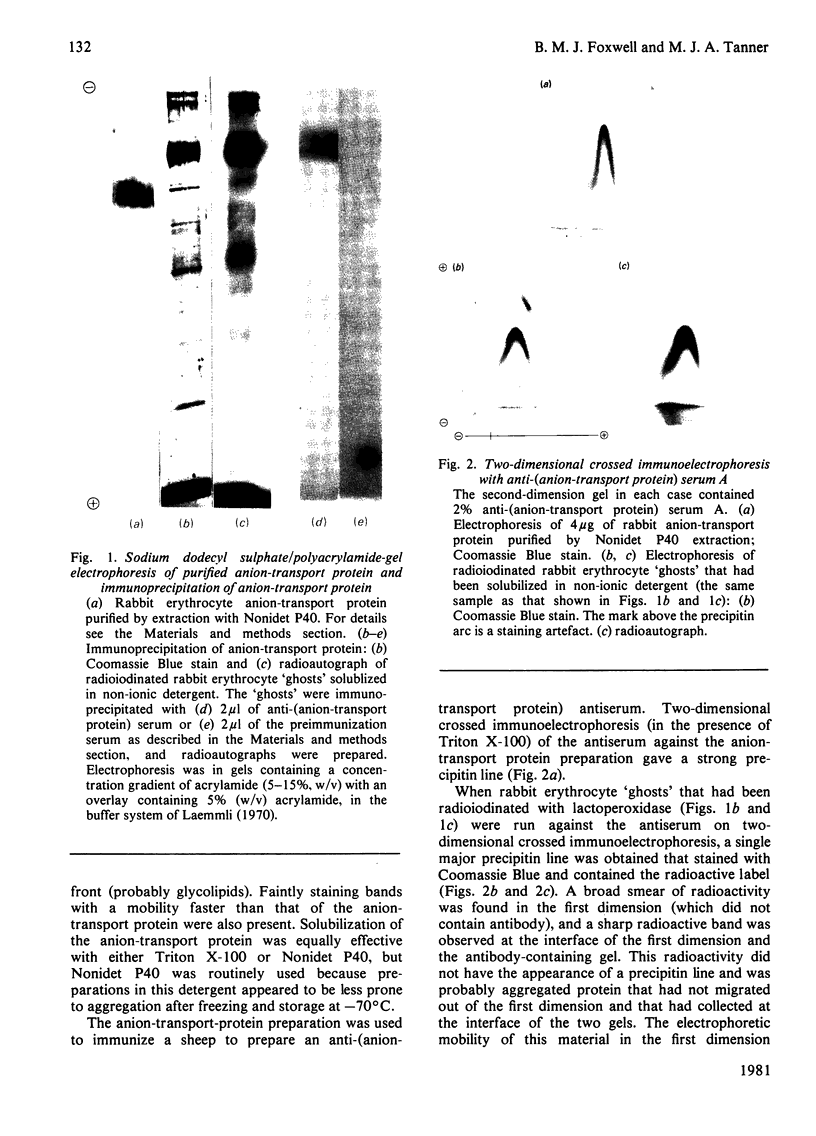
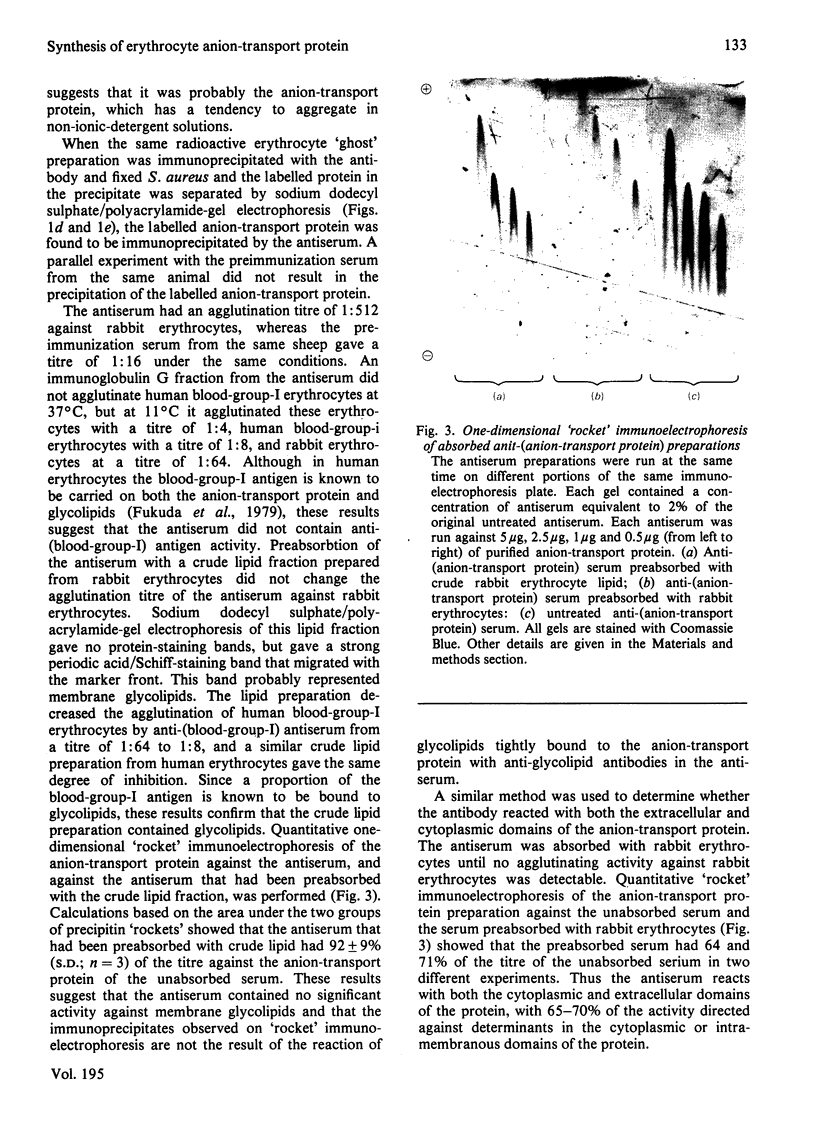
Abstract
We studied the surfaces of maturing rabbit bone-marrow erythroid cells for the presence of the erythrocyte anion-transport protein by using an immunochemical method. An antibody was raised against the purified anion-transport protein. The antibody was shown to react specifically with the anion-transport protein and it recognized determinants in the extracellular as well as the cytoplasmic or intramembranous domains of the protein. The binding of the antibody to the surface of intact rabbit bone-marrow erythroid cells was studied by using the Staphylococcus aureus 'rosette' technique described by Gahmberg, Jokinen & Andersson [(1978) Blood 52, 379-386]. Although pronormoblasts had little of the protein, there was a progressive increase in the amount of the protein at the surface of cells of increasing maturity up to the reticulocyte stage. Most of the protein is inserted into the plasma membrane between the polychromatic-normoblast and reticulocyte stage of the cell.
Full text
PDF


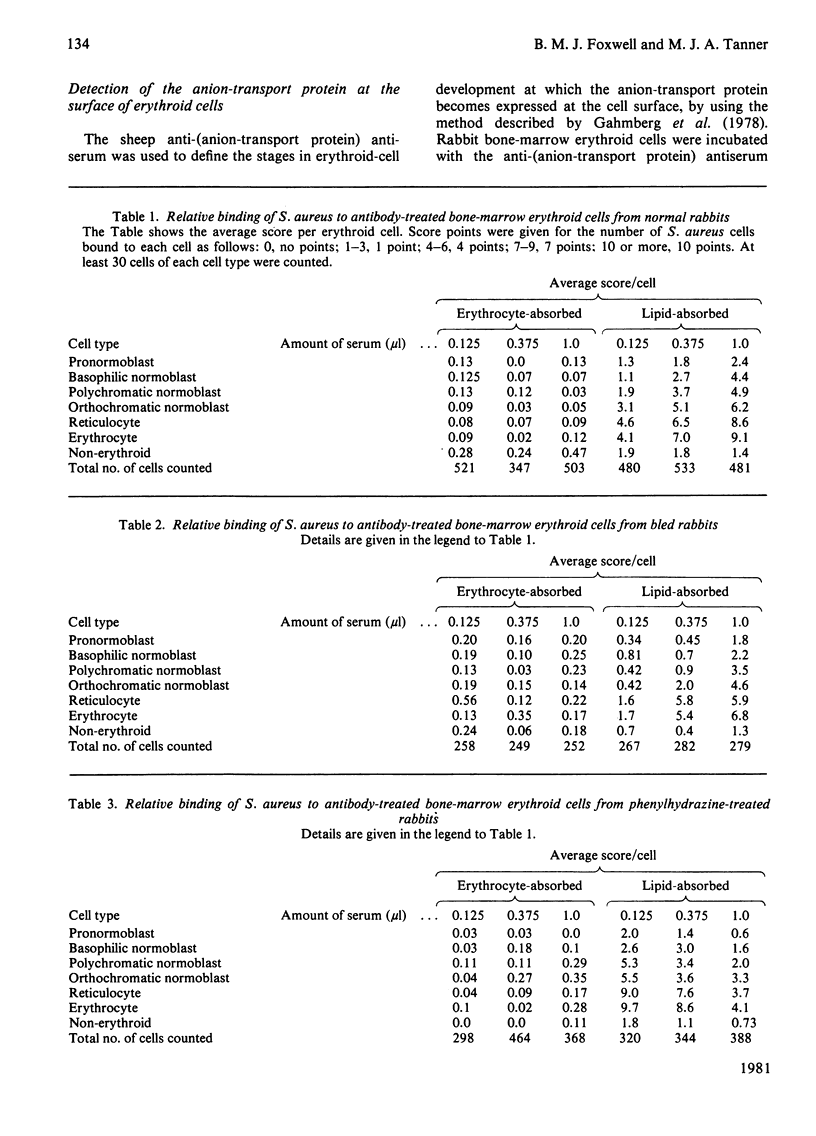
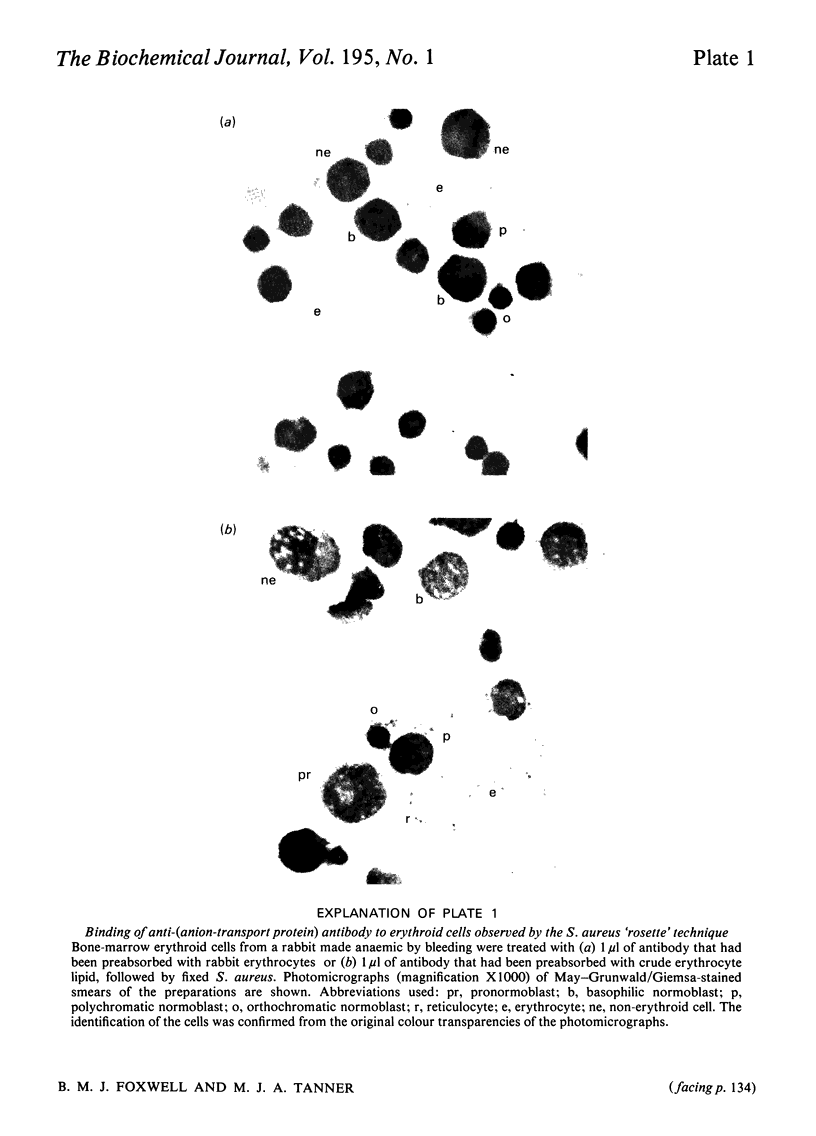
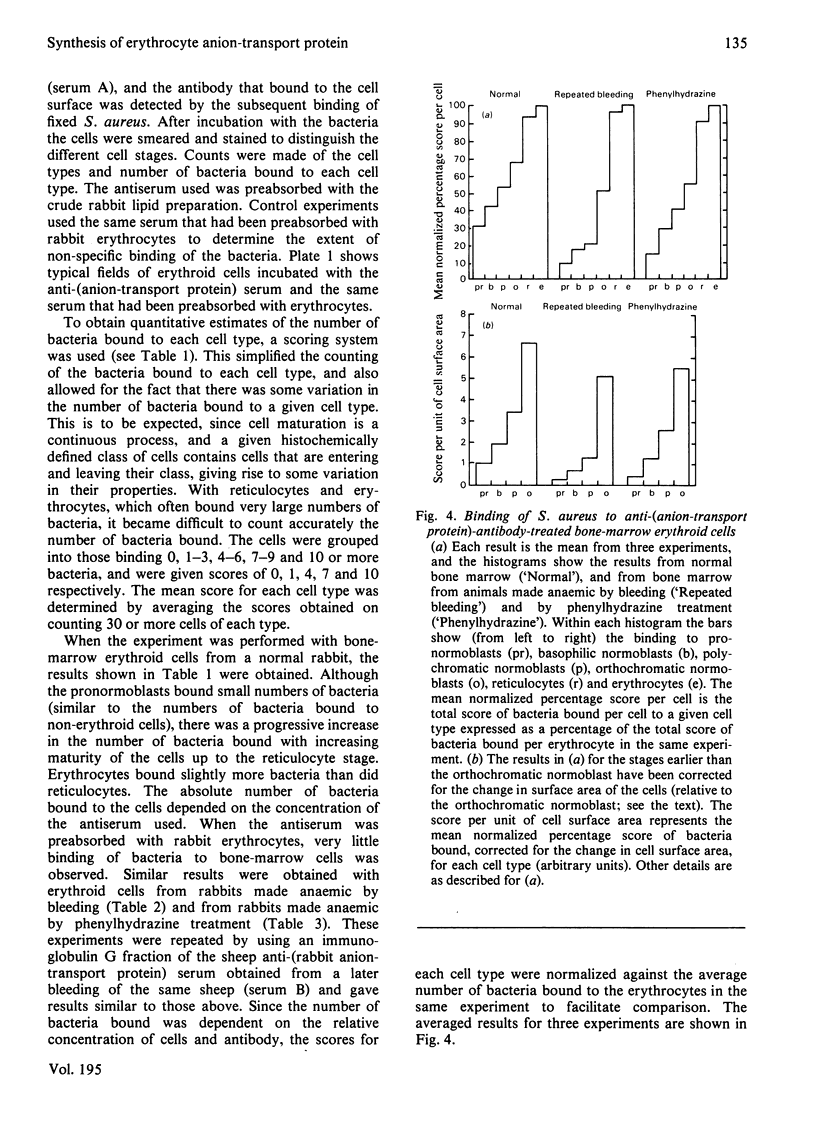


