Abstract
A method is described for the rapid preparation of electrophoretically pure troponin C from rabbit skeletal-muscle myofibrils that avoids the use of urea. The three-step procedure includes extraction od the myofibrils with EDTA-containing buffers, one-step elution from DEAE-Sephadex and Sephadex G-100 chromatography in the presence of EDTA. The procedure gives yields comparable with those of currently used methods that involve dissociation of the troponin complex with urea. Except for the thiol-group reactivity, troponin C produced by our method is physicochemically and functionally indistinguishable from that obtained by the classical procedure. Purified troponin C always contains traces of calmodulin. However, this contamination can be decreased to less than 0.02% by means of a second Sephadex G-100 chromatography step in the presence of EDTA.
Full text
PDF



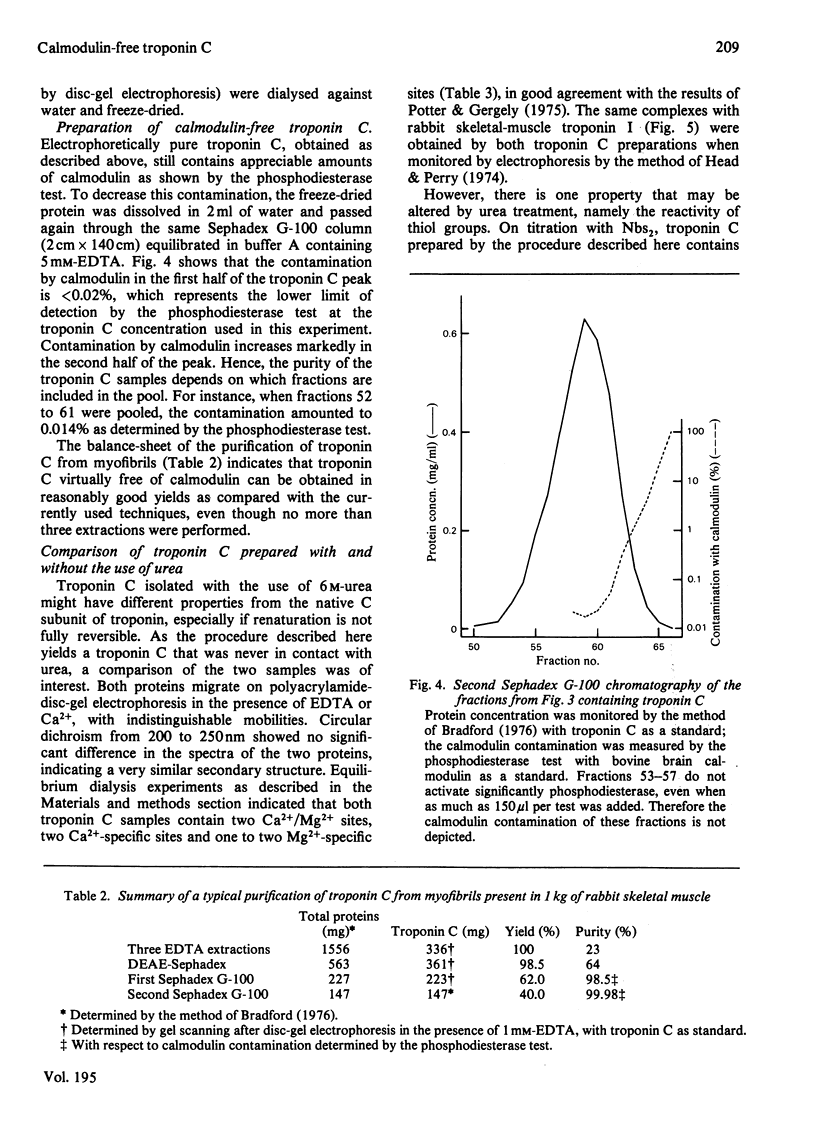

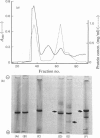
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amphlett G. W., Vanaman T. C., Perry S. V. Effect of the troponin C-like protein from bovine brain (brain modulator protein) on the Mg2+-stimulated ATPase of skeletal muscle actinomyosin. FEBS Lett. 1976 Dec 15;72(1):163–168. doi: 10.1016/0014-5793(76)80836-8. [DOI] [PubMed] [Google Scholar]
- Boudreau R. J., Drummond G. I. A modified assay of 3':5'-cyclic-AMP phosphodiesterase. Anal Biochem. 1975 Feb;63(2):388–399. doi: 10.1016/0003-2697(75)90361-9. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Dedman J. R., Potter J. D., Means A. R. Biological cross-reactivity of rat testis phosphodiesterase activator protein and rabbit skeletal muscle troponin-C. J Biol Chem. 1977 Apr 10;252(7):2437–2440. [PubMed] [Google Scholar]
- Ebashi S., Wakabayashi T., Ebashi F. Troponin and its components. J Biochem. 1971 Feb;69(2):441–445. doi: 10.1093/oxfordjournals.jbchem.a129486. [DOI] [PubMed] [Google Scholar]
- Eisenberg E., Kielley W. W. Troponin-tropomyosin complex. Column chromatographic separation and activity of the three, active troponin components with and without tropomyosin present. J Biol Chem. 1974 Aug 10;249(15):4742–4748. [PubMed] [Google Scholar]
- Greaser M. L., Gergely J. Reconstitution of troponin activity from three protein components. J Biol Chem. 1971 Jul 10;246(13):4226–4233. [PubMed] [Google Scholar]
- Head J. F., Perry S. V. The interaction of the calcium-binding protein (troponin C) with bivalent cations and the inhibitory protein (troponin I). Biochem J. 1974 Feb;137(2):145–154. doi: 10.1042/bj1370145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Head J. F., Weeks R. A., Perry S. V. Affinity-chromatographic isolation and some properties of troponin C from different muscle types. Biochem J. 1977 Mar 1;161(3):465–471. doi: 10.1042/bj1610465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Murray A. C., Kay C. M. Separation and characterization of the inhibitory factor of the troponin system. Biochem Biophys Res Commun. 1971 Jul 2;44(1):237–244. doi: 10.1016/s0006-291x(71)80184-5. [DOI] [PubMed] [Google Scholar]
