Abstract
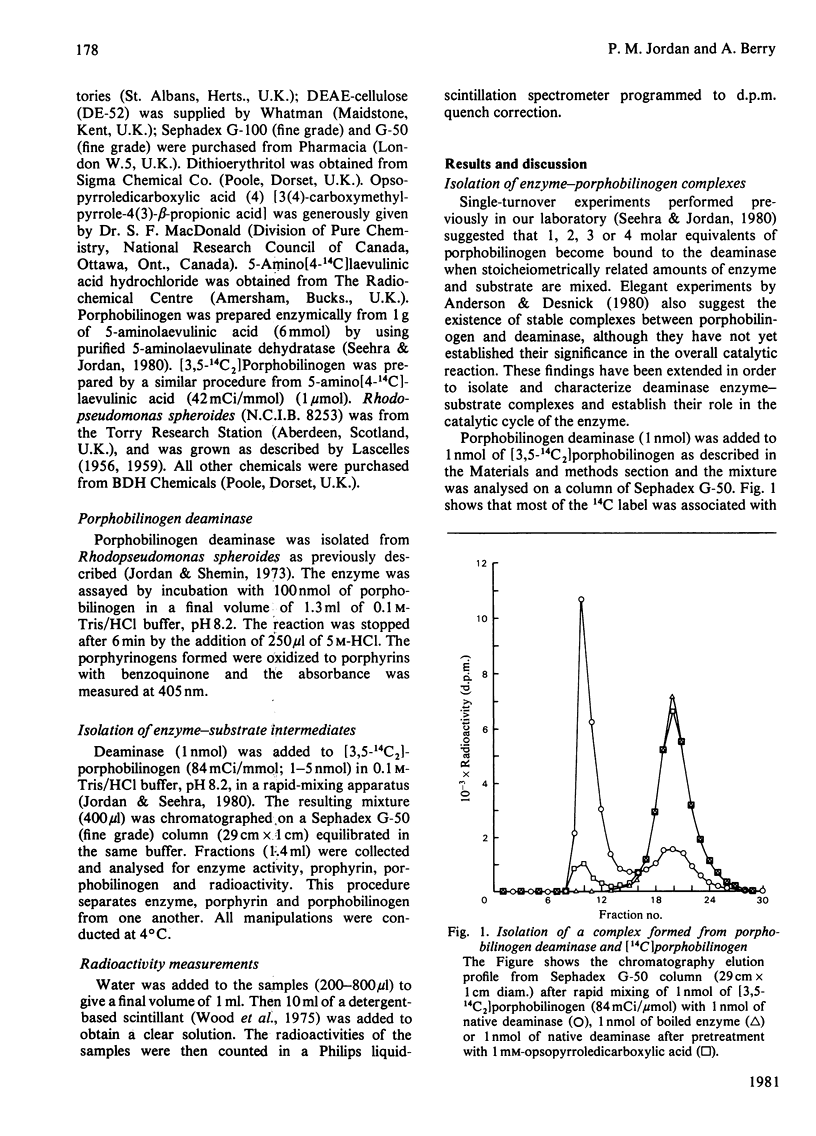
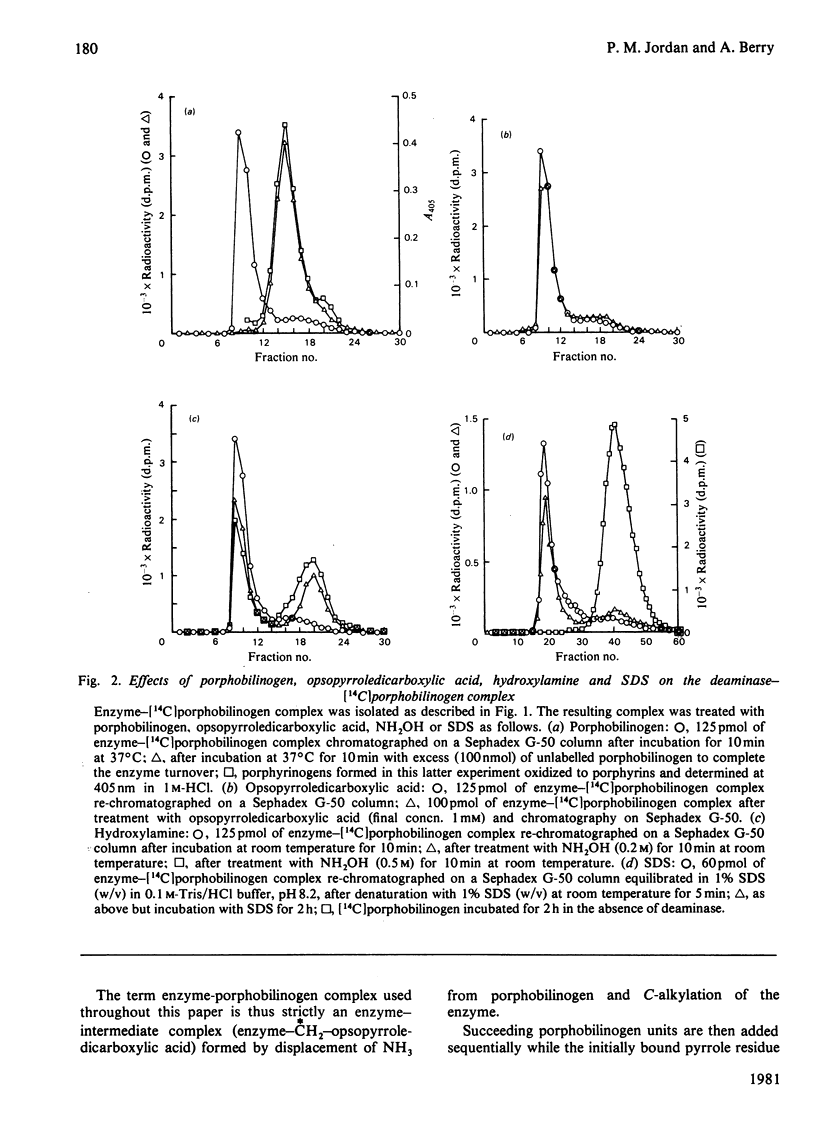
Highly stable labelled complexes are formed between porphobilinogen deaminase and stoicheiometric amounts of [14C]porphobilinogen. On completion of the catalytic cycle by the addition of excess of substrate, the complexes yield labelled product and display all the properties expected from covalently bound enzyme intermediates involved in the deaminase catalytic sequence.
Full text
PDF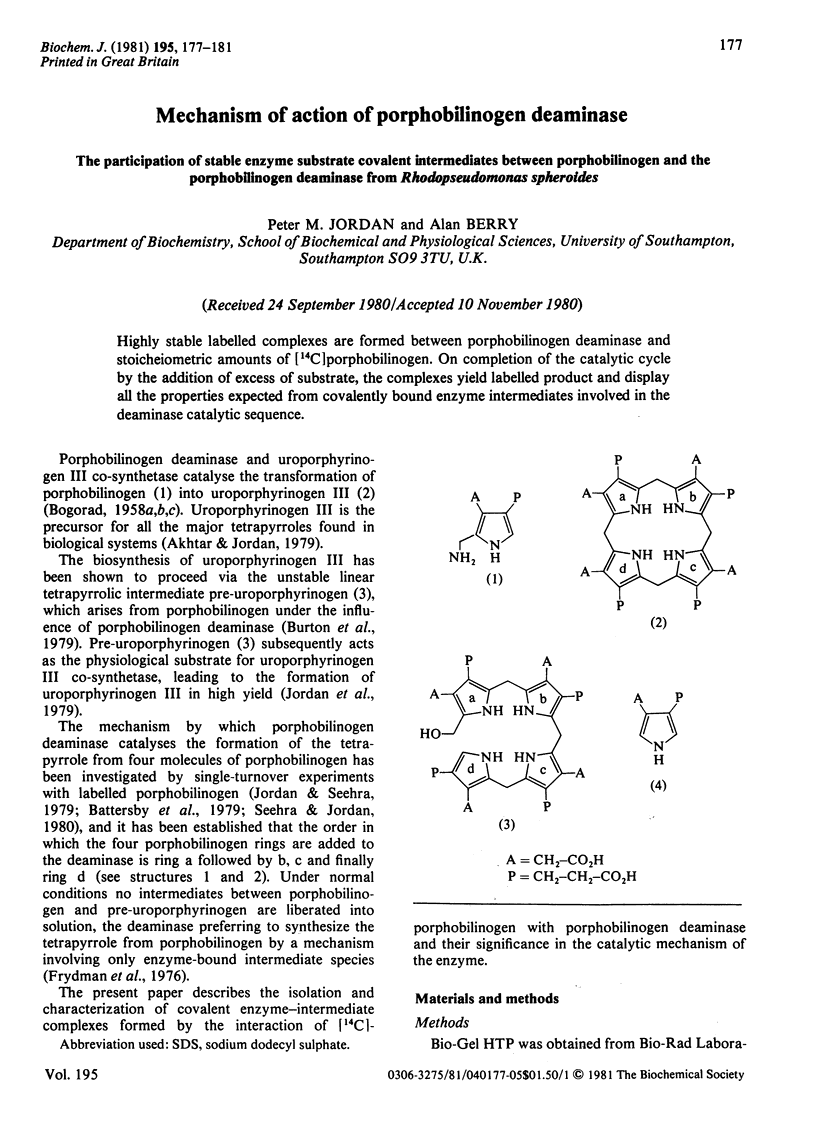


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson P. M., Desnick R. J. Purification and properties of uroporphyrinogen I synthase from human erythrocytes. Identification of stable enzyme-substrate intermediates. J Biol Chem. 1980 Mar 10;255(5):1993–1999. [PubMed] [Google Scholar]
- BOGORAD L. The enzymatic synthesis of porphyrins from porphobilinogen. I. Uroporphyrin I. J Biol Chem. 1958 Aug;233(2):501–509. [PubMed] [Google Scholar]
- BOGORAD L. The enzymatic synthesis of porphyrins from porphobilinogen. II. Uroporphyrin III. J Biol Chem. 1958 Aug;233(2):510–515. [PubMed] [Google Scholar]
