Abstract
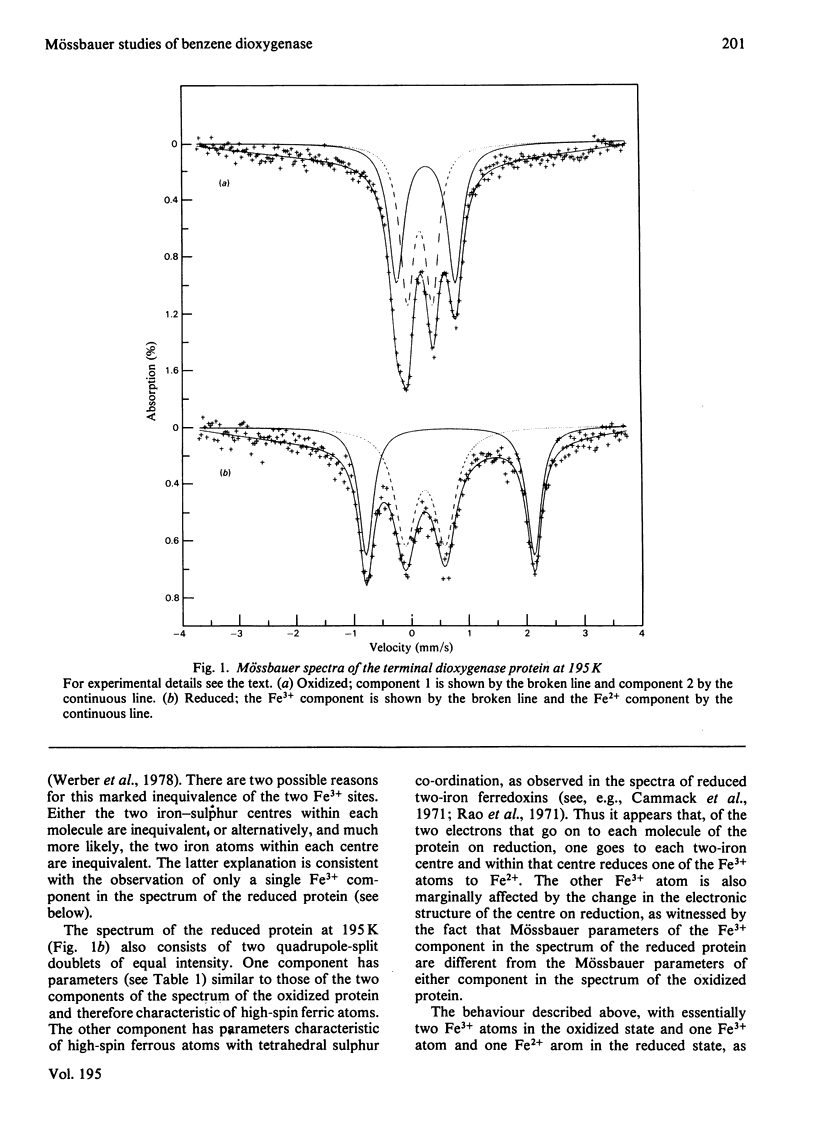
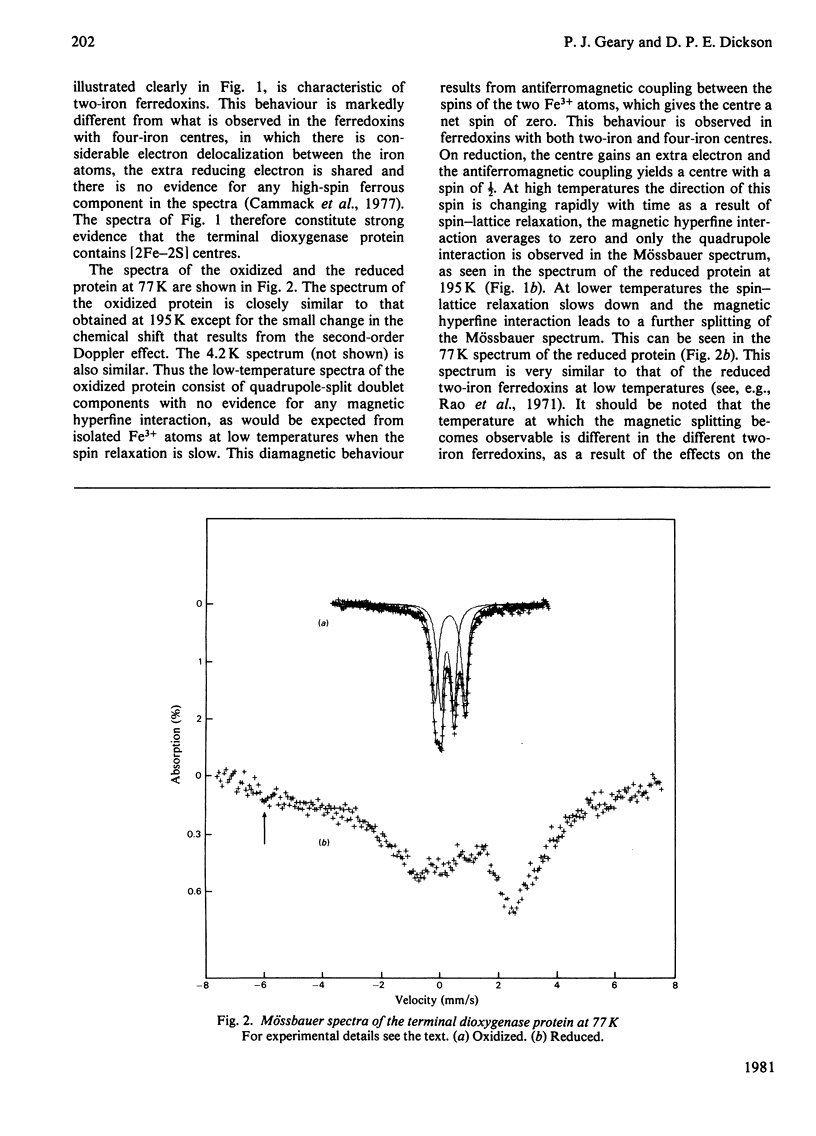
Mössbauer spectra obtained from the terminal dioxygenase protein of the benzene dioxygenase system from Pseudomonas putida show that it contains [2Fe--2S] centres similar to those of the two-iron plant-type ferredoxins. In the oxidized form the two iron atoms within the centre are high-spin ferric but with considerable inequivalence. In the reduced form the centre contains one extra electron, and this is localized on one of the iron atoms, which becomes high-spin ferrous.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Axcell B. C., Geary P. J. Purification and some properties of a soluble benzene-oxidizing system from a strain of Pseudomonas. Biochem J. 1975 Jan;146(1):173–183. doi: 10.1042/bj1460173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axcell B. C., Geary P. J. The metabolism of benzene by bacteria. Purification and some properties of the enzyme cis-1,2-dihydroxycyclohexa-3,5-diene (nicotinamide adenine dinucleotide) oxidoreductase (cis-benzene glycol dehydrogenase). Biochem J. 1973 Dec;136(4):927–934. doi: 10.1042/bj1360927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cammack R., Rao K. K., Hall D. O., Johnson C. E. Mössbauer studies of adrenodoxin. The mechanism of electron transfer in a hydroxylase iron-sulphur protein. Biochem J. 1971 Dec;125(3):849–856. doi: 10.1042/bj1250849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crutcher S. E., Geary