymes of D. simulans and D. melanogaster than with the enzyme of D. virilis.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown A. J., Lee C. Y. Purification of alcohol dehydrogenase from Drosophila by general-ligand affinity chromatography. Biochem J. 1979 Jun 1;179(3):479–482. doi: 10.1042/bj1790479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CRESTFIELD A. M., MOORE S., STEIN W. H. The preparation and enzymatic hydrolysis of reduced and S-carboxymethylated proteins. J Biol Chem. 1963 Feb;238:622–627. [PubMed] [Google Scholar]
- Day T. H., Hillier P. C., Clarke B. The relative quantities and catalytic activities of enzymes produced by alleles at the alcohol dehydrogenase locus in Drosophila melanogaster. Biochem Genet. 1974 Feb;11(2):155–165. doi: 10.1007/BF00485771. [DOI] [PubMed] [Google Scholar]
- Eisenthal R., Cornish-Bowden A. The direct linear plot. A new graphical procedure for estimating enzyme kinetic parameters. Biochem J. 1974 Jun;139(3):715–720. doi: 10.1042/bj1390715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher T. S., Ayala F. J., Thatcher D. R., Chambers G. K. Structural analysis of the ADHS electromorph of Drosophila melanogaster. Proc Natl Acad Sci U S A. 1978 Nov;75(11):5609–5612. doi: 10.1073/pnas.75.11.5609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juan E., González-Duarte R. Purification and enzyme stability of alcohol dehydrogenase from Drosophila simulans, Drosophila virilis and Drosophila melanogaster adhS. Biochem J. 1980 Jul 1;189(1):105–110. doi: 10.1042/bj1890105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T. Y., Chang Y. H. Hydrolysis of proteins with p-toluenesulfonic acid. Determination of tryptophan. J Biol Chem. 1971 May 10;246(9):2842–2848. [PubMed] [Google Scholar]
- Marchalonis J. J. Conservatism in the evolution of immunoglobulin. Nat New Biol. 1972 Mar 22;236(64):84–86. doi: 10.1038/newbio236084a0. [DOI] [PubMed] [Google Scholar]
- McDonald J. F., Chambers G. K., David J., Ayala F. J. Adaptive response due to changes in gene regulation: a study with Drosophila. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4562–4566. doi: 10.1073/pnas.74.10.4562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons P. A. The comparative evolutionary biology of the sibling species, Drosophila melanogaster and D. simulans. Q Rev Biol. 1975 Jun;50(2):151–169. doi: 10.1086/408437. [DOI] [PubMed] [Google Scholar]
- Thatcher D. R. Enzyme instability and proteolysis during the purification of an alcohol dehydrogenase from Drosophila melanogaster. Biochem J. 1977 May 1;163(2):317–323. doi: 10.1042/bj1630317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thatcher D. R., Sawyer L. Secondary-structure prediction from the sequence of Drosophila melanogaster (fruitfly) alcohol dehydrogenase. Biochem J. 1980 Jun 1;187(3):884–886. doi: 10.1042/bj1870884. [DOI] [PMC free article] [PubMed] [Google Scholar]