- BOGORAD L. The enzymatic synthesis of porphyrins from porphobilinogen. III. Uroporphyrinogens as intermediates. J Biol Chem. 1958 Aug;233(2):516–519. [PubMed] [Google Scholar]
- Davies R. C., Neuberger A. Polypyrroles formed from porphobilinogen and amines by uroporphyrinogen synthetase of Rhodopseudomonas spheroides. Biochem J. 1973 Jul;133(3):471–492. doi: 10.1042/bj1330471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frydman B., Frydman R. B., Valasinas A., Levy E. S., Feinstein G. Biosynthesis of uroporphyrinogens from porphobilinogen: mechanism and the nature of the process. Philos Trans R Soc Lond B Biol Sci. 1976 Feb 5;273(924):137–160. doi: 10.1098/rstb.1976.0006. [DOI] [PubMed] [Google Scholar]
- Jordan P. M., Seehra J. S. 13C NMR as a probe for the study of enzyme-catalysed reactions: mechanism of action of 5-aminolevulinic acid dehydratase. FEBS Lett. 1980 Jun 2;114(2):283–286. doi: 10.1016/0014-5793(80)81134-3. [DOI] [PubMed] [Google Scholar]
- Jordan P. M., Seehra J. S. The biosynthesis of uroporphyrinogen III: order of assembly of the four porphobilinogen molecules in the formation of the tetrapyrrole ring. FEBS Lett. 1979 Aug 15;104(2):364–366. doi: 10.1016/0014-5793(79)80853-4. [DOI] [PubMed] [Google Scholar]
- Jordan P. M., Shemin D. Purification and properties of uroporphyrinogen I synthetase from Rhodopseudomonas spheroides. J Biol Chem. 1973 Feb 10;248(3):1019–1024. [PubMed] [Google Scholar]
- LASCELLES J. Adaptation to form bacteriochlorophyll in Rhodopseudomonas spheroides: changes in activity of enzymes concerned in pyrrole synthesis. Biochem J. 1959 Jul;72:508–518. doi: 10.1042/bj0720508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LASCELLES J. The synthesis of porphyrins and bacteriochlorophyll by cell suspensions of Rhodopseudomonas spheroides. Biochem J. 1956 Jan;62(1):78–93. doi: 10.1042/bj0620078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radmer R., Bogorad L. A tetrapyrrylmethane intermediate in the enzymatic synthesis of uroporphyrinogen. Biochemistry. 1972 Feb 29;11(5):904–910. doi: 10.1021/bi00755a033. [DOI] [PubMed] [Google Scholar]
- Wood P., English J., Chakraborty J., Hinton R. The use of non-ionic industrial detergents in liquid scintillation counting. Lab Pract. 1975 Nov;24(11):739–740. [PubMed] [Google Scholar]