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BORSOOK H., LINGREL J. B., SCARO J. L., MILLETTE R. L. Synthesis of haemoglobin in relation to the maturation of erythroid cells. Nature. 1962 Oct 27;196:347–350. doi: 10.1038/196347a0. [DOI] [PubMed] [Google Scholar]
- BRECHER G., STOHLMAN F., Jr Reticulocyte size and erythropoietic stimulation. Proc Soc Exp Biol Med. 1961 Aug-Sep;107:887–891. doi: 10.3181/00379727-107-26785. [DOI] [PubMed] [Google Scholar]
- Bjerrum O. J. Immunochemical investigation of membrane proteins. A methodological survey with emphasis placed on immunoprecipitation in gels. Biochim Biophys Acta. 1977 Aug 9;472(2):135–195. doi: 10.1016/0304-4157(77)90016-8. [DOI] [PubMed] [Google Scholar]
- Boxer D. H., Jenkins R. E., Tanner M. J. The organization of the major protein of the human erythrocyte membrane. Biochem J. 1974 Mar;137(3):531–534. doi: 10.1042/bj1370531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabantchik Z. I., Knauf P. A., Rothstein A. The anion transport system of the red blood cell. The role of membrane protein evaluated by the use of 'probes'. Biochim Biophys Acta. 1978 Sep 29;515(3):239–302. doi: 10.1016/0304-4157(78)90016-3. [DOI] [PubMed] [Google Scholar]