P. J. Properties of the iron--sulphur proteins of the benzene dioxygenase system from Pseudomonas putida. Biochem J. 1979 Feb 1;177(2):393–400. doi: 10.1042/bj1770393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunham W. R., Bearden A. J., Salmeen I. T., Palmer G., Sands R. H., Orme-Johnson W. H., Beinert H. The two-iron ferredoxins in spinach, parsley, pig adrenal cortex, Azotobacter vinelandii, and Clostridium pasteurianum: studies by magnetic field Mössbauer spectroscopy. Biochim Biophys Acta. 1971 Nov 2;253(1):134–152. doi: 10.1016/0005-2728(71)90240-4. [DOI] [PubMed] [Google Scholar]
- Mullinger R. N., Cammack R., Rao K. K., Hall D. O., Dickson D. P., Johnson C. E., Rush J. D., Simopoulos A. Physicochemical characterization of the four-iron-four-sulphide ferredoxin from Bacillus stearothermophilus. Biochem J. 1975 Oct;151(1):75–83. doi: 10.1042/bj1510075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao K. K., Cammack R., Hall D. O., Johnson C. E. Mössbauer effect in Scenedesmus and spinach ferredoxins. The mechanism of electron transfer in plant-type iron-sulphur proteins. Biochem J. 1971 Apr;122(3):257–265. doi: 10.1042/bj1220257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werber M. M., Bauminger E. R., Cohen S. G., Ofer S. Ferredoxin from Halobacterium of the Dead Sea--Mössbauer and EPR spectra and comparison with Mössbauer spectrum of whole cells. Biophys Struct Mech. 1978 Apr 13;4(2):169–177. doi: 10.1007/BF00539230. [DOI] [PubMed] [Google Scholar]