- Nagy B., Gergely J. Extent and localization of conformational changes in troponin C caused by calcium binding. Spectral studies in the presence and absence of 6 M urea. J Biol Chem. 1979 Dec 25;254(24):12732–12737. [PubMed] [Google Scholar]
- Nairn A. C., Perry S. V. Calmodulin and myosin light-chain kinase of rabbit fast skeletal muscle. Biochem J. 1979 Apr 1;179(1):89–97. doi: 10.1042/bj1790089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter J. D., Gergely J. The calcium and magnesium binding sites on troponin and their role in the regulation of myofibrillar adenosine triphosphatase. J Biol Chem. 1975 Jun 25;250(12):4628–4633. [PubMed] [Google Scholar]
- Potter J. D., Gergely J. Troponin, tropomyosin, and actin interactions in the Ca2+ regulation of muscle contraction. Biochemistry. 1974 Jun 18;13(13):2697–2703. doi: 10.1021/bi00710a007. [DOI] [PubMed] [Google Scholar]
- Potter J. D., Seidel J. C., Leavis P., Lehrer S. S., Gergely J. Effect of Ca2+ binding on troponin C. Changes in spin label mobility, extrinsic fluorescence, and sulfhydryl reactivity. J Biol Chem. 1976 Dec 10;251(23):7551–7556. [PubMed] [Google Scholar]
- STARK G. R. ON THE REVERSIBLE REACTION OF CYANATE WITH SULFHYDRYL GROUPS AND THE DETERMINATION OF NH2-TERMINAL CYSTEINE AND CYSTINE IN PROTEINS. J Biol Chem. 1964 May;239:1411–1414. [PubMed] [Google Scholar]
- Schaub M. C., Perry S. V. The regulatory proteins of the myofibril. Characterization and properties of the inhibitory factor (troponin B). Biochem J. 1971 Jul;123(3):367–377. doi: 10.1042/bj1230367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens F. C., Walsh M., Ho H. C., Teo T. S., Wang J. H. Comparison of calcium-binding proteins. Bovine heart and brain protein activators of cyclic nucleotide phosphodiesterase and rabbit skeletal muscle troponin C. J Biol Chem. 1976 Aug 10;251(15):4495–4500. [PubMed] [Google Scholar]
- Sutoh K. Direct evidence for the calcium-induced change in the quaternary structure of troponin in situ. Millisecond cross-linking of troponin components by a photosensitive heterobifunctional reagent. Biochemistry. 1980 Apr 29;19(9):1977–1983. doi: 10.1021/bi00550a038. [DOI] [PubMed] [Google Scholar]
- Van Eerd J. P., Kawasaki Y. Effect of calcium(II) on the interaction between the subunits of troponin and tropomyosin. Biochemistry. 1973 Nov 20;12(24):4972–4980. doi: 10.1021/bi00748a024. [DOI] [PubMed] [Google Scholar]
- Watterson D. M., Harrelson W. G., Jr, Keller P. M., Sharief F., Vanaman T. C. Structural similarities between the Ca2+-dependent regulatory proteins of 3':5'-cyclic nucleotide phosphodiesterase and actomyosin ATPase. J Biol Chem. 1976 Aug 10;251(15):4501–4513. [PubMed] [Google Scholar]
- Wnuk W., Cox J. A., Kohler L. G., Stein E. A. Calcium- and magnesium-binding properties of a high affinity calcium-binding protein from crayfish sarcoplasm. J Biol Chem. 1979 Jun 25;254(12):5284–5289. [PubMed] [Google Scholar]
- Yagi K., Yazawa M., Kakiuchi S., Ohshima M., Uenishi K. Identification of an activator protein for myosin light chain kinase as the Ca2+-dependent modulator protein. J Biol Chem. 1978 Mar 10;253(5):1338–1340. [PubMed] [Google Scholar]