- DODGE J. T., MITCHELL C., HANAHAN D. J. The preparation and chemical characteristics of hemoglobin-free ghosts of human erythrocytes. Arch Biochem Biophys. 1963 Jan;100:119–130. doi: 10.1016/0003-9861(63)90042-0. [DOI] [PubMed] [Google Scholar]
- Denton M. J., Arnstein H. R. Characterization of developing adult mammalian erythroid cells separated by velocity sedimentation. Br J Haematol. 1973 Jan;24(1):7–17. doi: 10.1111/j.1365-2141.1973.tb05722.x. [DOI] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Fukuda M., Eshdat Y., Tarone G., Marchesi V. T. Isolation and characterization of peptides derived from the cytoplasmic segment of band 3, the predominant intrinsic membrane protein of the human erythrocyte. J Biol Chem. 1978 Apr 10;253(7):2419–2428. [PubMed] [Google Scholar]
- Fukuda M., Fukuda M. N., Hakomori S. Developmental change and genetic defect in the carbohydrate structure of band 3 glycoprotein of human erythrocyte membrane. J Biol Chem. 1979 May 25;254(10):3700–3703. [PubMed] [Google Scholar]
- Fukuda M., Fukuda M. N., Papayannopoulou T., Hakomori S. Membrane differentiation in human erythroid cells: unique profiles of cell surface glycoproteins expressed in erythroblasts in vitro from three ontogenic stages. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3474–3478. doi: 10.1073/pnas.77.6.3474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gahmberg C. G., Jokinen M., Andersson L. C. Expression of the major sialoglycoprotein (glycophorin) on erythroid cells in human bone marrow. Blood. 1978 Aug;52(2):379–387. [PubMed] [Google Scholar]
- Gerritsen W. J., Verkley A. J., Zwaal R. F., Van Deenen L. L. Freeze-fracture appearance and disposition of band 3 protein from the human erythrocyte membrane in lipid vesicles. Eur J Biochem. 1978 Apr;85(1):255–261. doi: 10.1111/j.1432-1033.1978.tb12234.x. [DOI] [PubMed] [Google Scholar]
- Hillman R. S., Giblett E. R. Red cell membrane alteration associated with "marrow stress". J Clin Invest. 1965 Oct;44(10):1730–1736. doi: 10.1172/JCI105280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler S. W. Rapid isolation of antigens from cells with a staphylococcal protein A-antibody adsorbent: parameters of the interaction of antibody-antigen complexes with protein A. J Immunol. 1975 Dec;115(6):1617–1624. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Light N. D., Tanner M. J. Changes in surface-membrane components during the differentation of rabbit erythroid cells. Biochem J. 1977 Jun 15;164(3):565–578. doi: 10.1042/bj1640565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Light N. D., Tanner M. J. Erythrocyte membrane proteins. Sequential accumulation in the membrane during reticulocyte maturation. Biochim Biophys Acta. 1978 Apr 20;508(3):571–576. doi: 10.1016/0005-2736(78)90101-3. [DOI] [PubMed] [Google Scholar]
- Steck T. L. The organization of proteins in the human red blood cell membrane. A review. J Cell Biol. 1974 Jul;62(1):1–19. doi: 10.1083/jcb.62.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steck T. L., Yu J. Selective solubilization of proteins from red blood cell membranes by protein perturbants. J Supramol Struct. 1973;1(3):220–232. doi: 10.1002/jss.400010307. [DOI] [PubMed] [Google Scholar]
- Yataganas X., Gahrton G., Thorell B. DNA, RNA and hemoglobin during erythroblast maturation. A cytophotometric study. Exp Cell Res. 1970 Sep;62(1):254–261. doi: 10.1016/0014-4827(79)90526-3. [DOI] [PubMed] [Google Scholar]
- Yu J., Fischman D. A., Steck T. L. Selective solubilization of proteins and phospholipids from red blood cell membranes by nonionic detergents. J Supramol Struct. 1973;1(3):233–248. doi: 10.1002/jss.400010308. [DOI] [PubMed] [Google Scholar]